16 February 2021: Original Paper
Risk of Thrombus Formation in Patients on Mechanical Circulatory Support with POLVAD-MEV
Tomasz Urbanowicz1ABDEF*, Anna Olasińska-Wiśniewska1CDEF, Michał Michalak2CDE, Michał Bociański1BCD, Dariusz Krawczyk3CD, Ewa Straburzyńska-Migaj4DEF, Hanna Wachowiak-Baszyńska1ABC, Marek Jemielity1ACDEFGDOI: 10.12659/AOT.926555
Ann Transplant 2021; 26:e926555
Abstract
BACKGROUND: Congestive heart failure is a challenging problem due to increasing prevalence in developed countries. Patients admitted due to decompensated congestive heart failure symptoms who do not respond to medical treatment require mechanical circulatory support. Patients with biventricular failure are at particularly high mortality risk.
MATERIAL AND METHODS: We analyzed the function of 49 pumps (POLVAD-MEV, FRK Intra-cordis, Poland) implanted to rescue INTERMACS 1 and 2 profile patients referred to our department due to severe congestive heart failure. All patients were waiting for heart transplantation and were readmitted due to acute decompensations of congestive biventricular heart failure with resistance to medical therapy.
RESULTS: During the observational period, there were no technical problems in pump function. The mean duration of pump therapy was 30.6±8.3 (5–49) days. The risk for right-sided pump complications included clots formation on the following parts of the pump: outflow tract (1, 2%), membrane (13, 27%), dome (6, 12.5%), and periphery (1, 2%). The overall risk for device thrombosis was 41%. The risk for thromboembolic complications was CRP-dependent regarding conglomerates of fibrin and platelets formation (p<0.05). The risk for left-sided pump complications included clots formation on the outflow tract (1, 2%), membrane (9, 19%) and dome (3, 6%). The overall risk for device thrombosis was 27%. The risk for clots formation on the membrane (P<0.05) and dome of the pump depended on time (P<0.07).
CONCLUSIONS: Mechanical circulatory support with a paracorporeal pump is a safe option for biventricular heart dysfunction as a bridge to heart transplantation. The risk for thrombi formation is relatively high but acceptable within 30 days after implantation.
Keywords: Advanced Cardiac Life Support, Embolism and Thrombosis, Heart-Assist Devices, Pulsatile Flow, Heart Transplantation, Thrombosis
Background
Congestive heart failure is a challenging problem due to increasing prevalence in developed countries [1]. Acute decompensations are the major indication for hospitalization with the symptoms of fluid retention as the result of dysregulation of neurohumoral compensatory mechanisms [2,3]. Disease is characterized by an insidious course and may lead to severe circulatory decompensation [4]. Repeated hospitalizations are associated with worse outcomes, including decreased 1-year survival to as low as 20–40% [5,6]. Progressive decompensations of congestive heart failure can be pharmacologically treated based on results of VERITAS (Value of Endothelin Receptor Inhibition With Tezosentan in Acute Heart Failure), REVIVE (Randomized Evaluation of Intravenous Levosimendan Efficacy), PROTECT (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist), RELAX-AHF (Relaxin in Acute Heart Failure), and TRUEAHF (Trial to Evaluate the Efficacy and Safety of Ularitide Intravenous Infusion in Patients Suffering From Acute Decompensated Heart Failure) trials. However, the results are still insufficient. Patients admitted due to decompensated congestive heart failure symptoms who do not respond to medical therapy including diuretics, pressor amins, or levosimendan infusion require mechanical circulatory support. Patients with biventricular failure are at particularly high mortality risk. The ultimate therapeutic solution is heart transplantation, which has good long-term results, or a left ventricle system device, with midterm results comparable to transplantation when the right ventricle is not dysfunctional [7,8].
Material and Methods
We analyzed the function of 49 pumps (POLVAD-MED, FRK Intra-cordis, Poland) implanted to rescue INTERMACS 1 and 2 profile patients referred to our department due to severe congestive heart failure. The study was approved from the local Institutional Ethics Committee.
All patients were waiting for heart transplantation and were readmitted due to acute decompensations of congestive biventricular heart failure with resistance to medical therapy.
We evaluated pumps by screening after explantation to assess safety in terms of device thrombosis and risk factors for complications.
The pumps were implanted through median sternotomy. The left-sided pumps were placed into the left atrium (inflow canula) and ascending aorta (outflow canula) with pledget sutures. The right-sided pumps were placed into the right atrium (inflow canula) and pulmonary truck (outflow canula) with pledget sutures.
The INR ratio (international normalized ratio) and CRP (C-reactive protein) were estimated every second day after surgery. The pumps were checked by physicians daily.
Results
During the observational period, there were no technical problems regarding pump function. The mean duration of pump therapy was 30.6±8.3 (5–49) days. The pumps were replaced by physicians if thrombi were detected.
The risk for right-sided pump complications included clots formation on the following parts of the pump: outflow tract (1, 2%), membrane (13, 27%), dome (6, 12.5%), and periphery (1, 2%). The overall risk for device thrombosis was 41%. The risk for thromboembolic complications was CRP-dependent regarding conglomerates of fibrin and platelets formation (
The risk for left-sided pomp complications included clots formation on the outflow tract (1, 2%), membrane (9, 19%), and dome (3, 6%). The overall risk for device thrombosis was 27%. The risk for clots formation on the membrane (
Comparing the risk for thrombus formation between both pumps, we found statistically significant differences in the risk for thrombus formation on the membrane and dome. The mechanical valves and outflow or inflow tracts were not found to be significantly affected. The detailed data are presented in Table 1. Thrombus deposits on the right and left pump membranes were found in 13 (27%) and 9 (19%), respectively. Thrombus deposits on the right and left pump dome were found in 6 (13%) and 3 (6%), respectively.
Thrombi formation was not related to lower INR ratios. This may be explained by the fact that the dual therapy with vitamin K antagonists (VKA) and antiplatelets drugs was enhanced by low-molecular-weight heparin (LMWH) additions when INR decreased below 2.5.
Inflammatory markers were found to be risk factor for thrombi formation in multivariate analysis. The mean C-reactive protein (CRP) in the non-thrombotic subgroup was 10.3±4.4 mg/L vs 60.5±8.1 mg/L in the thrombotic group (
Discussion
The growing number of left ventricular assist devices (LVAD) implanted worldwide for long-term circulatory support and extracorporeal membrane-oxygenation (ECMO) for short-term support have decreased the utility of paracorporeal VADs [9]. However, paracorporeal pneumatic pumps are still the best option for biventricular dysfunction, which accounts for 10–20% of the entire VAD population [10]. The number of BIVAD implantations is currently decreasing, mainly due to earlier LVAD implantation along with perioperative right ventricle function management [11]. Use of BIVADs consisting of 2 HeartWare HVAD® (HVADs) is associated with high mortality and thrombus formation risk [12].
Biventricular dysfunction is a major indication for implantation pulsatile paracorporeal pumps. One of the advantages of pneumatic pumps is microcirculation preservation by pulsatile flow. Maintaining physiological blood flow may improve tissue perfusion and capillary flow and decrease the risk for acquired von Willebrand syndrome.
ECMO is relatively easy and available but has some disadvantages, including the lack of ventricular unloading compounded with creation of high afterload and a secondary decrease in coronary perfusion [13]. A sequence of therapies from ECMO through POLVAD-MED and finally into solid organ transplantation were documented, providing possible solutions while transplant centers struggle with the organ donor shortage [14]. The multicenter ECMELLA study (ECLS and Impella application) demonstrated a significant improvement in outcomes in patients receiving short-term mechanical support therapy [15]. Impella allows for left ventricle unloading. Both devices are effective for a very short time (5–6 days) after application [16].
Paracorporeal pneumatic biventricular support is a life-saving technology while bridging to heart transplantation. The major disadvantages are bleeding, thrombosis, and infection [17]. POLVAD-MED generates pulsatile blood flow and is comparable to left ventricle system devices (LVADs) in producing continuous blood flow [18]. The main advantage is that it provides the possibility to replace function of both ventricles.
The major disadvantage of any mechanical support implantation device is the risk for thrombosis [19,20]. We evaluated the risk for thrombus formation by visual assessment after device explantation. Coagulopathy related to mechanical support was reported previously and was called the Vroman effect [20]. It is related to absorption of activated contact proteins on mechanical support device surfaces.
In our study, we found the risk for thrombus formation on certain parts of pumps depends on the CRP ratio. The right pump, especially the membrane and dome of the pump, had high risk of thrombus formation during the study. Daily visual inspection for thrombus formations is a standard clinical practice. We evaluated the risk for thrombi what was non-visually detected but was demonstrated after pump explantation. We found that the major risk for any regional coagulopathies is related to inflammatory state. During the POLVAD patients’ follow-up, the high-sensitive C-reactive protein was checked every second day. The mean CRP values throughout the pump function periods were considered in analysis. We found that statistically significant risk for thrombi formation is related to increased CRP values, as previously reported in LVAD patients [21].
Systemic inflammation following LVAD implantation can be fatal when systemic inflammatory reaction develops, especially during surgical procedures in cardiopulmonary bypass operations [22]. The overall results of LVAD patients are related to preoperative and postoperative inflammatory triggers. Inflammatory reactions in patients on mechanical support can cause right ventricle failure, which complicates up to 40% of implantations [23]. Midterm mortality rates are also affected by preoperative increased inflammatory parameters [24]. Moreover, the risk for cerebral microembolization in relation to C-reactive protein has been proposed [25].
In the present study, the INR ratio was checked every second day and vitamin K antagonist doses were adjusted. Patients were on dual therapy with oral anticoagulants and antiplatelet agents. If INR results were below 2.5, we followed our protocol to add low-molecular-weight heparin (LMWH) to therapy and increased oral anticoagulants.
Although the use of external pulsatile pumps is decreasing, we want to highlight their advantages in certain patients. Patients referred for mechanical circulatory support in end-stage biventricular dysfunction may benefit from therapy. Moreover, paracorporeal pneumatic pumps are postulated to prevent collapse of capillaries and nitric oxide release in the microcirculation due to the pulsatile pattern of blood flow [26]. Use of BIVAD can be especially helpful for patients admitted due to circulatory collapse caused by fulminant myocarditis, as recovery of the failing myocardium was reported to be more effective in pulsatile mechanical therapy [27]. Recently, third-generation LVADs have a speed pump modulation algorithm, termed the Lavare cycle, to partially mimic pulsatile flow and prevent thrombus formation [28,29].
Conclusions
Mechanical circulatory support with a paracorporeal pump is a safe option for biventricular heart dysfunction as a bridge to heart transplantation. The risk for thrombi formation is relatively high but acceptable within 30 days after implantation. The risk for thrombi formation in our group was lower than that reported in the Euromacs Registry (27% and 41% vs 48%, respectively). We found that inflammatory activity was the highest within 30 days of use, and was a risk factor for thrombotic complications.
Figures
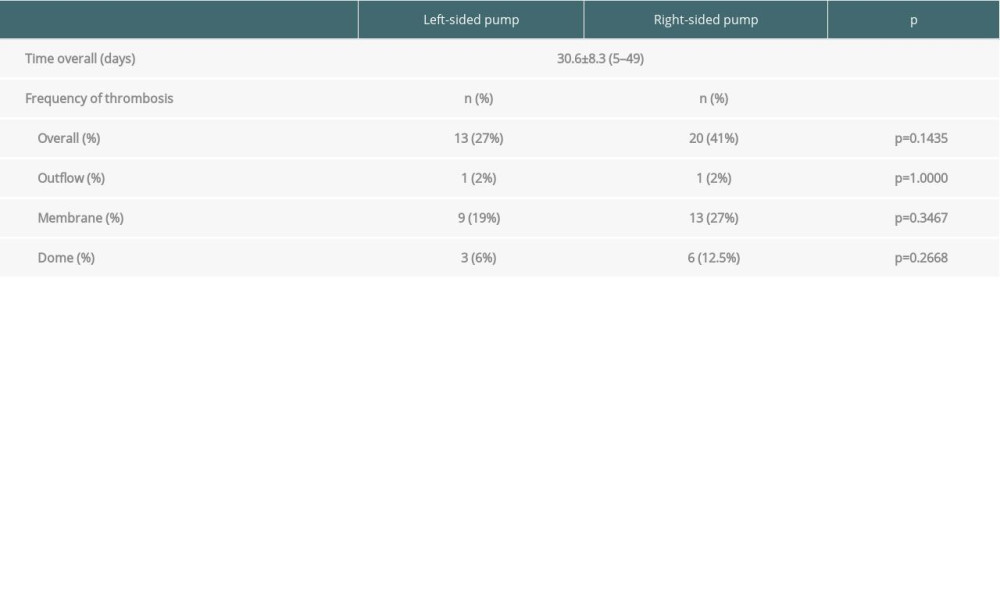
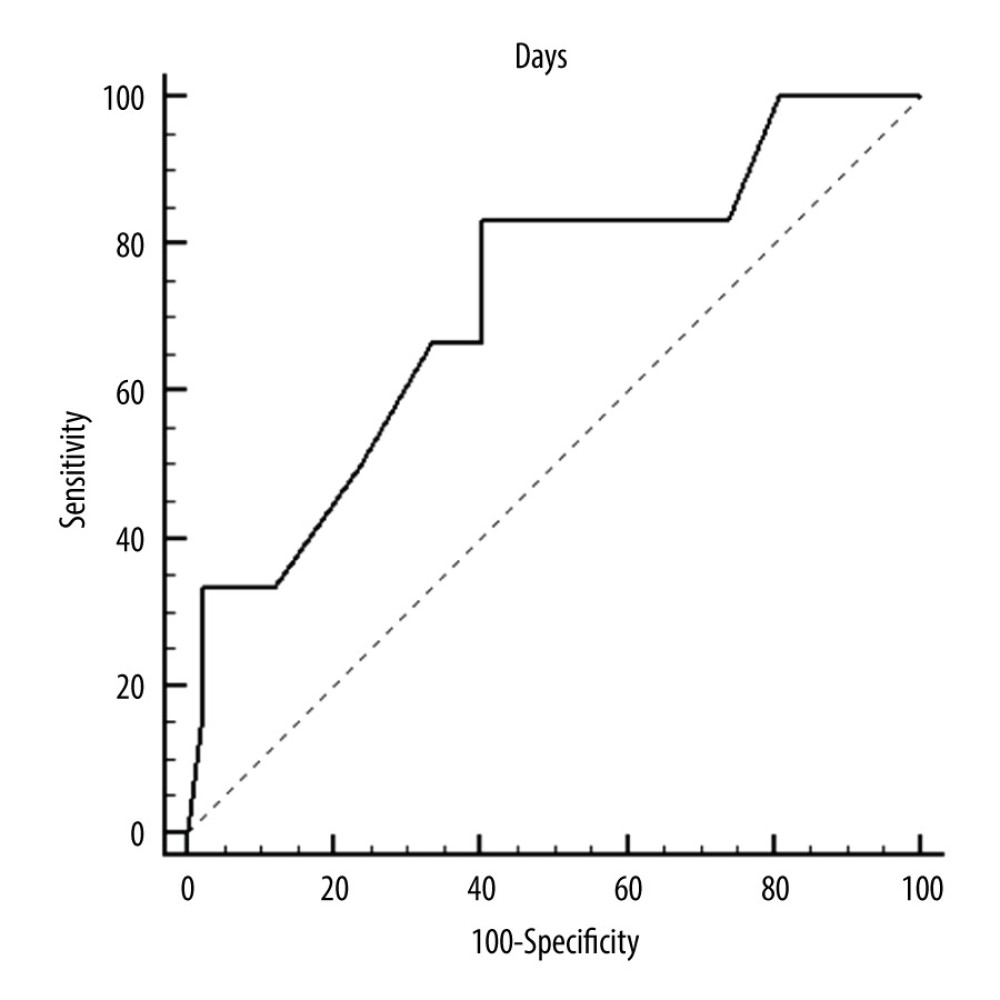 Figure 1. ROC curves of thrombotic events on right pumps. Overall risk for thrombus formation.
Figure 1. ROC curves of thrombotic events on right pumps. Overall risk for thrombus formation. 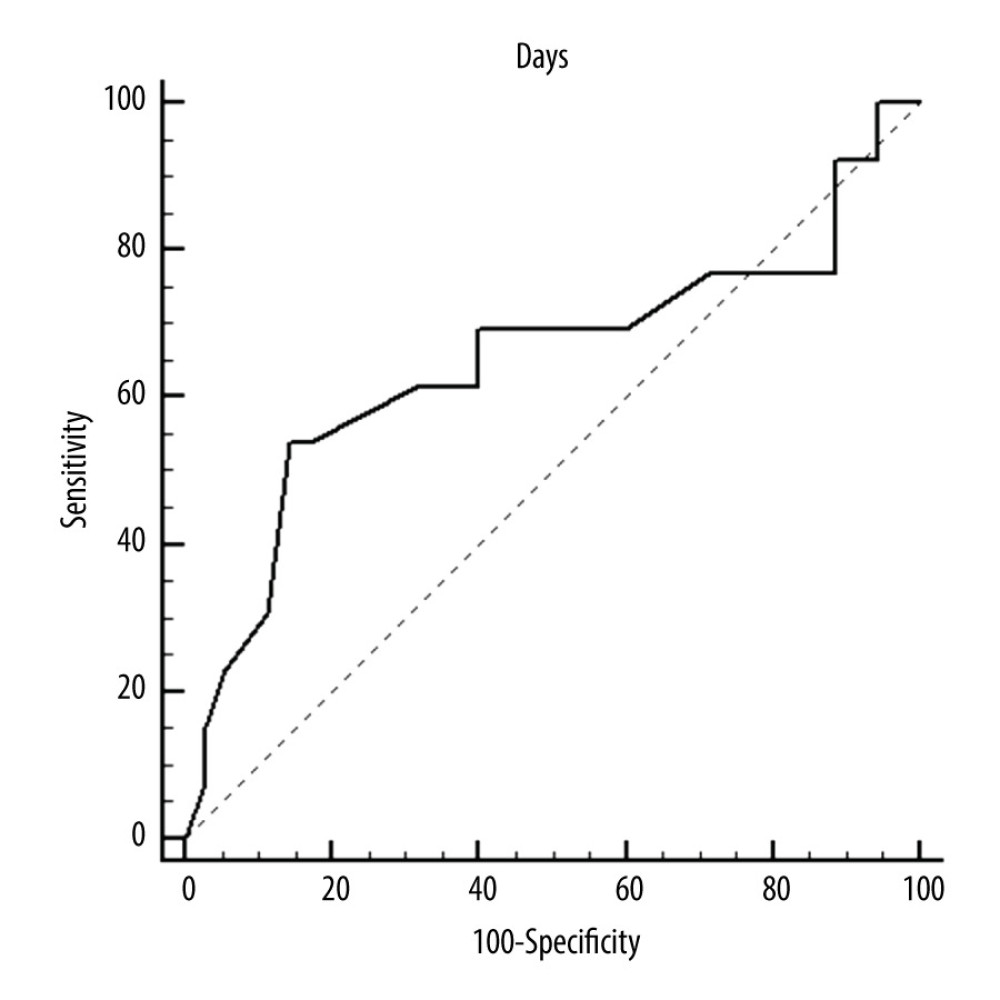 Figure 2. ROC curves of thrombotic events on right pumps. Risk of thrombosis depending on CRP.
Figure 2. ROC curves of thrombotic events on right pumps. Risk of thrombosis depending on CRP. 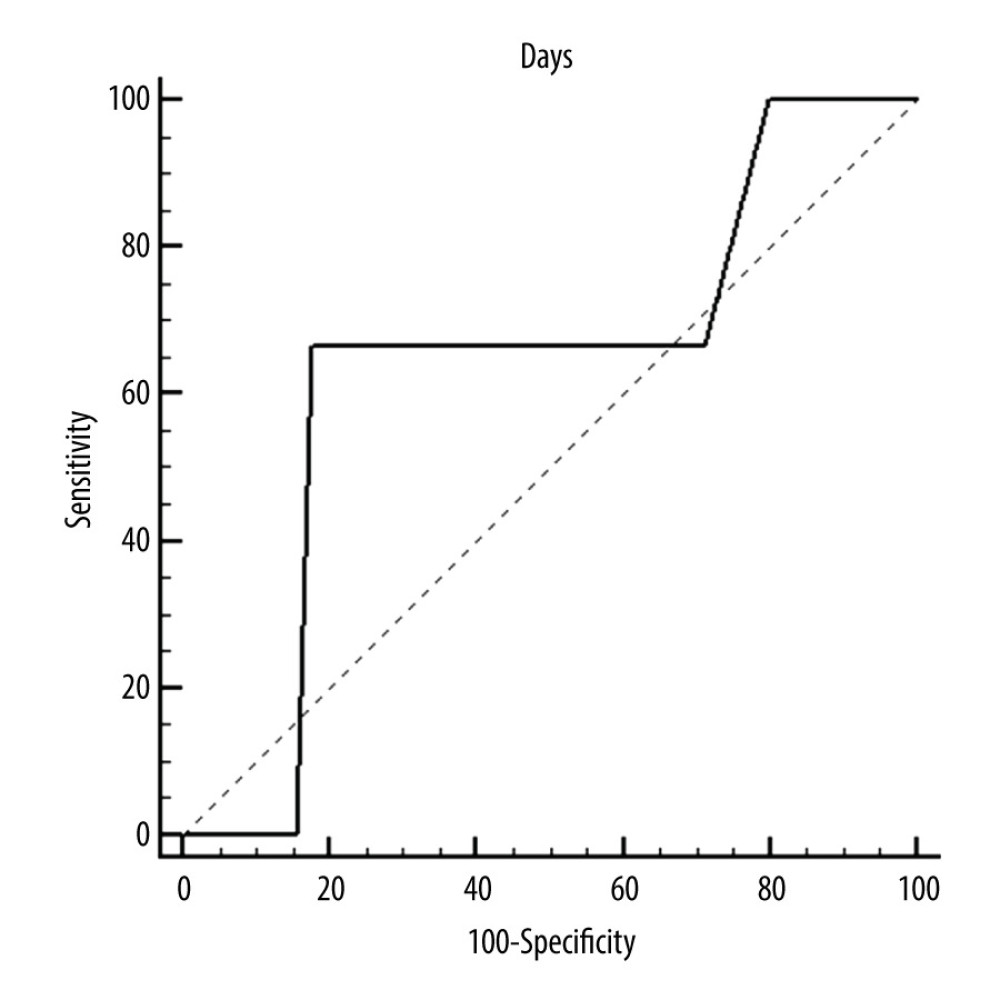 Figure 3. ROC curves of thrombotic events on left pumps. Overall risk for thrombus formation.
Figure 3. ROC curves of thrombotic events on left pumps. Overall risk for thrombus formation. 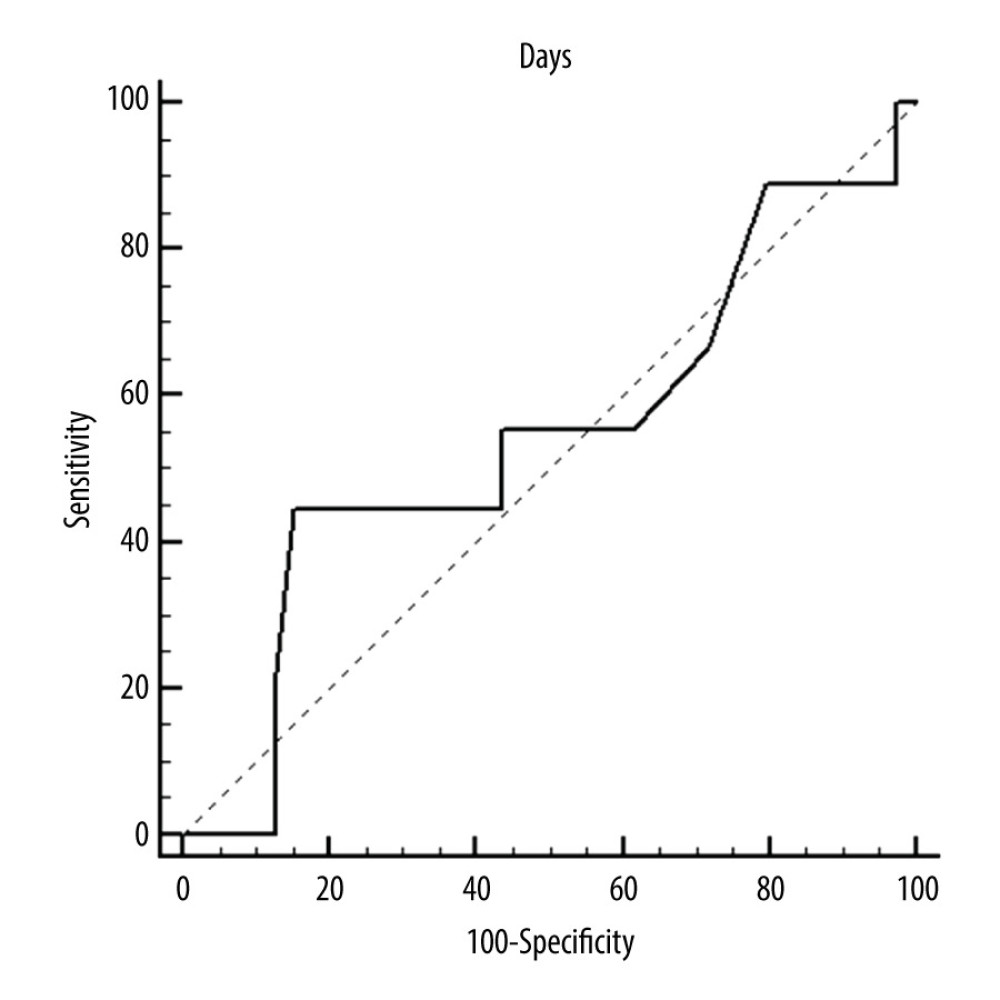 Figure 4. ROC curves of thrombotic events on left pumps. Risk of thrombosis depending on CRP.
Figure 4. ROC curves of thrombotic events on left pumps. Risk of thrombosis depending on CRP. 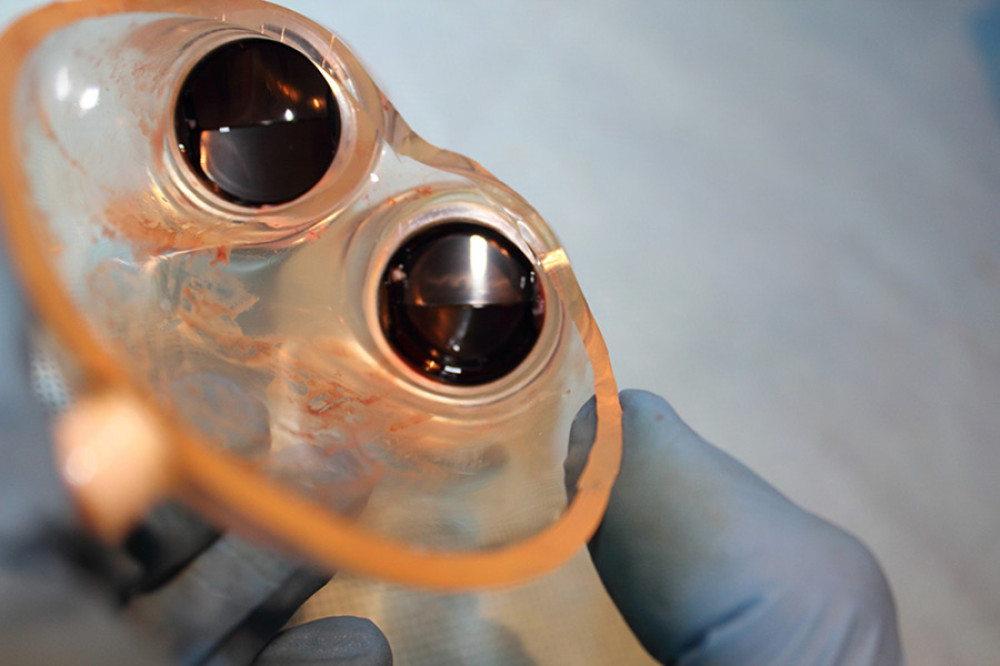 Figure 5. Deposits on the pump dome.
Figure 5. Deposits on the pump dome.  Figure 6. Deposits on the pump membrane.
Figure 6. Deposits on the pump membrane. 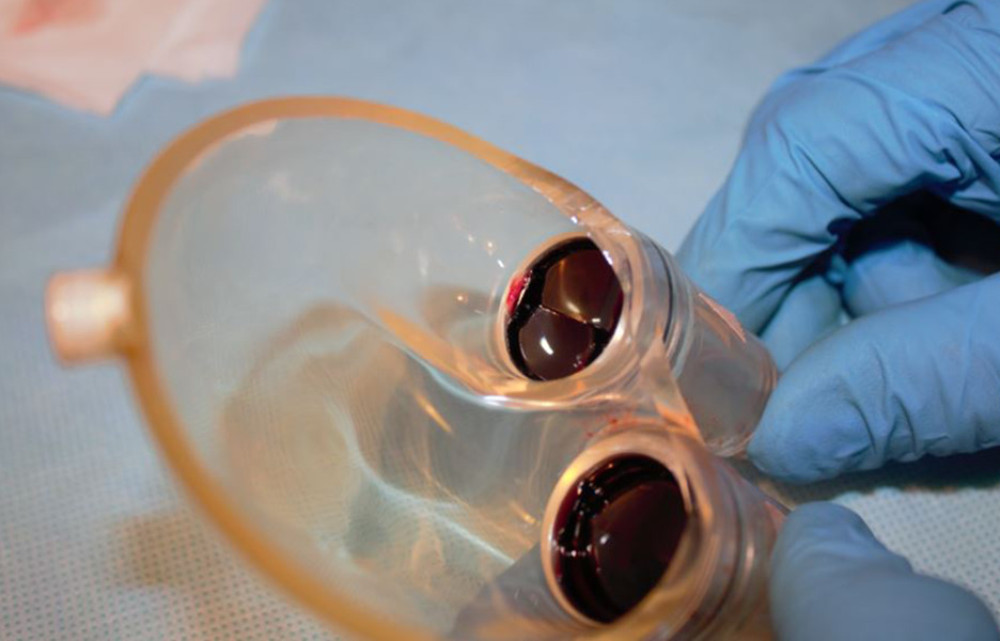 Figure 7. Deposits on mechanical valves of the pump.
Figure 7. Deposits on mechanical valves of the pump. References
1. Farré N, Vela E, Clèries M, Real world heart failure epidemiology and outcome: A population-based analysis of 88,195 patients: PLoS One, 2017; 12(2); 1-3
2. Teerlink JR, Alburikan K, Metra M, Rodgers JE, Acute decompensated heart failure update: Curr Cardiol Rev, 2015; 11; 53-2
3. Kurmani S, Squire I, Acute heart failure: Definition, classification and epidemiology: Curr Heart Fail Rep, 2017; 14; 385-2
4. O’Connor CM, Stough WG, Gallup DS, Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure. Observations from the IMPACT-HF registry: J Card Fail, 2005; 11(3); 200
5. Nichols GA, Reynolds K, Kimes TM, Comparison of risk of re-hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved versus reduced ejection fraction: Am J Cardiol, 2015; 116; 1088-2
6. Cheng RK, Cox M, Neely ML, Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population: Am Heart J, 2014; 168; 721
7. Urbanowicz T, Staburzyńska-Migaj E, Pawłowska M, EuroSCORE is a predictor of postoperative pericardial effusion following heart transplantation: Ann Transplant, 2015; 20; 193-7
8. Mehra MR, Uriel N, Naka Yfor the MOMENTUM 3 Investigators, A fully magnetically levitated left ventricular assist device–final report: N Engl J Med, 2019; 380; 1618-7
9. Sponga S, Benedetti G, Livi U, Short-term mechanical circulatory support as bridge to heart transplantation: Paracorporeal ventricular assist device as alternative to extracorporeal life support: Ann Cardiothorac Surg, 2019; 8(1); 143
10. Takeda K, Naka Y, Yang JA, Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion: J Heart Lung Transplant, 2014; 33; 141-8
11. Dykes JC, Maeda K, Commentary: To BiVAD or not to BiVAD… that is the question?: J Thorac Cardiovasc Surg, 2020; 160(5); 1310-1
12. Shah P, Ha R, Singh R, Multicenter experience with durable biventricular assist devices: J Heart Lung Transplant, 2018; 37(9); 1093-01
13. den Uil CA, Akin S, Jewbali LS, Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: A systematic review and meta-analysis: Eur J Cardiothorac Surg, 2017; 52; 14-5
14. Religa G, Jasińska M, Czyżewski Ł, The effect of the sequential therapy in end-stage heart failure (ESHF) – from ECMO, through the use of implantable pump for a pneumatic heart assist system, Religa Heart EXT, as a bridge for orthotopic heart transplant (OHT). Case study: Ann Transplant, 2014; 21(19); 537
15. Nersesian G, Hennig F, Müller M, Temporary mechanical circulatory support for refractory heart failure: The German Heart Center Berlin experience: Ann Cardiothorac Surg, 2019; 8(1); 76-3
16. Tschöpe C, Van Linthout S, Klein O, Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts: J Cardiovasc Transl Res, 2019; 12(2); 116-3
17. Demirozu ZT, Kucukaksu DS, Bridging to heart transplantation from the biventricular pulsatile Berlin Heart EXCOR assist device support in a patient with advanced end-organ failure: J Teh Univ Heart Ctr, 2015; 10(4); 201
18. Nadziakiewicz P, Pacholewicz J, Zakliczynski M, Comparison of mechanical circulatory support by the use of pulsatile left ventricular assist devices Polvad MEV and continuous flow heart ware and Heart Mate II in a single-center experience: Transplant Proc, 2016; 48(5); 1770-4
19. Nielsen VG, Kirklin JK, Holman WL, Mechanical circulatory device thrombosis: A new paradigm linking hypercoagulation and hypofibrinolysis: ASAIO J, 2008; 54(4); 351-8
20. Rosenthal JL, Starling RC, Coagulopathy in mechanical circulatory support: A fine balance: Curr Cardiol Rep, 2015; 17(12); 114-9
21. Walenga JM, Torres TA, Jeske WP, Protein C pathway, inflammation, and pump thrombosis in patients with left ventricular assist devices: Clin Appl Thromb Hemost, 2020; 26 1076029620959724
22. Mondal NK, Sorensen EN, Pham SM, Systemic inflammatory response syndrome in end-stage heart failure patients following continuous-flow left ventricular assist device implantation: Differences in plasma redox status and leukocyte activation: Artif Organs, 2016; 40(5); 434-3
23. Tang PC, Haft JW, Romano MA, Right ventricular failure following left ventricular assist device implantation is associated with a preoperative pro-inflammatory response: J Cardiothorac Surg, 2019; 14(1); 80-8
24. Tsyganenko D, Gromann TW, Schoenrath F, Predictors of mid-term outcomes in patients undergoing implantation of a ventricular assist device directly after extracorporeal life support: Eur J Cardiothorac Surg, 2019; 55(4); 773-9
25. Thoennissen NH, Allroggen A, Ritter M, Influence of inflammation and pump dynamic on cerebral microembolization in patients with Continuous-Flow DeBakey LVAD: ASAIO J, 2006; 52(3); 243-7
26. Baric D, Why pulsatility still matters: A review of current knowledge: Croat Med J, 2014; 55(6); 609
27. Dandel M, Hetzer R, Myocardial recovery during mechanical circulatory support: Weaning and explantation criteria: Heart Lung Vessel, 2015; 7(4); 280-8
28. Kumar J, Elhassan A, Dimitrova G, Essandoh M, The Lavare Cycle: A novel pulsatile feature of the HVAD continuous-flow left ventricular assist device: J Cardiothorac Vasc Anesth, 2019; 33(4); 1170-1
29. Granegger M, Choi Y, Locher B, Comparative analysis of cardiac mechano-energetics in isolated hearts supported by pulsatile or rotary blood pumps: Sci Rep, 2019; 9(1); 20058
Figures
 Figure 1. ROC curves of thrombotic events on right pumps. Overall risk for thrombus formation.
Figure 1. ROC curves of thrombotic events on right pumps. Overall risk for thrombus formation. Figure 2. ROC curves of thrombotic events on right pumps. Risk of thrombosis depending on CRP.
Figure 2. ROC curves of thrombotic events on right pumps. Risk of thrombosis depending on CRP. Figure 3. ROC curves of thrombotic events on left pumps. Overall risk for thrombus formation.
Figure 3. ROC curves of thrombotic events on left pumps. Overall risk for thrombus formation. Figure 4. ROC curves of thrombotic events on left pumps. Risk of thrombosis depending on CRP.
Figure 4. ROC curves of thrombotic events on left pumps. Risk of thrombosis depending on CRP. Figure 5. Deposits on the pump dome.
Figure 5. Deposits on the pump dome. Figure 6. Deposits on the pump membrane.
Figure 6. Deposits on the pump membrane. Figure 7. Deposits on mechanical valves of the pump.
Figure 7. Deposits on mechanical valves of the pump. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860