12 February 2021: Original Paper
Inferior Vena Cava and Venous Outflow Reconstruction in Living Donor Liver Transplantation in Children: A Single-Center Retrospective Study and Literature Review
Marek Szymczak1ABDEF, Piotr J. Kaliciński1ADE, Grzegorz Kowalewski1BCDEF*, Mateusz Ciopiński1B, Małgorzata Markiewicz-Kijewska1B, Dorota Broniszczak1BD, Bożenna Dembowska-Bagińska2AD, Andrzej Kościesza3BD, Grażyna Brzezińska-Rajszys4BD, Waldemar Patkowski5B, Marek Stefanowicz1BDDOI: 10.12659/AOT.926217
Ann Transplant 2021; 26:e926217
Abstract
BACKGROUND: In this report, we present technical problems and solutions used in the reconstruction of the inferior vena cava and graft venous outflow during living donor liver transplantation (LDLT) in children.
MATERIAL AND METHODS: In 65 grafts out of 379 liver transplantations from living donors, reconstruction of multiple hepatic venous branches and/or IVC was necessary. In 4 cases, cryopreserved deceased donor venous grafts were used for the reconstruction of the IVC and/or HV.
RESULTS: Follow-up ranged from 2 months to 17.8 years (median 7.2 years). In 4 children, liver re-transplantation was required for a reason not related to venous outflow (biliary complications in 3 patients, graft insufficiency caused by small-for-size syndrome). Two patients died: 1 due to tumor recurrence and 1 due to multi-organ failure. Fifty-nine patients are alive with good liver function. One patient (1.5%) after deceased donor venous graft reconstruction showed symptoms of venous outflow obstruction, which was successfully treated with endovascular balloon angioplasty and stent placement. The remaining 59 transplanted patients do not show any signs of venous outflow obstruction.
CONCLUSIONS: In most cases, the reconstruction of multiple hepatic veins of living donor allografts can successfully be done with local venoplasty, while using cold-stored vein grafts may be helpful in selected cases of LDLT.
Keywords: Allografts, Liver Transplantation, Living Donors, inferior vena cava, Child, Hepatic Veins, Vena Cava, Inferior
Background
Living donor liver transplantation in children is one of the most challenging surgical procedures due to high anatomical variability, the huge discrepancy between donor and recipient vessels size, and shortness of graft vessels. Any technical imperfection or complications in vascular anastomoses can cause severe adverse effects, graft loss, and even death of the recipient. Technical problems include the need for a recipient hepatic artery or portal vein inflow reconstruction, or reconstruction of graft hepatic veins to assure good blood outflow from the transplanted part of the liver. Technical problems can be related to the large distance between multiple hepatic venous branches of the graft, very narrow venous drainage, or extremely short cuff. Specific technical problems affect children with liver tumors. In the case of unresectable liver tumors in children, liver transplantation (LT), including LT from a living donor, is the only life-saving treatment. Removal of the retrohepatic IVC is sometimes mandatory for oncological reasons due to vascular invasion or compression [1–3]. In the case of LDLT and the need for removal of the retrohepatic IVC, reconstruction can be performed using either autologous, cryopreserved allogeneic, or synthetic vascular grafts [4–6]. In these situations, the necessity of reconstruction of venous outflow using vascular graft may arise.
This study aimed to analyze the technical problems encountered with hepatic veins outflow in pediatric living donor liver transplantations.
We present our experience with living donor liver transplantation performed in children, in which venous outflow of the liver graft or the retrohepatic IVC reconstruction was necessary.
Material and Methods
CHARACTERISTICS OF LIVER GRAFTS:
A fragment of the liver from a living donor was procured in an adult transplantation surgery unit with extensive experience in liver surgery which cooperates with our center.
The donors of the liver graft were the mother in 40 patients, the father in 20 patients, a grandmother in 2 patients, an uncle in 2 patients, and an aunt in 1 patient. Grafts consisted of segments II+III in 54 patients, the left lobe in 10 patients, and the right lobe in 1 patient. The graft-to-recipient weight ratio (GRWR) ranged from 0.9% to 5.4% (median 2.6%).
In 63 living donor grafts, multiple hepatic veins were observed on the transection surface (2 veins in 58 grafts, 3 veins in 3 grafts, and 4 veins in 2 grafts). In another 2 patients with unresectable hepatoblastoma and tumor invasion/infiltration of the IVC wall, resection of the retrohepatic IVC was necessary up to the diaphragm during the recipient liver hepatectomy (Table 1).
VENOUS OUTFLOW FROM GRAFTS WITH SINGLE HV:
In the case of a single graft’s HV, standard triangle-shaped “piggyback” anastomosis was routinely performed to the confluence of LHV and MHV, with 3 running non-absorbable 6/0 or 5/0 monofilament sutures (Figure 1).
VENOUS OUTFLOW RECONSTRUCTIONS:
In 2 children with unresectable HBL qualified for LDLT, compression and invasion of the IVC by tumor was found on CT examination and intraoperative imaging.
Therefore, own liver hepatectomy included retrohepatic vena cava resection and subsequent reconstruction of the IVC using a cryopreserved deceased donor venous graft.
An iliac venous graft from a tissue bank was used for caval reconstruction. The venous conduit after preparation on the back table was anastomosed end-to-end to the infrahepatic end of the IVC. The upper anastomosis of the interposition vein graft was created to also include the “piggyback” triangle anastomosis of the hepatic vein of the graft (Figures 2A–2C, 3).
In another patient, due to a large distance (approx. 30 mm) between 2 hepatic venous branches of the graft, a common outflow was created with a short venous graft from a tissue bank. Two separate hepatic veins were anastomosed on the back table, end-to-side to the venous graft. The reconstructed venous drainage was then anastomosed end-to-side to the recipient’s IVC (Figure 4).
Another reconstruction was performed in a BA patient whose graft had a very narrow and shallow confluence of the 2 hepatic veins. Venous outflow reconstruction on the back table was performed by creating a cuff extending the venous outlet from the frozen venous graft (Figure 5).
In 1 patient with ALF, urgent right liver lobe transplantation from a living donor was performed. Two hepatic veins were found on the graft surface at a distance of 8 cm. Relatively long graft venous cuffs allowed making 2 separate triangle-shaped end-to-side anastomoses to the recipient’s IVC (Figure 6).
In the remaining 60 patients with grafts with multiple veins, their wall was elongated until obtaining a cuff sufficient for their anastomosis. The above solution was possible for grafts with a distance of up to 2 cm between HV branches. Subsequently, anastomosis to the recipients’ IVC was performed in a triangle-shaped manner (Figure 7).
Portal vein, hepatic artery, and bile duct anastomoses were performed typically in all patients from the analyzed group. The bile duct of the graft was anastomosed in most cases end-to-side of the Roux-en-Y loop. In most patients, Vicryl mesh was inserted into the abdominal wall to avoid compression of the graft.
Standard immunosuppression consisted of tacrolimus and mycophenolate mofetil (MMF), with individual modifications depending on the patient’s characteristics. Routine anticoagulation was used postoperatively in all patients with enoxaparin, followed by acetylsalicylic acid at a dose of 1 mg/kg body weight once a day for 6 months. Doppler ultrasound was performed twice a day during the first week after surgery, then once a day for the next week and at every check-up in the clinic. In cancer patients, complementary chemotherapy was continued according to oncological protocols.
The consumption of blood products, duration of anhepatic phase, CIT, GRWR (graft-to-recipient weight ratio), total operation time, and hospitalization time in patients requiring complex IVC or venous outflow reconstruction were analyzed. These data were then compared with a group consisting of patients who required standard venous reconstruction at the time of LDLT.
STATISTICAL ANALYSIS:
Data were analyzed using Statistica 12 software (StatSoft, Inc.). The analysis involved the assessment of baseline demographics and clinical data using median ranges and distributions for categorical variables. The
Results
Patient age at transplantation ranged from 5 months to 14 years (median 15 months), and body mass ranged from 5.1 kg to 47 kg (median 9.7 kg). The indications for liver transplantation were: biliary atresia (BA) in 40 patients, acute liver failure (ALF) in 5 patients, hepatoblastoma (HBL) in 3 patients, hepatocarcinoma (HCC) in 2 patients, vascular tumor in 1 patient, cystic fibrosis in 2 patients, alpha-1 antitrypsin deficiency (A1AD) in 2 patients, re-transplantation in 2 patients, and other reasons in 8 patients.
PELD scores ranged from −10 to 35 (median 14) in all groups (including patients without liver insufficiency, eg, HBL or HCC), except for patients with liver insufficiency, in whom scores ranged from 4 to 35 (median 17). Clinical data of patients with complex vascular reconstructions and simple venous anastomosis with associated
Follow-up ranged from 2 months to 17.8 years (median 7.2 years). Two patients died 4 months and 8.4 years after LDLT, one due to tumor recurrence and the other due to multi-organ failure. In 4 patients, liver re-transplantation was necessary due to reasons unrelated to venous outflow reconstruction (biliary complications in 3 patients and graft insufficiency caused by small-for-size syndrome in 1). These 4 children were re-transplanted between 2 and 33 months after LDLT. Eleven patients were transferred to the care of the transplantation center for adults.
Patient and graft survival were 97% and 91.8%, respectively, within the follow-up period. None of the patients with graft HV reconstruction presented venous outflow obstruction or thrombosis. No surgical or radiological interventions regarding venous outflow from the liver were necessary in this group of patients. Kaplan-Meier plots for patient and graft survival are presented in Figures 8 and 9, respectively.
Four children were successfully transplanted with the use of a cold-stored vascular graft during reconstruction. In 2 cases, it was used for IVC replacement, while in the other 2 it was used for venous outflow reconstruction. In 1 BA patient after venous outflow reconstruction, Doppler ultrasound raised a suspicion of the stenosis of the graft anastomosis 3 months after LT. Angio-CT examination confirmed the suspicion. Percutaneous transvascular balloon dilation of the narrowed venous graft was done and a stent 8 mm in diameter and 20 mm in length was introduced into the hepatic vein, resulting in normalization of blood flow.
In 2 HBL patients after retrohepatic IVC replacement with frozen vein grafts, Doppler ultrasound showed good flow in the graft used. There have been no signs of tumor recurrence, the AFP concentration remains within the normal range, and the oncological treatment has been completed. HBL patients are managed according to SIOPEL guidelines for the treatment of hepatoblastoma. Before LTx, patients receive chemotherapy, depending on standard-risk, high-risk, and very high-risk tumors. The standard treatment is 4 cycles of preoperative chemotherapy followed by surgical resection and 2 postoperative cycles of therapy. Patients with IVC involvement meet the high-risk criteria and receive the dose-intensive “superPLADO” arm of the SIOPEL study before liver transplantation.
Discussion
Transplantation from a living donor carries the possibility of several technical problems concerning both vascular and biliary anastomoses. Good venous outflow from the partial graft, in children mostly consisting of segments II and III, is one of the key preconditions for successful transplantation. In the case of LDLT, a piggyback anastomosis is normally performed to the recipient’s retained retrohepatic caval vein. The main problem encountered with this anastomosis is its obstruction, causing acute or chronic graft congestion and damage [7]. The obstruction may be caused by an anastomosis that is too narrow, twisting of the anastomosis, or multiple tied, sutured, or not precisely reconstructed hepatic veins on the graft cut surface.
According to a recent study, veno-occluded regions have approximately 40% of the maximum function of the corresponding regions [8]. With all the above factors in mind, a thorough knowledge of the vascular system of the liver is essential in the aspect of organ procurement in living organ donation. Sometimes, despite the development of visualization techniques, unexpected intraoperative decision-making is required due to various anomalies.
Tani et al [8] conducted thorough research on the venous system of the liver, showing that the total amount of blood drained by the left hepatic vein, the middle hepatic vein, and the right hepatic vein accounts for 93% of liver outflow; the remaining 7.0% of the liver is drained by short hepatic veins and accessory veins. Different variants also apply (to a lesser extent) to the left and middle hepatic vein region, which are especially important during the procurement of the II and III liver segments, and sometimes also segment IV, for a pediatric recipient. In the case of multiple venous branches found on the surface of the liver graft, the best solution is to bring them close together and anastomose them to one orifice at the back table. This requires some lengthening of the cuff of individual veins and subsequent wide, side-to-side triangular anastomosis to the IVC; in our experience, usually to the recipient’s LHV and MHV confluence. A different, more complex problem arises in the case of multiple veins with a long distance between them. There are only a few reports in the literature about the reconstruction of venous outflow from the liver graft. They mainly concentrate on adult patients and transplantation of the right hepatic lobe from living donor or domino liver transplantation [9,10]. In the presented material, in 1 patient who was transplanted with the right lobe from a live donor, it was decided to perform 2 separate end-to-side anastomoses to the retrohepatic IVC. Hepatic outflow reconstructions in modified right liver lobe grafts without the MHV are performed in many centers [10]. In the case of multiple left or right lobe hepatic veins and the inability to combine them into a single outflow channel, back-table venoplasty may be a suitable procedure. It can be accomplished using venous conduits such as cryopreserved venous allografts, autologous venous grafts, donors’ or recipients’ umbilical vein, or synthetic grafts [4–6]. Polytetrafluoroethylene (PTFE) is the most commonly used artificial prosthetic material. However, the use of synthetic vascular grafts in LDLT remains limited to emergencies in the absence of other vascular grafts, mainly due to a greater risk of thrombosis [11]. Many authors consider vascular autologous or frozen grafts as the best solution in the case of planned reconstruction [11,12,13].
Sato applied a living donor’s superficial femoral venous (SFV) graft in a 12-year-old girl with post-Kasai BA who underwent right-lobe LDLT. The donor right liver graft had 3 major hepatic veins. He performed hepatic venous reconstruction by creating a large, wide triple orifice consisting of the RHV and 2 SFVs [7].
Despite the progress in chemotherapy, the prognosis for patients with PRETEXT III and particularly PRETEXT IV tumors remains uncertain or poor [14]. Specific problems may occur in patients with unresectable tumors of the liver qualified for LT. In some cases, radical resection of the infiltrated or compressed retrohepatic IVC is crucial to complete microscopic surgical excision and ensure a successful treatment of HBL [12,14,15].
There are various techniques of IVC reconstruction. Chardot et al [1] published a series of 4 cases of HBL in children who underwent LDLT with IVC removal. For the IVC reconstruction, they used tissue-banked iliac veins in 2 cases and a jugular vein procured from a living donor in 2 recipients. In one of them, 2 years after LT and IVC reconstruction with tissue-banked iliac veins, asymptomatic thrombosis occurred. Hort et al [12] used a venous graft from a deceased donor to reconstruct IVC in an 18-month-old child with HBL. This child received 2 lateral segments from the deceased donor (in situ split graft).
Hsu et al [6] published their IVC reconstruction experience in adult patients, transplanted for various reasons, in which reconstruction of the IVC with PTFE was performed after retrohepatic IVC resection. They found that such grafts are safe, provide satisfactory outflow, and require the use of standard anticoagulation only. The thrombosis risk would probably be much higher despite using much smaller diameter PTFE graft in an infant undergoing LDLT. Shinkai et al [13] reported experiences with patients undergoing vascular reconstruction using a recanalized umbilical vein (rUV) during hepatic surgery. In 1 patient in their series, who underwent living donor liver transplantation for recurrent hepatoblastoma and tumor involvement of IVC, reconstruction was made by transposition of the infrahepatic IVC and interposition of rUV obtained from the donor liver graft to bridge the remaining gap.
On the other hand, Hasegava et al [2] published the case of a 2-year-old boy with an unresectable HBL tumor involving the IVC. Despite retrohepatic IVC resection, reconstruction was not performed in this patient. The collaterals were well developed and the patient tolerated IVC closure (CVP control showed only a slight increase after infrahepatic IVC was clamped).
There is no one ideal way to reconstruct the IVC. PTFE grafts are readily available in a variety of diameters and lengths but in opinion of many authors they have the potential to cause infection and thrombosis. Their use remains limited mainly to emergencies in the absence of other vascular grafts [4]. Biological grafts are recommended by some authors because of their biocompatibility, long-term patency, and low risk of infection [11]. However, cryopreserved vessel grafts from the tissue bank are also not without flaws, as reports of high incidences of aneurysm, thrombosis, and strictures have been described [12]. In 2 of our patients, a cryopreserved iliac venous graft was used and the surgical procedure was uneventful. The presence of a tissue bank in the hospital is an important factor of preoperative preparation as it allows the proper selection of a suitable vascular graft for use in transplantation.
Conclusions
Careful creation of blood outflow from the graft in LDLT with multiple hepatic veins is crucial and results in a very low rate of obstruction or thrombosis. If there is a short distance between multiple hepatic venous branches, venous outflow can be reconstructed by back-table anastomosis between them and the creation of a triangle-shaped orifice for anastomosis with the IVC. In case of the need for more complex reconstruction of allograft venous outflow or retrohepatic IVC, a cold-stored venous graft may be helpful.
Figures
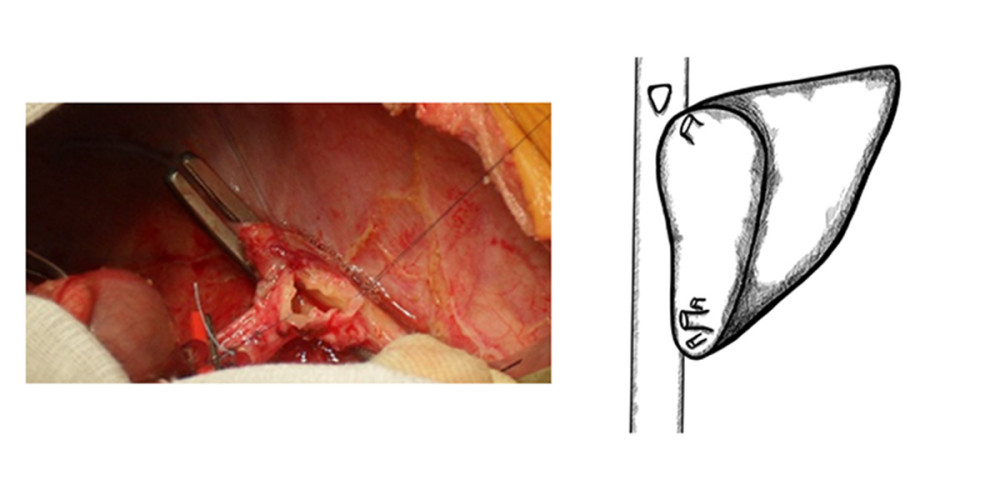 Figure 1. Typical anastomosis of graft HV to the recipient’s IVC – triangle-shaped anastomosis.
Figure 1. Typical anastomosis of graft HV to the recipient’s IVC – triangle-shaped anastomosis. 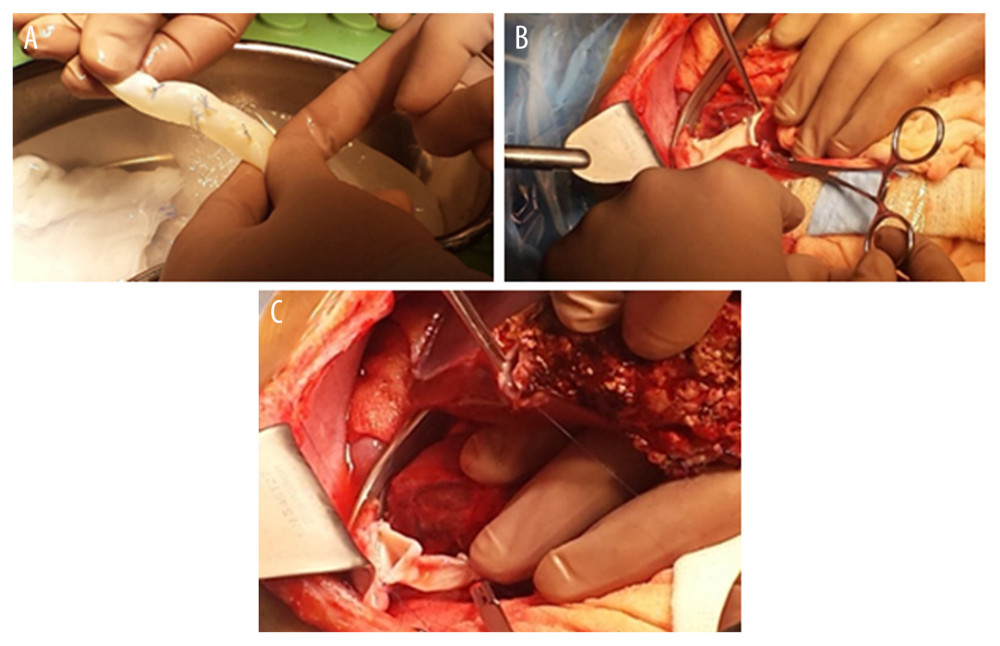 Figure 2. (A–C) Retrohepatic IVC after reconstruction.
Figure 2. (A–C) Retrohepatic IVC after reconstruction. 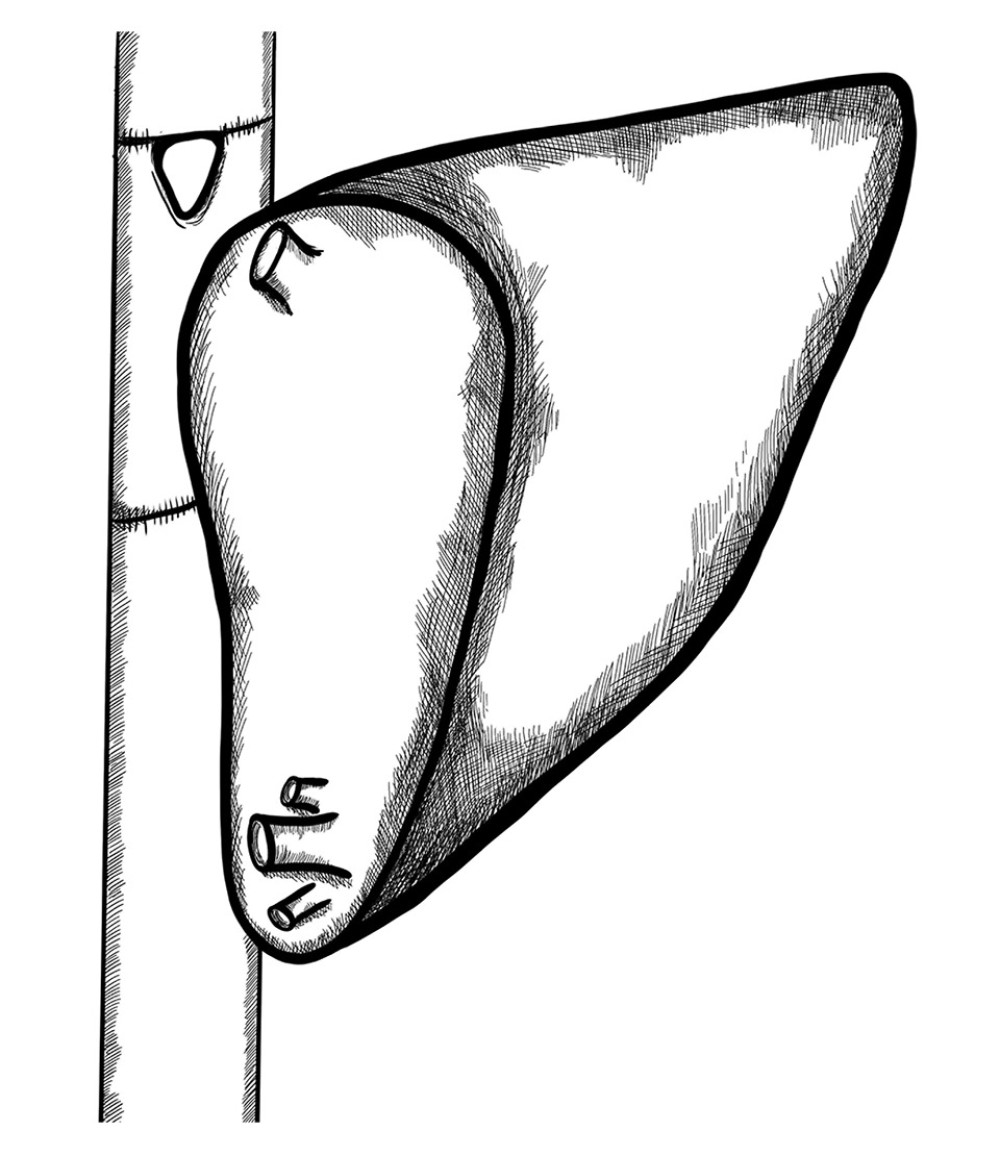 Figure 3. Retrohepatic vena cava reconstruction using a deceased donor venous graft.
Figure 3. Retrohepatic vena cava reconstruction using a deceased donor venous graft. 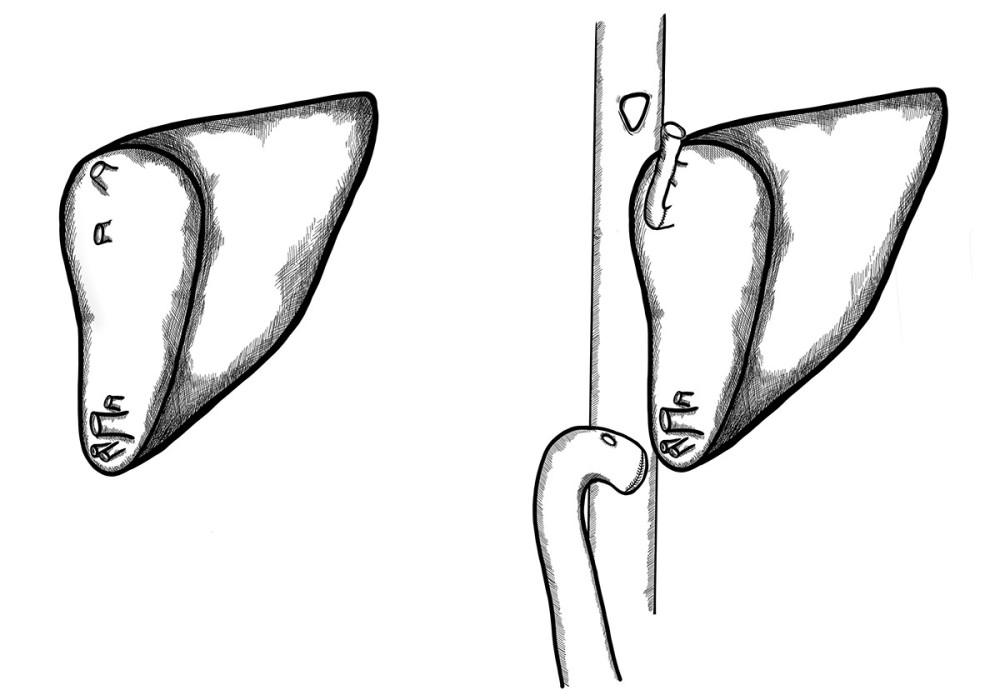 Figure 4. Long distance between 2 hepatic venous branches of the graft (segment II, III), reconstruction using a deceased donor venous graft.
Figure 4. Long distance between 2 hepatic venous branches of the graft (segment II, III), reconstruction using a deceased donor venous graft. 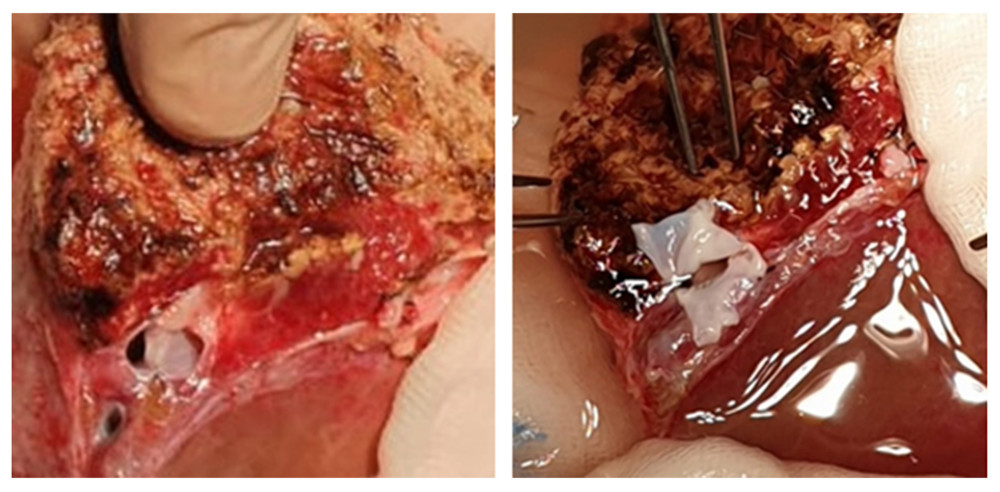 Figure 5. A very deep confluence of the 2 hepatic veins with an extremely short cuff. Reconstruction of the cuff of hepatic veins – back table.
Figure 5. A very deep confluence of the 2 hepatic veins with an extremely short cuff. Reconstruction of the cuff of hepatic veins – back table. 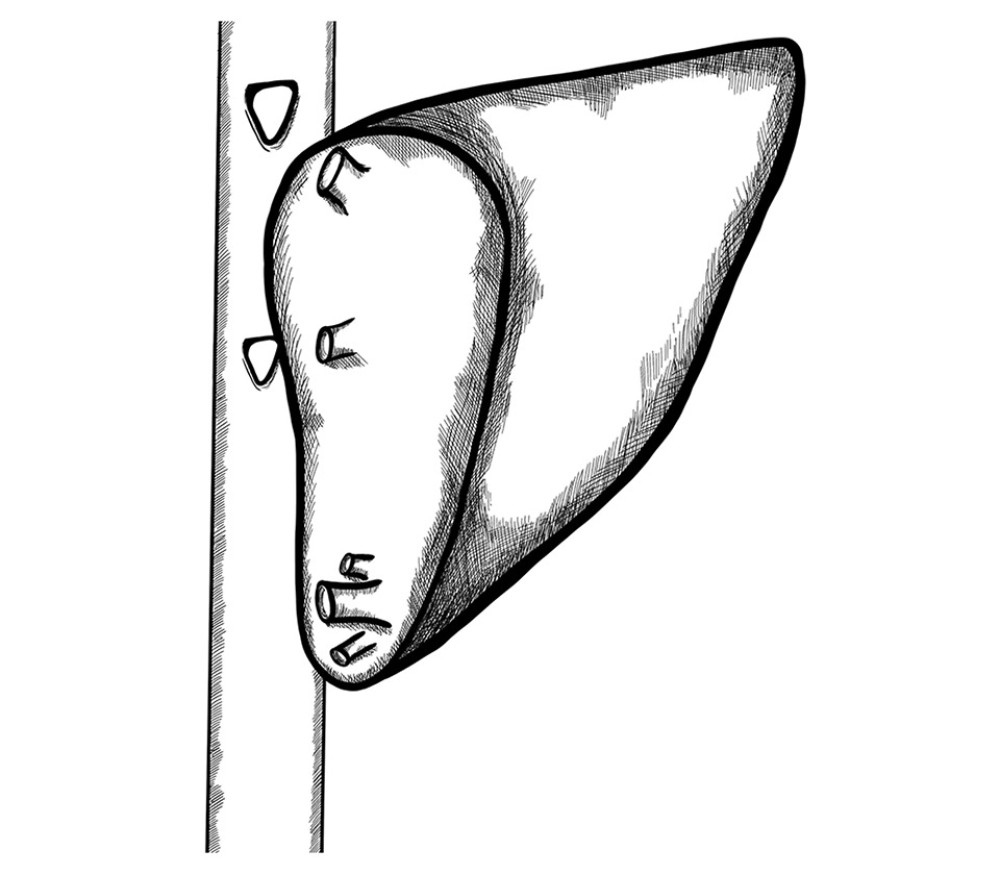 Figure 6. Two separate triangle-shaped end-to-side anastomoses to the recipient’s IVC.
Figure 6. Two separate triangle-shaped end-to-side anastomoses to the recipient’s IVC. 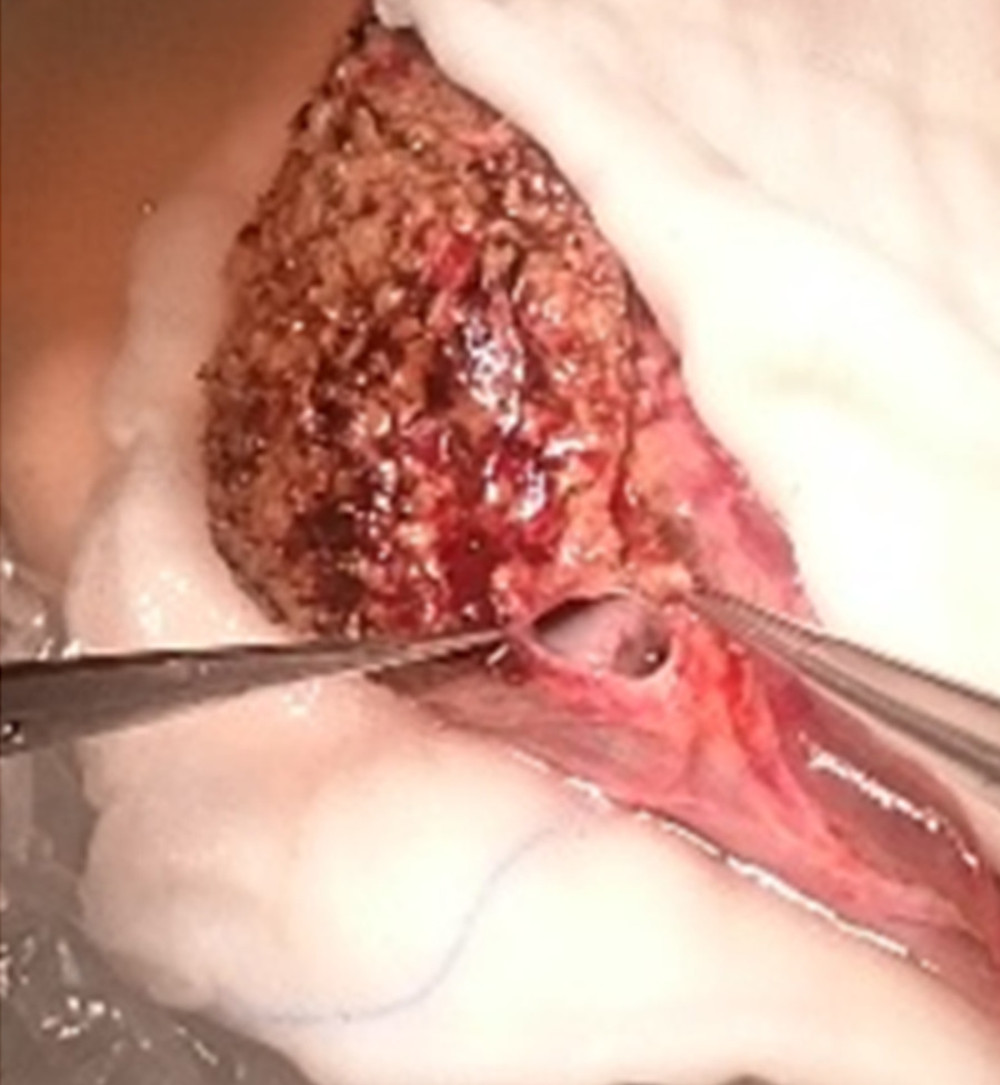 Figure 7. Back-table reconstruction of venous outflow in the case of multiple hepatic veins – triangle-shaped anastomosis.
Figure 7. Back-table reconstruction of venous outflow in the case of multiple hepatic veins – triangle-shaped anastomosis. 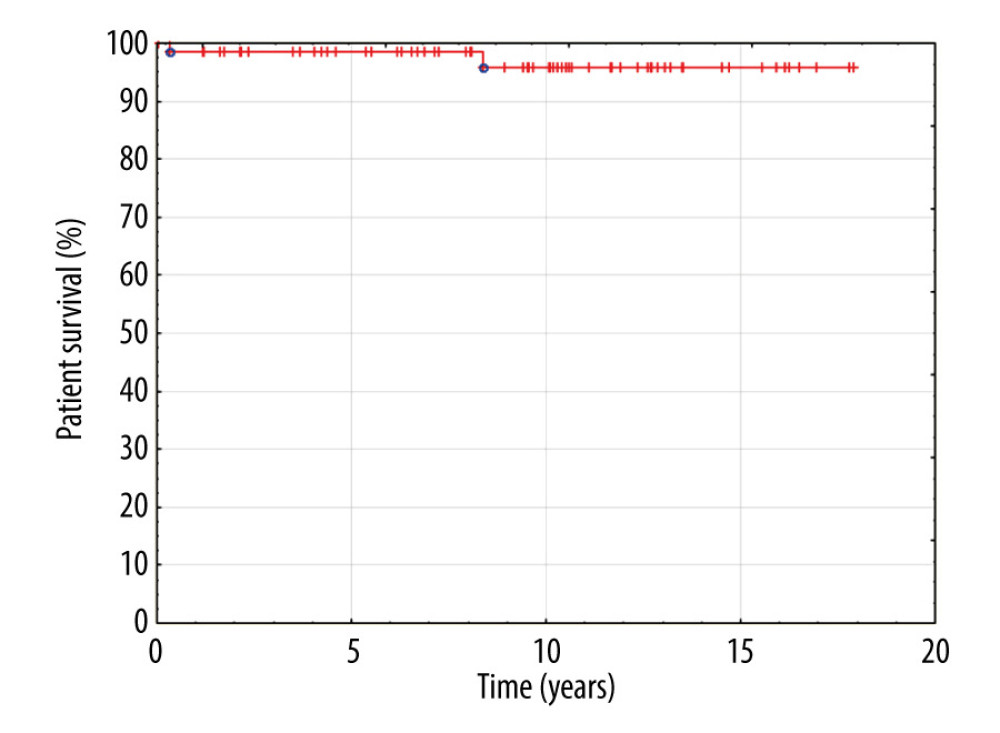 Figure 8. Kaplan-Meier patient survival curve.
Figure 8. Kaplan-Meier patient survival curve. 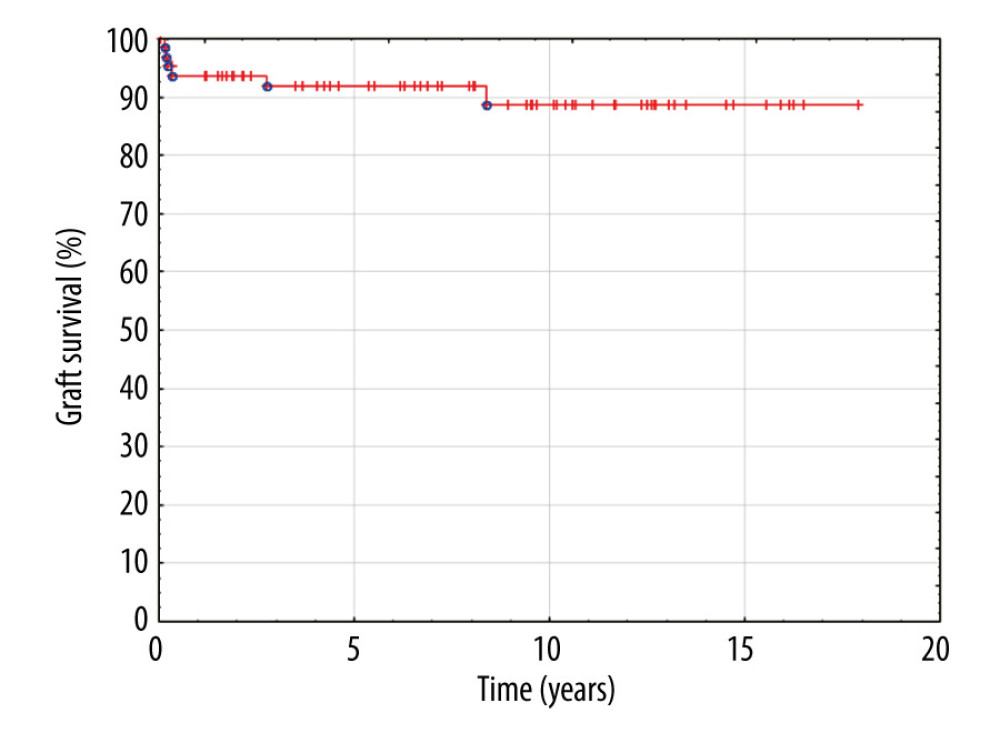 Figure 9. Kaplan-Meier graft survival curve.
Figure 9. Kaplan-Meier graft survival curve. References
1. Chardot C, Saint Martin C, Gilles A, Living-related liver transplantation and vena cava reconstruction after total hepatectomy including the vena cava for hepatoblastoma: Transplantation, 2002; 73; 90-92
2. Hasegawa T, Kimura T, Ihara Y, Living-related liver transplantation with removal of inferior vena cava for unresectable hepatoblastoma: Pediatr Transplant, 2006; 10(4); 521-24
3. Vladov NN, Mihaylov VI, Belev NV, Resection and reconstruction of the inferior vena cava for neoplasms: World J Gastrointest Surg, 2012; 4(4); 96-101
4. Jeng LB, Li PC, Yang MD, Artificial vascular graft for inferior vena cava reconstruction in living donor liver transplantation: A case report: Transplant Proc, 2008; 40(8); 2527-28
5. Chan T, DeGirolamo K, Chartier-Plante S, Buczkowski AK, Comparison of three caval reconstruction techniques in orthotopic liver transplantation: A retrospective review: Am J Surg, 2017; 213(5); 943-49
6. Hsu SC, Jeng LB, Thorat A, Management of extensive retrohepatic vena cava defect in recipients of living donor liver transplantation: Transplant Proc, 2014; 46(3); 699-704
7. Sato K, Sekiguchi S, Kawagishi N, Hepatic venous reconstruction using the superficial femoral vein in a right-lobe living donor liver transplant patient with interrupted inferior vena cava: Pediatr Transplant, 2014; 18(1); E13-17
8. Tani K, Shindoh J, Akamatsu N, Venous drainage map of the liver for complex hepatobiliary surgery and liver transplantation: HPB (Oxford), 2016; 18(12); 1031-38
9. Pinheiro RS, Lai Q, Dahrenmoller C, Lerut J, Complex hepatic outflow reconstruction in domino liver transplantation: Hepatobiliary Pancreat Dis Int, 2014; 13(1); 98-100
10. Kim BW, Park YK, Paik OJ, Effective anatomic reconstruction of the middle hepatic vein in modified right lobe graft living donor liver transplantation: Transplant Proc, 2007; 39(10); 3228-33
11. Pulitanó C, Crawford M, Ho P, The use of biological grafts for reconstruction of the inferior vena cava is a safe and valid alternative: Results in 32 patients in a single institution: HPB (Oxford), 2013; 15(8); 628-32
12. Hort A, Karpelowsky J, Shun A, Thomas G, Use of a donor iliac vein graft for reconstruction of the inferior vena cava in liver transplantation for hepatoblastoma with caval extension: Pediatr Transplant, 2019; 23(4); e13409
13. Shinkai M, Mochizuki K, Kitagawa N, Usefulness of a recanalized umbilical vein for vascular reconstruction in pediatric hepatic surgery: Pediatr Surg Int, 2016; 32(6); 553-58
14. Aronson DC, Czauderna P, Maibach R, The treatment of hepatoblastoma: Its evolution and the current status as per the SIOPEL trials: J Indian Assoc Pediatr Surg, 2014; 19(4); 201-7
15. Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, Long-term outcomes of liver transplantation for hepatoblastoma: A single-center 14-year experience: Pediatr Transplant, 2018 [Online ahead of print]
Figures
 Figure 1. Typical anastomosis of graft HV to the recipient’s IVC – triangle-shaped anastomosis.
Figure 1. Typical anastomosis of graft HV to the recipient’s IVC – triangle-shaped anastomosis. Figure 2. (A–C) Retrohepatic IVC after reconstruction.
Figure 2. (A–C) Retrohepatic IVC after reconstruction. Figure 3. Retrohepatic vena cava reconstruction using a deceased donor venous graft.
Figure 3. Retrohepatic vena cava reconstruction using a deceased donor venous graft. Figure 4. Long distance between 2 hepatic venous branches of the graft (segment II, III), reconstruction using a deceased donor venous graft.
Figure 4. Long distance between 2 hepatic venous branches of the graft (segment II, III), reconstruction using a deceased donor venous graft. Figure 5. A very deep confluence of the 2 hepatic veins with an extremely short cuff. Reconstruction of the cuff of hepatic veins – back table.
Figure 5. A very deep confluence of the 2 hepatic veins with an extremely short cuff. Reconstruction of the cuff of hepatic veins – back table. Figure 6. Two separate triangle-shaped end-to-side anastomoses to the recipient’s IVC.
Figure 6. Two separate triangle-shaped end-to-side anastomoses to the recipient’s IVC. Figure 7. Back-table reconstruction of venous outflow in the case of multiple hepatic veins – triangle-shaped anastomosis.
Figure 7. Back-table reconstruction of venous outflow in the case of multiple hepatic veins – triangle-shaped anastomosis. Figure 8. Kaplan-Meier patient survival curve.
Figure 8. Kaplan-Meier patient survival curve. Figure 9. Kaplan-Meier graft survival curve.
Figure 9. Kaplan-Meier graft survival curve. Tables
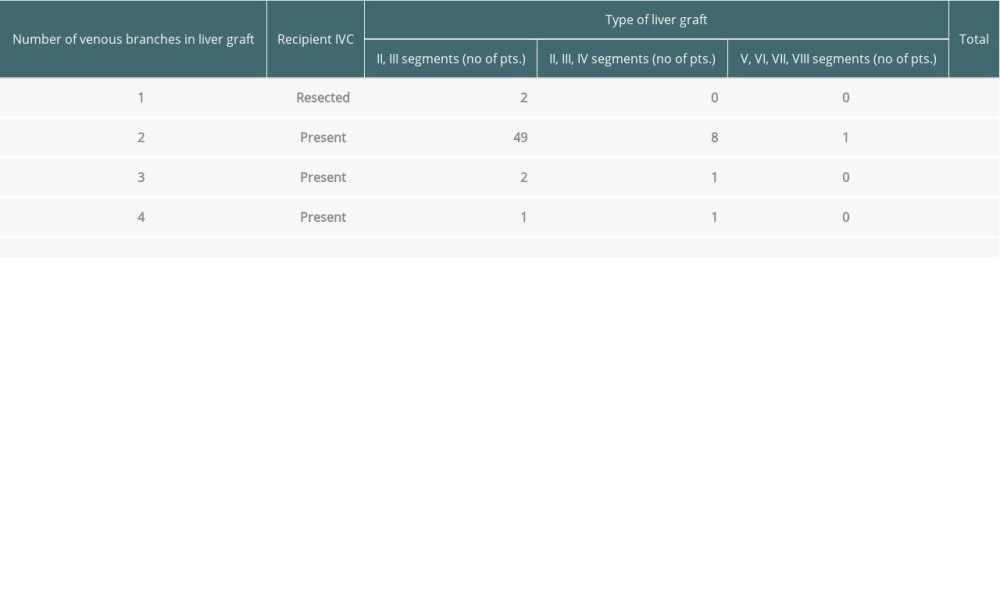 Table 1. Characteristics of liver grafts in which the reconstruction of venous outflow or retrohepatic IVC was necessary.
Table 1. Characteristics of liver grafts in which the reconstruction of venous outflow or retrohepatic IVC was necessary.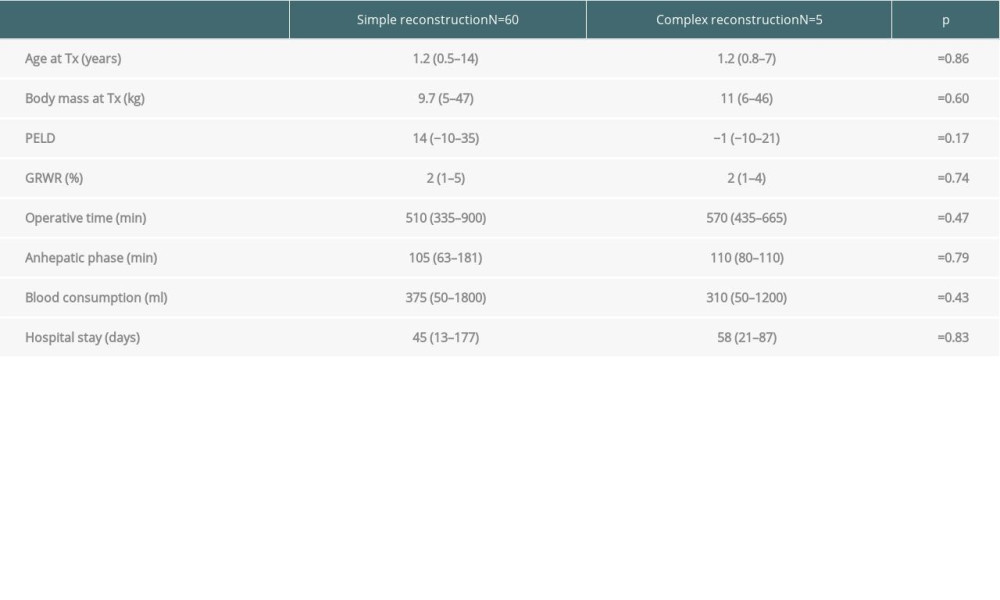 Table 2. Comparison of complex vascular and simple venous reconstructions.
Table 2. Comparison of complex vascular and simple venous reconstructions. Table 1. Characteristics of liver grafts in which the reconstruction of venous outflow or retrohepatic IVC was necessary.
Table 1. Characteristics of liver grafts in which the reconstruction of venous outflow or retrohepatic IVC was necessary. Table 2. Comparison of complex vascular and simple venous reconstructions.
Table 2. Comparison of complex vascular and simple venous reconstructions. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








