19 January 2021: Original Paper
Sirolimus Adverse Event Profile in a Non-Clinical Trial Cohort of Heart Transplantation Patients
Karim Sallam12DEFG, Geetha P. Bhumireddy2B, Vishnu D. Evuri2B, Joshua P. Abella2BC, Francois Haddad3D, Hannah A. Valentine3AD, Patricia K. Nguyen32CDE, Michael X. Pham4ADEG*DOI: 10.12659/AOT.923536
Ann Transplant 2021; 26:e923536
Abstract
BACKGROUND: Sirolimus has been used increasingly in heart transplantation for its ability to reduce acute rejection, prevent the progression of cardiac allograft vasculopathy (CAV), and preserve renal function. We sought to assess the adverse reactions associated with the use of sirolimus compared to mycophenolate mofetil (MMF).
MATERIAL AND METHODS: We retrospectively reviewed the charts of 221 adult heart transplant patients who received either sirolimus or MMF as part of their immunosuppression from June 1, 2001 to April 1, 2005. Patients were assigned to 2 groups based upon immunosuppression use. The prevalence and types of complications were recorded in each group.
RESULTS: Sirolimus was received by 109 patients and 112 patients received MMF during the study period. Seventy-seven patients (71%) in the sirolimus group experienced adverse reactions compared to 45 patients (40%) in the MMF group (P<0.01). Compared to MMF, the use of sirolimus was associated with a higher prevalence of elevated triglyceride levels, lower-extremity edema, and oral ulcerations. Sirolimus was discontinued due to adverse reactions in 22% of patients, whereas no patients in the MMF group experienced adverse effects requiring drug discontinuation.
CONCLUSIONS: Compared to MMF, sirolimus use is associated with a higher prevalence of adverse reactions requiring drug discontinuation, but most patients were able to stay on therapy despite adverse effects.
Keywords: Drug-Related Side Effects and Adverse Reactions, Heart Transplantation, Immunosuppression, Sirolimus, Immunosuppressive Agents
Background
The proliferation signal inhibitors (PSI) sirolimus and everolimus are used in heart transplantation as part of maintenance immunosuppression regimens in selected recipients with cardiac allograft vasculopathy (CAV), frequent cutaneous malignancies, or significant renal impairment. This class of drugs prevents B and T cell proliferation in response to cytokine signals by binding to the FK-binding protein and inhibiting the activity of the mammalian target of rapamycin (mTOR), thus blocking interleukin-2-mediated signal transduction and causing cell cycle arrest at the G1 to S phase. They also exhibit anti-proliferative activity in vascular smooth muscle cells and fibroblasts [1,2]. Clinical studies have demonstrated decreased allograft rejection rates and reduced incidence of CAV when used in a
However, the utility of these agents to date has been limited by the high incidence of drug-related adverse effects observed with sirolimus and by the need to discontinue the drug prior to major surgery due to its effects on delayed wound healing [11,12]. In this retrospective study, we sought to describe the indications and prevalence of sirolimus-related adverse reactions observed in clinical practice.
Material and Methods
PATIENT POPULATION:
After obtaining Institutional Review Board approval, we retrospectively reviewed the medical records of all adult heart transplant recipients who received care at Stanford University Medical Center between June 1, 2001 and April 1, 2005. Patients were classified into 2 groups based on receiving sirolimus or mycophenolate mofetil (MMF) as part of their immunosuppression regimen. Patients who were concurrently on sirolimus and MMF were assigned to the sirolimus group. Patients on azathioprine were not included in the study. We included patients who were started on sirolimus immediately following transplant or at a later date.
IMMUNOSUPPRESSION:
Although our center employed a standard protocol for immunosuppression, there was variability in the immunosuppression applied owing to patient characteristics necessitating tailoring of therapy or changes to institutional immunosuppression protocol over time. Induction therapy was used in most patients and consisted of either the interleukin-2 receptor antagonist daclizumab, the monoclonal mouse antibody OKT3, or with polyclonal antithymocyte globulin. Maintenance immunosuppression consisted of a calcineurin inhibitor (either cyclosporine or tacrolimus) and either MMF or sirolimus. A subset of patients were maintained on a calcineurin inhibitor-free regimen consisting of sirolimus and mycophenolate mofetil. One patient in the study was maintained on a calcineurin inhibitor in addition to both sirolimus and mycophenolate mofetil. The choice of immunosuppression regimen was left to the primary provider based on patient characteristics and perceived benefits of each regimen. Corticosteroids were initiated immediately postoperatively and progressively tapered and discontinued by 9–12 months in most patients per standard protocol; however, a subset of patients was maintained on long-term low-dose corticosteroids due to the presence of steroid withdrawal symptoms or recurrent rejection. Sirolimus was administered at a starting dose of 1–3 mg daily, without a loading dose, and targeted to achieve a 24-h trough level of approximately 5–10 ng/mL. MMF was administered at a typical starting dose of 1000 mg twice daily, and the dose was down-titrated due to adverse effects or up-titrated due to recurrent rejection. Mycophenolic acid levels were not routinely obtained during the study period.
DEFINITIONS:
The primary outcome in this study was the composite incidence of adverse events defined as: elevated triglycerides (TG), lower-extremity edema, wound healing complications, leukopenia, anemia, elevated low-density lipoprotein (LDL), pneumonitis, alveolar hemorrhage, thrombocytopenia, oral ulcerations, pleural effusion requiring drainage, retrosternal fluid collection, recurrent pericardial effusion, joint pain, gastrointestinal intolerance, thrombosis, or dermatitis.
STATISTICAL ANALYSIS:
Statistical analysis was performed using Stata (StataCorp, College Station, Texas). Comparison between groups was performed using the Wilcoxon rank test and Fisher exact test, as appropriate. Based on the Shapiro-Wilk test, continuous variables were not normally distributed and are expressed as median and interquartile range. A multivariate logistic regression analysis was performed to assess whether patients treated with sirolimus had a higher risk of developing any complication than those treated with MMF. Dependent variables included in the model were age, body mass index, diabetes, transplant indication, CMV recipient, renal insufficiency, CMV donor, CAV, and other immunosuppressive agents (cyclosporine, tacrolimus, mycophenolate mofetil, and prednisone).
Results
PATIENT CHARACTERISTICS:
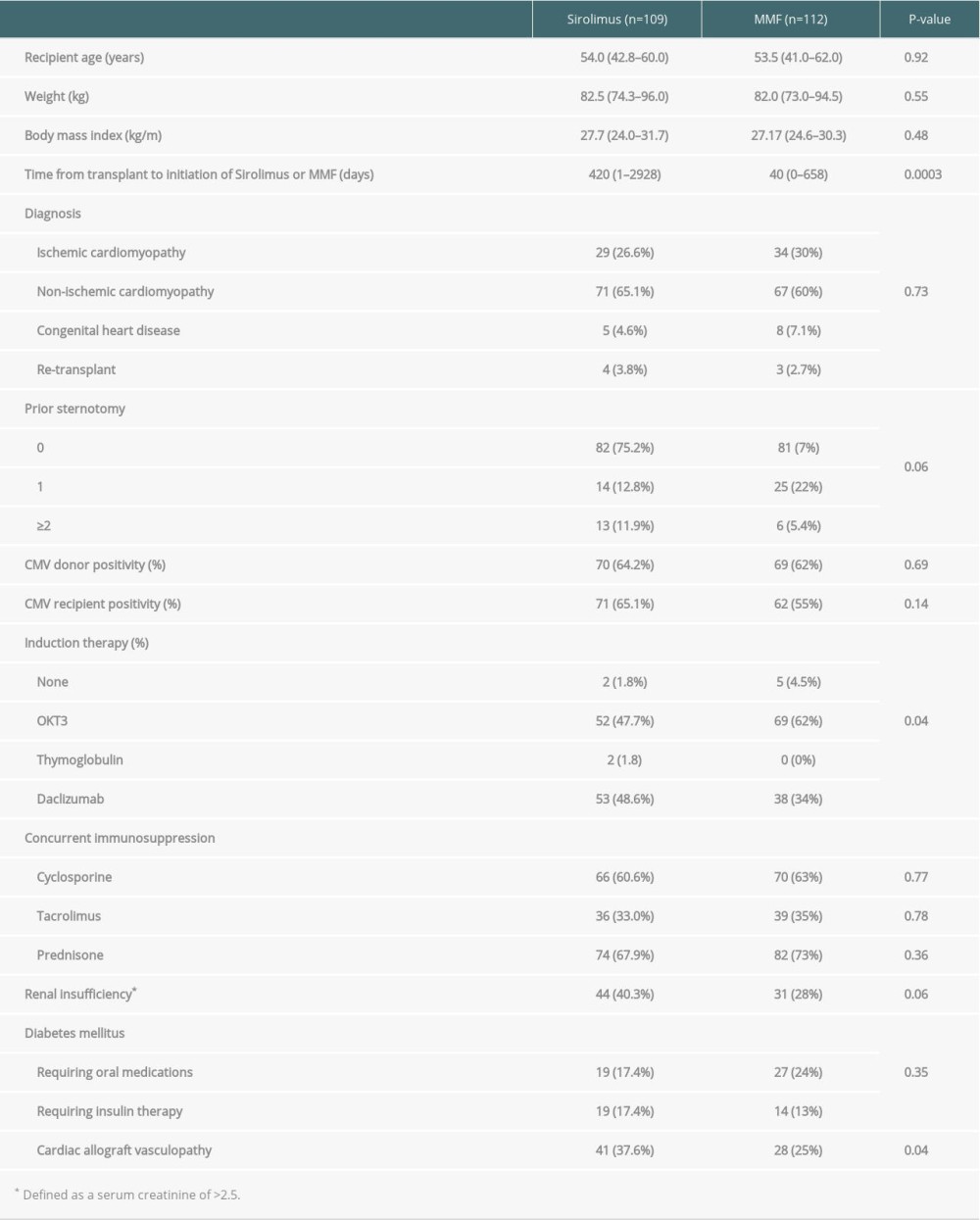
A total of 221 patients with available follow-up data were identified during the study period. Patients were transplanted between 1982 and 2005, and the median follow-up was 50.8 months (range, 11–216 months). Patients on sirolimus and MMF immunosuppression were similar with respect to age, weight, pre-transplant diagnosis, time after transplantation, and baseline immunosuppression (Table 1).
Compared to the MMF group, patients in the sirolimus group were more likely to have CAV, more likely to have received daclizumab induction versus OKT3, and with a notable trend of having more underlying renal insufficiency. During the study period, sirolimus was most commonly used as part of
ADVERSE REACTIONS:
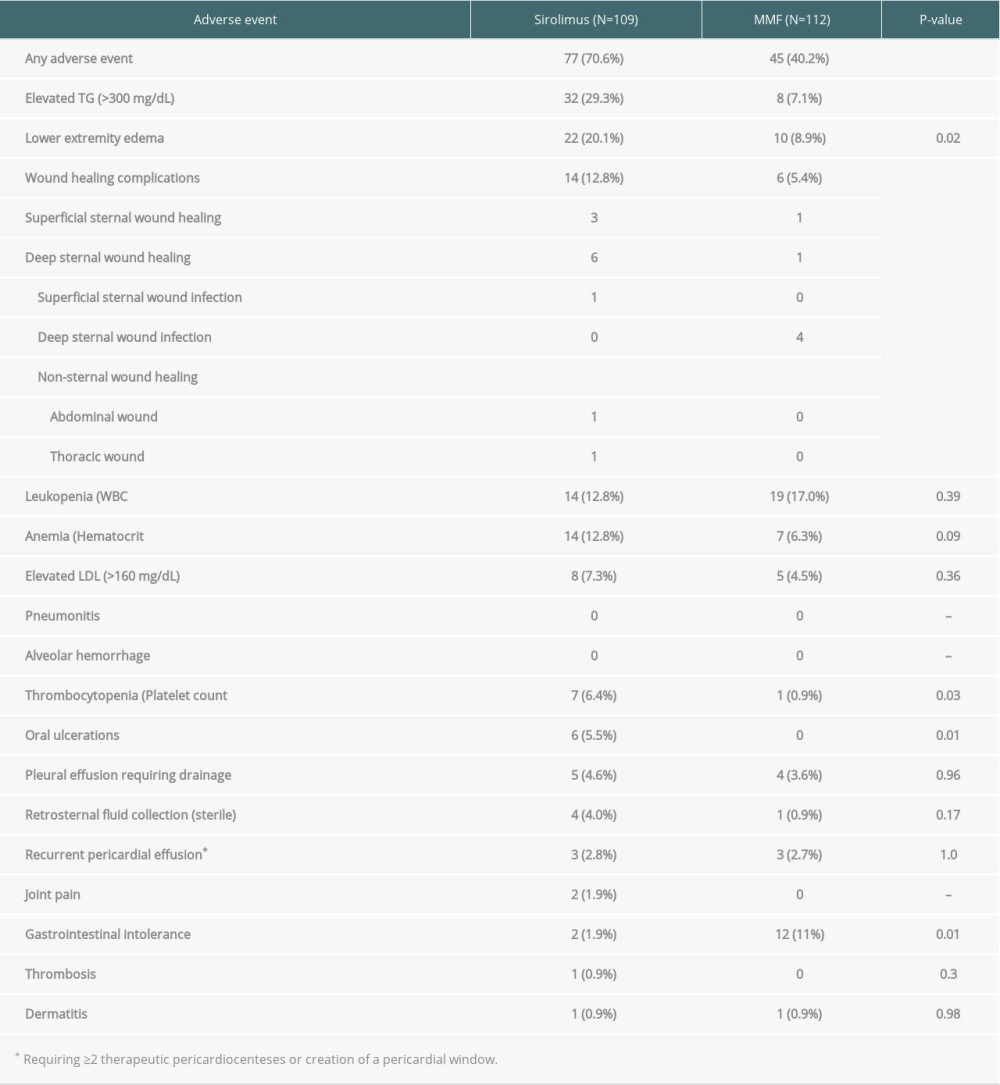
Overall, 122 patients (55%) developed drug-related adverse reactions during the study period (Table 2). Adverse reactions were more common in the sirolimus group compared to the MMF group (71% vs. 40%, P <0.01). Statistically significant complications in the sirolimus group were: elevated triglycerides (29%), lower-extremity edema (20%), wound healing complications (13%), thrombocytopenia (6%), and oral ulcerations (5.5%). The median times to the development of complications were: elevated triglycerides 126 days, lower-extremity edema 311 days, wound healing complications 37 days, thrombocytopenia 15 days, and oral ulcers 101 days. The most common reason for discontinuation of sirolimus was drug-related adverse events (36%), followed by anticipated surgical procedures (22%). The median time from drug initiation to discontinuation was 320 days (range, 6–763 days). In comparison, the only statistically significant adverse reaction in the MMF group was gastrointestinal intolerance (11%). No patients in the MMF group developed an adverse reaction requiring drug discontinuation, although dose adjustments were frequently made in response to these complications.
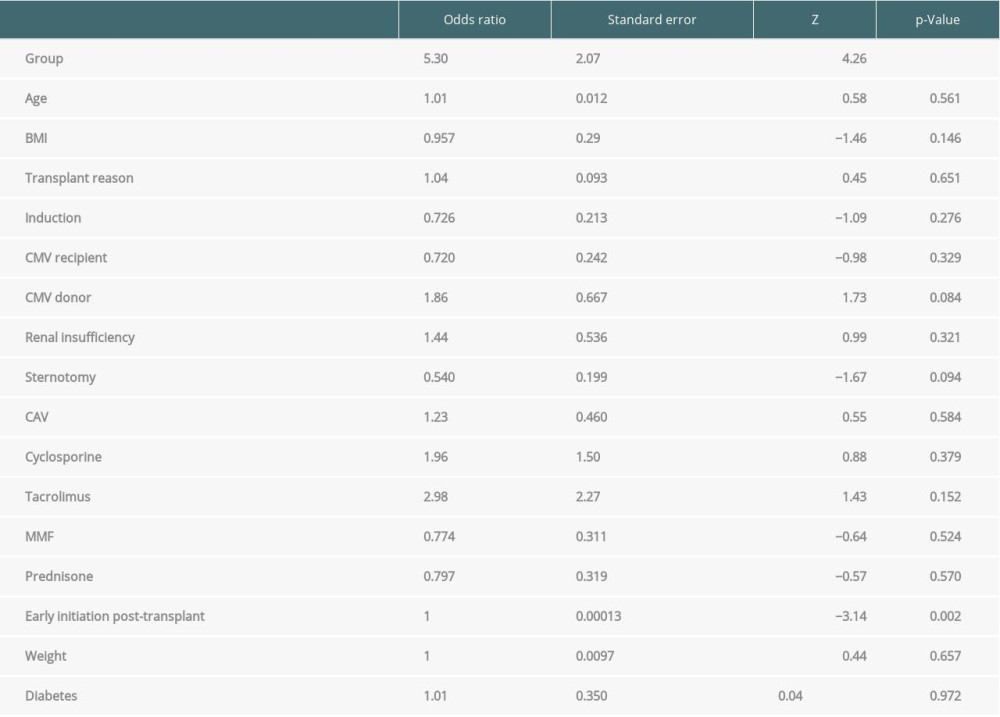
A secondary analysis was performed to evaluate if any clinical differences explain the univariate differences in rates of adverse outcomes between groups. No association was identified between demographics or comorbid conditions and the probability of experiencing adverse outcomes (Table 3).
Discussion
In this retrospective analysis of heart transplant patients, we observed a statistically significant higher incidence of adverse events in patients receiving sirolimus compared to MMF as part of their immunosuppression regimen. We also found that more patients required discontinuation of sirolimus due to the adverse events compared to the MMF group. The most frequent adverse events identified in the sirolimus group were hypertriglyceridemia and lower-extremity edema. The difference in the incidence of adverse outcomes between immunosuppression regimens was not explained by patients’ demographics or clinical characteristics.
Sirolimus has been extensively used in heart transplantation immunosuppression owing to its lower nephrotoxicity profile, favorable impact on post-transplant CAV, and malignancy outcomes. The adverse effects profile of sirolimus has been reported by other groups [3,13,14], but the present analysis represents a real-world population beyond the limitations of clinical trials. Furthermore, prior published trials compared sirolimus to azathioprine, which is rarely clinically used in this era; this study compares sirolimus to MMF, which is a more contemporary alternative to sirolimus in post-transplant immunosuppression regimens. The population of patients included present mixed groups that were started on sirolimus immediately after transplant and those who were started late.
More recently, everolimus, another mTOR inhibitor, has had increased use, but widespread clinical adoption of the drug remains limited by cost and barriers to measuring drug levels. Thus, sirolimus remains the primary mTOR inhibitor in clinical use in heart transplant patients, and research of the adverse events associated with its use is of great clinical importance.
Patients initiated on sirolimus were more likely to have been started on the drug later after transplant compared to MMF initiation, have been induced daclizumab, and have a higher incidence of CAV and renal insufficiency. The delay in initiation of sirolimus therapy after transplant likely reflects a change in clinical practice based on clinical trial results describing sternal wound healing complications associated with early initiation [6]. The fact that sirolimus has been associated with improved outcomes in patients with CAV and renal insufficiency explains the trend of clinicians preferentially initiating sirolimus and enriching the sirolimus cohort with those patients. In our cohort, patients in the sirolimus group more frequently received induction with daclizumab compared to the MMF group owing to an institutional shift in induction therapy that coincided with increased use of sirolimus.
Sirolimus was associated with a 71% incidence of adverse events compared with 40% of patients receiving an MMF-based immunosuppression regimen. Sirolimus was associated with a statistically significant higher incidence in hypertriglyceridemia, lower-extremity edema, wound healing complications, thrombocytopenia, and oral ulcers, whereas MMF was associated with a higher incidence of gastrointestinal intolerance. The profile of sirolimus-associated adverse effects is largely similar to what has been reported, although at much lower frequencies than what has been reported in clinical trials for heart and kidney transplantation [3,10,11]. However, the rate of discontinuation of sirolimus in this study was similar to previous reports, suggesting that higher frequencies of adverse events reported in clinical trials may be in part due to reporting bias in a clinical trial setting.
Contrary to prior reports, in our population, sirolimus was associated with an overall low incidence of GI intolerance; 1.9% in our study versus 49% in other studies. While this may be in part due to reporting bias in clinical trials, it is noteworthy that the incidence of GI intolerance was actually higher in the MMF group. Similarly, the incidence of anemia and leukopenia reported by other groups was lower in our population and was in fact no worse than in the MMF group. We did not observe the pulmonary toxicity reported by other studies, and the overall incidence of pericardial or pleural effusions was low. Such adverse effects are rare, with an incidence below 5%, explaining why this was not observed in our population.
Almost one-third of patients with sirolimus-associated complications required drug discontinuation, whereas no patients in the MMF group required discontinuation, but some patients had their MMF dose reduced. This discordance is explained by a number of factors. First, sirolimus has a more frequent and broader profile of adverse effects, making it more likely to be discontinued. Second, there is greater clinical experience in utilizing reduced-dose MMF as part of maintenance immunosuppression compared to reduced-dose sirolimus.
Examining demographic interactions with adverse effect likelihood did not reveal significant interactions. Age, BMI, history of diabetes mellitus, reason for transplant, prior sternotomy, use of induction therapy, CMV status, renal insufficiency, CAV, or type of secondary immunosuppression agent used did not influence risk of sirolimus adverse events.
The aggregate of our data indicates that overall sirolimus does carry a more burdensome adverse effect profile compared to MMF, and more patients required discontinuation of therapy due to adverse events. Nonetheless, given the favorable outcome sirolimus has on CAV, nephrotoxicity, and malignancy in post-transplant patients, a large group of patients likely warrant a trial of the drug. Based on our data, the incidence of adverse events is lower than described in clinical trials, and over two-thirds of patients will continue on the drug despite adverse effects.
There are limitations to this study. First, the adverse event reporting primarily relies on clinician documentation and patients were not routinely screened for adverse outcomes. Additionally, this analysis is several years old, making the clinical setting slightly older than current standard practice. Furthermore, this was a single-center experience subject to the biases and unique practices represented in this program. This was not a clinical trial in which a standard study protocol was applied between groups. Although we did not identify an interaction between steroid use or type of calcineurin inhibitor used, we acknowledge that the variability in treatment may have created an unrecognized bias driving the differences in adverse events between groups. However, the strength of the dataset is that it predates changes in the publication of clinical trial data that influenced clinical practice, providing an unbiased perspective on the adverse outcome profile of sirolimus. Additionally, the length of follow-up for adverse events (over 4 years) provides insight beyond that detailed by clinical trials of the drug. Lastly, everolimus is a another mTOR inhibitor that has been clinically adopted as part of clinical practice and may have a different adverse effect profile; thus, extrapolation of our data for everolimus should not be attempted [15,16].
Conclusions
Overall, our findings show that sirolimus is associated with more adverse events compared to MMF-based regimens requiring discontinuation of the drug. There are no clinical characteristics that predict a higher incidence of adverse events. Nonetheless, 69% continued on the drug to receive clinical benefit. With less than 20% of heart transplant patients receiving sirolimus, more patients stand to benefit with more widespread use in post-transplant patients with renal dysfunction, CAV, or malignancy [6,17]. Our data provide insight into the real-world adverse events profile to be expected with expanded clinical use.
References
1. Akselband Y, Harding MW, Nelson PA, Rapamycin inhibits spontaneous and fibroblast growth factor beta-stimulated proliferation of endothelial cells and fibroblasts: Transplant Proc, 1991; 23(6); 2833-36
2. Marx SO, Jayaraman T, Go LO, Marks AR, Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells: Circ Res, 1995; 76(3); 412-17
3. Keogh A, Richardson M, Ruygrok P: Circulation, 2004; 110(17); 2694-700
4. Eisen HJ, Kobashigawa J, Starling RC, Everolimus versus mycophenolate mofetil in heart transplantation: A randomized, multicenter trial: Am J Transplant, 2013; 13(5); 1203-16
5. Eisen HJ, Tuzcu EM, Dorent R, Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients: N Engl J Med, 2003; 349(9); 847-58
6. Asleh R, Briasoulis A, Kremers WK, Long-term sirolimus for primary immunosuppression in heart transplant recipients: J Am Coll Cardiol, 2018; 71(6); 636-50
7. Jennings DL, Lange N, Shullo M, Outcomes associated with mammalian target of rapamycin (mTOR) inhibitors in heart transplant recipients: A meta-analysis: Int J Cardiol, 2018; 265; 71-76
8. Mancini D, Pinney S, Burkhoff D, Use of rapamycin slows progression of cardiac transplantation vasculopathy: Circulation, 2003; 108(1); 48-53
9. Gleissner CA, Doesch A, Ehlermann P, Cyclosporine withdrawal improves renal function in heart transplant patients on reduced-dose cyclosporine therapy: Am J Transplant, 2006; 6(11); 2750-58
10. Potter BJ, Giannetti N, Edwardes MD: Clin Transplant, 2007; 21(3); 305-8
11. Zuckermann A, Osorio-Jaramillo E, Aliabadi-Zuckermann AZ, mTOR Inhibition and clinical transplantation: Heart: Transplantation, 2018; 102(2S Suppl 1); S27-29
12. Kuppahally S, Al-Khaldi A, Weisshaar D: Am J Transplant, 2006; 6(5 Pt 1); 986-92
13. Kaplan B, Qazi Y, Wellen JR, Strategies for the management of adverse events associated with mTOR inhibitors: Transplant Rev, 2014; 28(3); 126-33
14. Machado PG, Felipe CR, Hanzawa NM: Clin Transplant, 2004; 18(1); 28-38
15. Potena L, Pellegrini C, Grigioni F: Transplantation, 2018; 102(3); 493-501
16. Rashidi M, Esmaily S, Fiane AE: Int J Cardiol, 2016; 210; 80-84
17. Asleh R, Clavell AL, Pereira NL, Incidence of malignancies in patients treated with sirolimus following heart transplantation: J Am Coll Cardiol, 2019; 73(21); 2676-88
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860