23 February 2021: Original Paper
Kidney Function After Liver Transplantation in a Single Center
Grzegorz Niewiński1ADEFG, Wiktor Smyk2B, Agata Graczyńska1B, Konrad Kostrzewa3C, Joanna Raszeja-Wyszomirska2ADEF*, Urszula Ołdakowska-Jedynak4DF, Jolanta Małyszko4DF, Maciej Wójcicki2DF, Krzysztof Zieniewicz5DEFDOI: 10.12659/AOT.926928
Ann Transplant 2021; 26:e926928
Abstract
BACKGROUND: Renal dysfunction in the peri-transplant period appears to complicate both short- and long-term outcome of liver transplantation (LT). The aim of this study was to analyze the impact of selected clinical features in the peri-liver transplant period, as well calcineurin inhibitor, particularly tacrolimus given after LT, on kidney function in a single liver transplant center’s experience.
MATERIAL AND METHODS: A total 125 consecutive liver-grafted individuals (82 M, 43 F), mean age 50±13 y (with alcohol-related liver disease in 48 (38%) patients) were included into the study. Their clinical data were collected in the database until 46 months of follow-up, and the Python packages Pandas (version 0.22.0) and scikit-learn (version 0.21.3) were used for data analysis.
RESULTS: More advanced liver disease as judged by Child-Pugh class and MELD score differed significantly patients with preserved (serum creatinine SCr <1.5 mg/dL) and impaired (SCr ≥1.5 mg/dL) kidney function before LT. Older age and higher SCr pre-LT were associated with higher levels of SCr after LT in 2 time-points. SCr before LT was correlated with delta SCr for the highest and last recorded value (P<0.0001). Higher amounts of transfused colloids during surgery were associated with increased delta SCr for the highest value (P=0.019) after grafting in logistic regression analysis. There were no associations between SCr after LT and duration of anhepatic phase, urine output ≤100 mL/h, or post-reperfusion syndrome during transplantation (all P>0.05). There were no associations between SCr after LT and tacrolimus trough levels in analyses of correlations and linear regression analyses (all P>0.05).
CONCLUSIONS: We found that pretransplant serum creatinine was the only factor affecting kidney function after LT in our liver transplant center. The restricted fluid policy was safe and effective in terms of long-term renal function. The role of kidney-saving immunosuppressive protocols in preserving renal function long-term after LT was also confirmed.
Keywords: Creatine, Liver Transplantation, Renal Insufficiency, Chronic, calcineurin inhibitors, Creatinine, Kidney, Kidney Function Tests, Risk Factors, Tacrolimus
Background
Despite the major progress in liver transplantation, renal dysfunction (RD) before and after liver transplantation (LT) is still a factor complicating both short- and long-term outcomes of LT, increasing health care costs. Acute kidney injury (AKI), with reported incidence ranging from 10% to 20% in the pretransplant setting, may reach even 64% after LT, with a known impact on morbidity and mortality [1–13]. The development of RD before and/or after liver grafting is complicated and multifactorial. However, currently available methods of assessing RD in the setting of liver cirrhosis are not sufficient or practical enough [14]. Thus, in clinical practice, serum creatinine (SCr) testing is widely available and inexpensive, although the limitations of SCr are well known. In cirrhotic patients, lower levels of SCr are related to sarcopenia, decreased production of creatine by the liver, and potentially increased tubular secretion [15]. Notably, many cirrhotic patients have actively fluctuating renal function or normal renal function despite existing histological findings in the kidneys [16].
Serum creatinine is an over-weighted component of model for end-stage liver disease (MELD) score, but MELD score cannot distinguish between acute and chronic kidney dysfunction [5,17]. The spectrum of renal dysfunction before LT varies from minimal increase in serum creatinine to full-blown renal failure requiring renal replacement therapy (RRT) [17]. To emphasize the impact of kidney dysfunction on liver-transplanted patient survival, post-transplant sepsis and longer intensive care unit (ICU) stay [18], the International Ascites Club (IAC) redefined the criteria of RD in cirrhotic individuals and introduced the concept of acute kidney injury (AKI) [19,20] as well as chronic kidney disease (CKD) and acute-on-chronic kidney disease [21] to include both functional and structural renal diseases [18–20]. Subsequently, more precise criteria of AKI and CKD were introduced [22]. Impaired renal function at transplantation was associated with a higher risk of CKD after transplantation [18,19,23–25]. Giusto et al found the dominant role of lower pre-transplantation estimated glomerular filtration rate (GFR) being the only independent risk factor of CKD after LT [26]. Additionally, pretransplant AKI is associated with post-transplant morbidity, including post-LT AKI, which then predisposes to post-transplant CKD. Risk factors of post-LT AKI were identified [27] and post-LT AKI itself is a risk factor of 30-day mortality in liver graft recipients and can lead to CKD in a substantial proportion of affected individuals [1,18,28]. Thus, prevention of pre-LT or early post-transplant AKI is essential to reduce the burden of CKD after grafting [29]. Of note, the prevalence of CKD in LT recipients ranges from 20% up to 80%, and 10 years after grafting, patients have a 30% to 50% risk of chronic kidney disease development [7,18,28]. In this cohort, the risk of death is 4 times higher [30]. Development of arterial hypertension, episodes of severe infections, and reduced glomerular filtration rate may help to estimate the risk of post-LT CKD [23,26]. Arterial hypertension after LT occurs in more than 50% of recipients, which is significantly higher than in the general population [31], similar to the higher prevalence of CKD after LT [32]. However, the complexity of post-LT CKD development reflects the Renal Risk index, developed and validated by Sharma et al, which consists of 14 patient-specific factors, even without the role of immunosuppressive regimen [17].
Calcineurin inhibitors (CNI)-based immunosuppressive regimens, including cyclosporine, and mainly tacrolimus (TAC), play important roles in long-term liver graft outcome. However, CNI nephrotoxicity is still considering a major contributing factor for chronic kidney dysfunction, with up to 20% of liver graft recipients developing chronic renal disease within 3 years of LT [25,33]. These findings highlight the importance of wide-ranging strategies to balance the risk of liver graft rejection and chronic RD with the known impact of both on LT results.
Thus, the aim of this study was to analyze the impact of selected clinical features in the peri-grafting period on kidney function, including renal function before LT and surgery characteristics. The second end-point of the analysis was the role of immunosuppressive TAC-based regimen on SCr after the procedure.
Material and Methods
ETHICS:
The study protocol was approved by the Ethics Committee of the Medical University of Warsaw (AKBE/130/2020) and conformed with the ethics guidelines of the 1975 Declaration of Helsinki (6th revision, 2008).
STATISTICS:
Data are presented as mean±standard deviation (SD) and median±interquartile range (IQR). The Pearson’s correlation coefficients were calculated in order to investigate the relationship between SCr and the remaining variables. For numeric variables, the Mann-Whitney U test was performed. Normally distributed data, as well as randomly and independently distributed non-numeric data, were assessed with the Z test. The linear regression model was also used. For data analysis, the Python packages Pandas (version 1.1.0) and scikit-learn (version 0.23.2) and NumPy (version 1.18.5) and SciPy (version 1.4.1) were used. A
Results
The analyzed cohort of patients consisted of 82 males and 44 females. Their selected clinical features before LT are presented in Table 1. There were no significant differences between groups in age, sex, MELD, Child-Pugh class, baseline SCr, or serum sodium.
Before LT, there were 26 individuals with SCr level ≥1.5 mg/dL and 100 individuals with SCr <1.5 mg/dl in the entire cohort of liver graft recipients. Their clinical characteristics are summarized in Table 2. Patients with impaired and preserved renal function in respect to pretransplant SCr differed significantly in MELD score and Child-Pugh classes.
The mean volume of crystalloids and colloids transfused during surgery were 4019±1094.41 mL and 1033.98±492.50 mL, respectively. The mean anhepatic phase lasted 98.26±37.45 minutes. In n=28 (22.4%) of grafted individuals, post-reperfusion syndrome occurred. There were 110 (88%) individuals with oliguria during surgery, and 12 individuals required intraoperative dialysis. There were 72 patients with hypotonia (MAP ≤80 mmHg) immediately before LT, with an indication to catecholamine usage in 60 of them (F17, M43). The mean highest SCr during the entire observation period was 1.66±0.94 mg/dL (1.35±0.47 in females and 1.83±1.09 mg/dL in males) and at the last recorded follow-up period it was – 1.07±0.32 mg/dL (in females 0.94±0.29, and in males 1.15±0.31 mg/dL).
The factor most strongly associated with highest SCr after surgery during the observational period was the age of the recipients at LT, but not sex (p=0.09), and their SCr level before LT. In patients with preserved renal function before LT, there was a trend to intravenous administration of lower amounts of colloids during LT. The roles of arterial pressure (p=0.985) and aminopressors usage (p=0.13) were not significant. Tacrolimus blood trough level was also not a significant factor (
This trend was also observed for the last recorded SCr during the whole follow-up period of 46 months. However, the trend of benefit of lower colloids infusion during surgery reached statistical significance, with the role of hypotension during surgery (MAP ≤80 mmHg) (p=0.016) and the positive trend of catecholamines usage (
When we analyzed data regarding the impact of selected features on the changes of highest SCr (D SCr) during follow-up, only SCr before LT and the amount of intravenously administered colloids during surgery were associated with changes of creatinine level. However, the role of hypotonia and catecholamines usage was minor (both
We found an impact of pre-LT SCr on changes in the last creatinine value as well as a role of pre-LT serum sodium (
Discussion
In the present study, we found that SCr level before liver transplantation was the only risk factor for renal dysfunction after LT pretransplant and intra-operatively. In this single-center study of LT recipients, we did not find as many individuals with significant renal dysfunction as previously described in the literature. Moreover, the results questioned the leading role of CNI as a major nephrotoxic agent and showed good long-term control of both graft and kidney functions in our experience.
Post-transplant renal dysfunction is one of the most important and common complications experienced by LT recipients, leading to increased morbidity and mortality [17,33,34]. Major risk factors contributing to post-LT end-stage renal disease include recipient factors at LT as well as immunosuppression-related adverse effects [17]. However, many of the risk factors that predict chronic kidney disease after LT are associations, and not always causative [7,17]. Thus, early identification of patients at risk as well as their modification before, during, and after LT are challenging because of the narrow window of opportunity [17].
Renal dysfunctions, both AKI and CKD, in liver graft recipients, are clearly associated with post-LT morbidity and mortality [35]. Of note, RD after LT is associated with prolonged hospitalization and significant increase in hospitalization costs and ICU readmissions, and was reported to be the major risk factor for cardiac events [14]. However, despite the previously reported widely increasing problem of more severely ill cirrhotic patients with more comorbidities, our results did not reflect this trend in respect to MELD score and RD. However, more advanced liver dysfunction as judged by MEDL score and Child-Pugh class were features distinguished significantly patients with impaired or preserved renal function in the present study, similar to findings of Weber et al [18]. Of note, normal creatinine levels in the study group may be center-specific, reflecting the number of patients without comorbidities resulting in RD (eg, diabetes and NAFLD) as well as the number of individuals with HCC and well-preserved liver function, and the number of patients without significant liver function impairment or ascites (eg, in primary sclerosing cholangitis (PSC) or with alveococcosis). Wong et al found an association between the severity of ascites and increasing prevalence of AKI despite minimal baseline liver and renal dysfunction [36].
Serum creatinine before liver transplantation was the only factor associated with the levels of SCr after grafting as well as changes of creatinine levels over time. Both findings were in line with the results of recently published data of Bassegoda et al, who found that CKD commonly developed after an episode of AKI in cirrhotic individuals [37]. Baseline serum creatinine was among the factors associated with AKI progressing to CKD, but not with the etiology of kidney injury [37]. Our findings are also consistent with those of Wong et al, who showed that baseline serum creatinine might be a risk factor of AKI [20], with higher SCr associated with higher risk of AKI development and progression to CKD with reduced survival [20]. Of note, renal dysfunction defined as SCr >1.5 mg/dl lasting longer than 2 weeks was reported to be associated with the risk of CKD after liver transplantation at 1 year [19,23,24], with no role of the origin of RD. It is postulated that progression of renal damage after AKI might be related to a maladaptive repair in the tubular, vascular, and interstitial compartments of the kidney, leading to interstitial fibrosis [38]. Although the authors did not show the impact of age on delta SCr in the 2 analyzed time-points, this effect was observed for the highest and last recorded SCr. The postulated maladaptive regeneration might be derivative of age. Recipient age together with preoperative kidney function were the risk factors of CKD in the study of Kalisvaart et al [39].
Liver transplantation is a major surgery associated with intraoperative massive fluid shift. The higher amounts of intravenously administered colloids were associated with higher levels of SCr as well as increased delta SCr in the present study. However, our liver transplant center used a restrictive fluid/volume policy during grafting to decrease blood transfusion requirements, as previously described [40]. Lekerika et al [40] and the present study found that this strategy was effective in terms of kidney function, probably due to reduced bleeding during LT. Moreover, blood loss during surgery was independently associated with the risk of post-LT AKI in the study of Zongyi et al [27] as well as in previously published research [20,23]. The role of intraoperative blood loss was also found to be a significant risk factor of chronic RD 1 year after LT in univariate regression analysis of 500 cases [41]. Recently, Mrzljak et al published Crostians’ data regarding early AKI after LT, indicating the negative role of red blood cell transfusion [42]. The majority of patients developed AKI within the first 2 days after LT; thus, the major determinant of AKI development had to be procedure-related, with kidney hypoperfusion, large intraoperative fluid shifts, and blood loss. Red blood cells transfusion might indicate a pro-inflammatory state and increasing concentration of nephrotoxic free hemoglobin and iron [42–44]. Thus, intraoperative volume shifts might be a contributor to post-LT renal dysfunction, as suggested by Mrzljak et al [42]. Additionally, recipient transfused with crystalloid only had less intraoperative ascites and blood loss, with more stable hemodynamics and early extubation [45].
The highest and last recorded tacrolimus blood trough levels were not associated with SCr during the long follow-up period in the present study. Although calcineurin inhibitors in the post-LT immunosuppressive regimen have been associated with nephrotoxicity [18], it is postulated that their role may be overestimated [46]. The results of the present study are in keeping with these suppositions [47,48]. Notably, kidney-saving immunosuppressive regimens based on induction and delayed TAC introduction with lower TAC doses/blood trough levels have been applied in our center, in line with the recent recommendations [49]. We believe that more attention should be paid to acquired interstitial/post-inflammatory changes in the kidneys after LT.
The most important finding of the present study is the SCr level in the end of observation period; after 46 months of follow-up, the last recorded SCr was within normal ranges in the entire cohort. This is the achievement reflecting our center’s experience with immunosuppressive kidney-sparing protocols as well as with CNI therapy. Of note, in one of the largest studies, with 69 321 participants, 16.5% of recipients of non-renal organs developed chronic kidney disease, and among them, RRT or renal transplant were required in 28.9% [30]. Again, among the other factors, recipients age and pretransplant kidney function were risk factors for post-surgery CKD [30].
Although the presented results are encouraging in terms of long-term kidney function in liver graft recipients, our study has certain limitations, including the lack of proper collection of some important data affecting long-term kidney function, such as arterial hypertension or diabetes mellitus development after LT in the analyzed cohort. However, the main goal of the study was to analyze the selected peri-transplant features causing peri-operative hemodynamic insult to the kidney.
Conclusions
We found less renal dysfunction in our single liver transplant center than previously reported, and pretransplant serum creatinine was the only factor significantly associated with post-LT kidney function. A restricted fluid policy is safe and effective in terms of short- and long-term renal function. We confirmed the role of kidney-saving immunosuppressive protocols in preserving long-term renal function. A rational drug use policy might reduce CNI-associated nephrotoxicity. Some other important factors, such as arterial hypertension or diabetes mellitus development after LT, may affect long-term kidney function, but the main goal of the study was to analyze the selected peri-transplant features causing peri-operative hemodynamic insult to the kidney.
Our results show the experience of a high-volume LT center with long-term treatment using drugs with a narrow therapeutic window.
Tables
Table 1. Selected clinical features of the study group.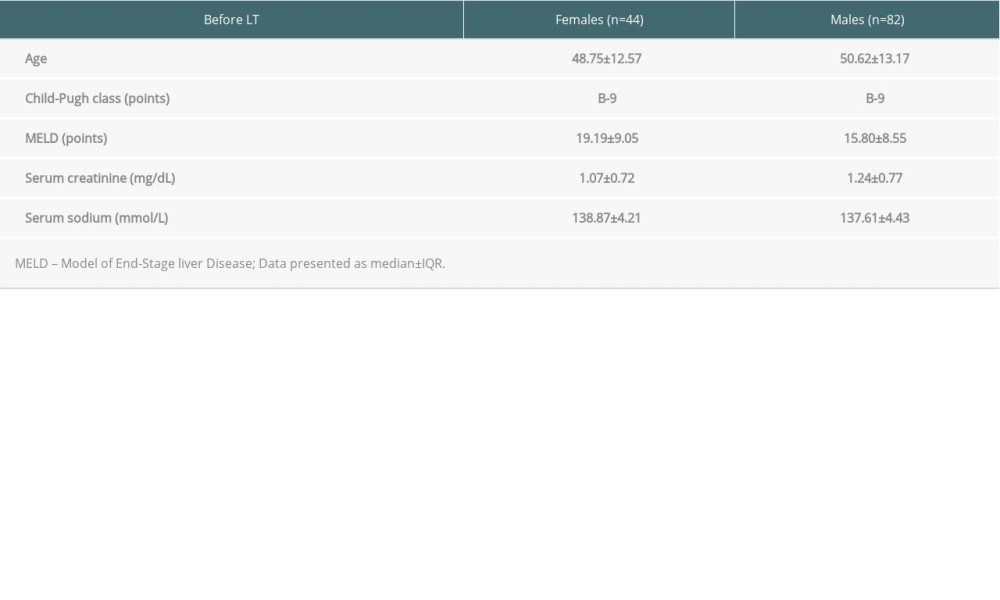 Table 2. Clinical differences between liver graft candidates in respect to baseline SCr.
Table 2. Clinical differences between liver graft candidates in respect to baseline SCr.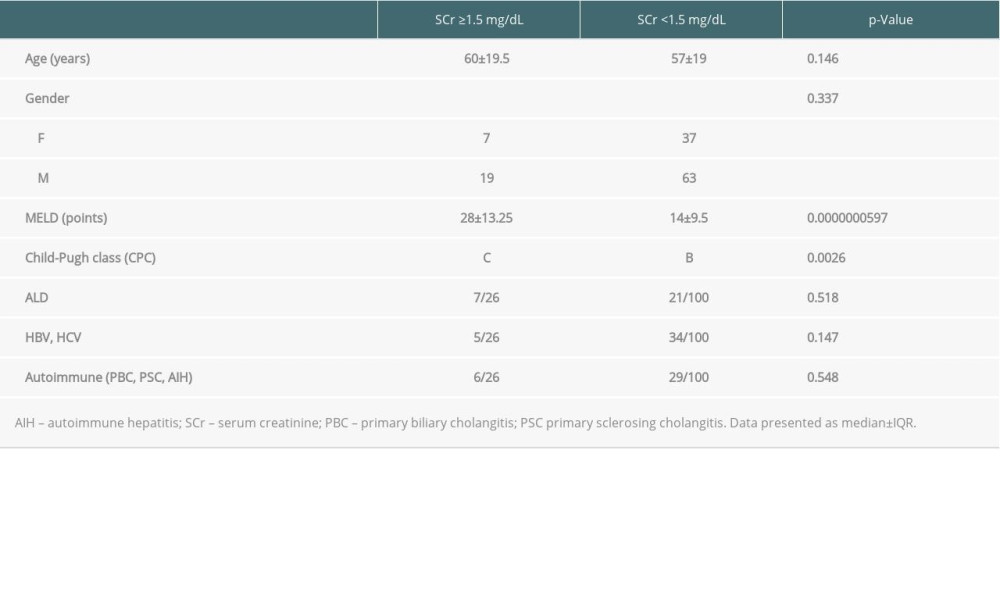 Table 3. Factors associated with the highest SCr during the whole analyzed period.
Table 3. Factors associated with the highest SCr during the whole analyzed period.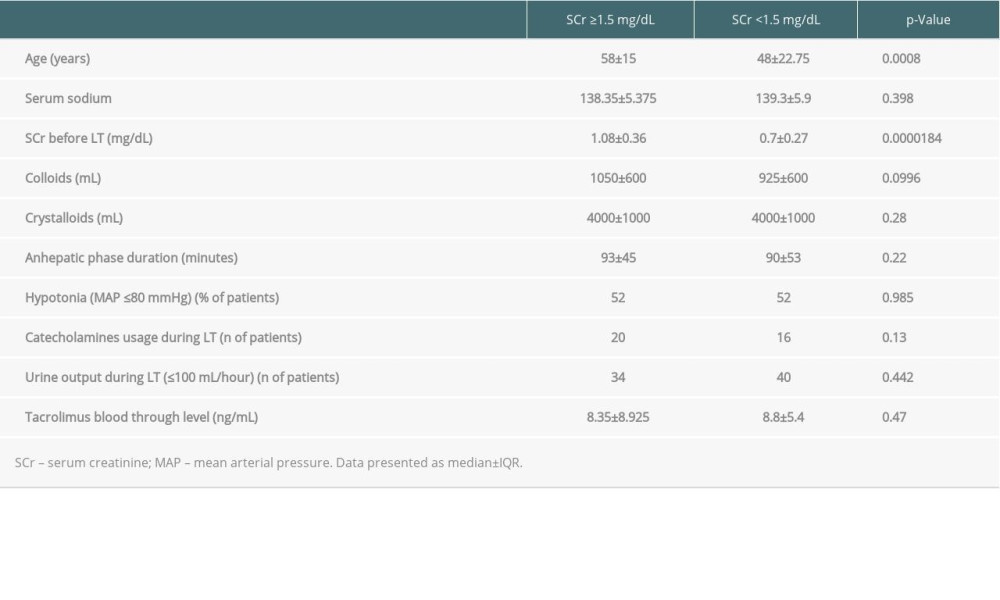 Table 4. Factors featuring the last recorded SCr during follow-up period.
Table 4. Factors featuring the last recorded SCr during follow-up period.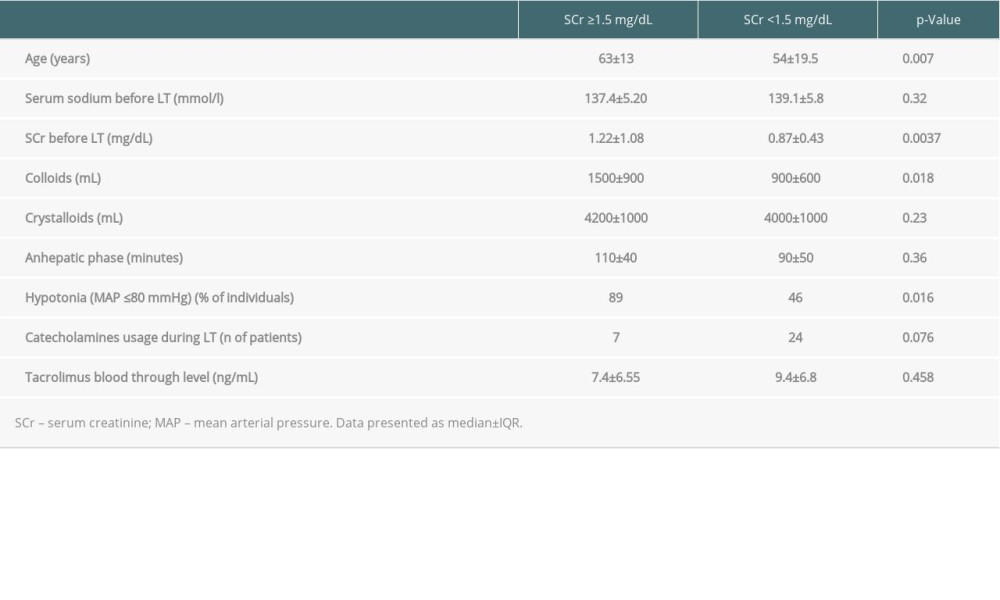 Table 5. Some characteristic impacted increasing or decreasing the highest level of SCr during observation.
Table 5. Some characteristic impacted increasing or decreasing the highest level of SCr during observation.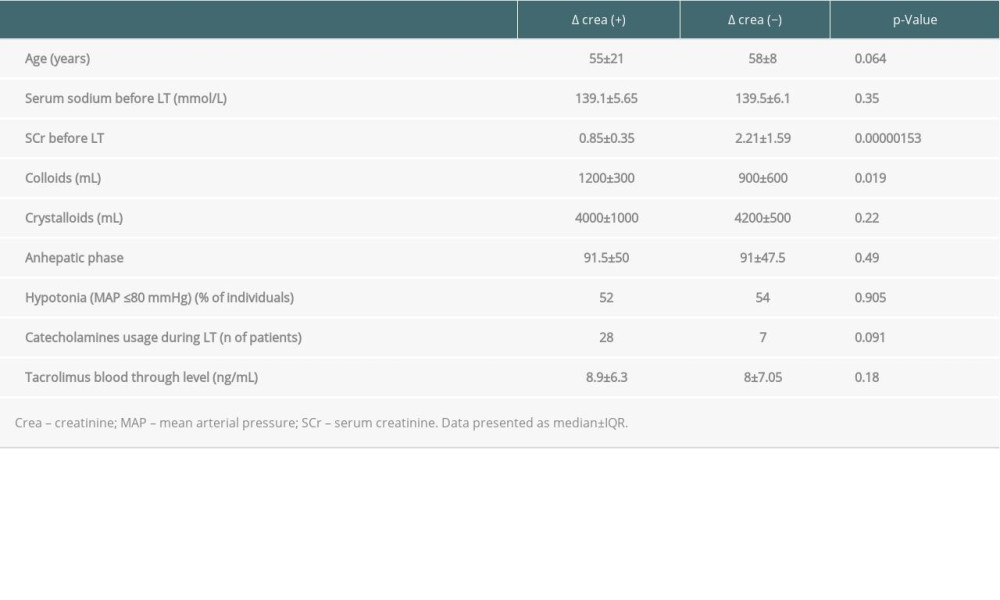 Table 6. The features impacted the changes in the last recorded SCr during follow-up period.
Table 6. The features impacted the changes in the last recorded SCr during follow-up period.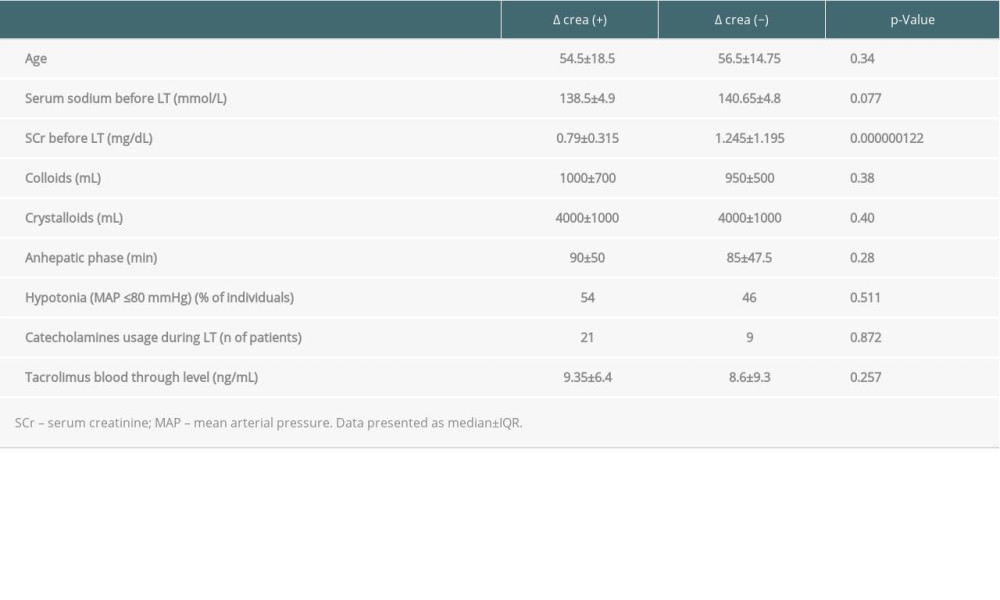
References
1. Barreto AG, Daher EF, Silva GB, Risk factors for acute kidney injury and 30-day mortality after liver transplantation: Ann Hepatol, 2015; 14; 688-94
2. Charlton MR, Wall WJ, Ojo AO, Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation: Liver Transpl, 2009; 15; S1-34
3. Chen J, Singhapricha T, Hu KQ, Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: A matched study: Transplantation, 2011; 91; 348-53
4. Gonwa TA, Klintmalm GB, Levy M, Impact of pretransplant renal function on survival after liver transplantation: Transplantation, 1995; 59; 361-65
5. Gonwa TA, McBride MA, Anderson K, Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: Where will MELD lead us?: Am J Transplant, 2006; 6; 2651-59
6. Hilmi IA, Damian D, Al-Khafaji A, Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes: Br J Anaesth, 2015; 114; 919-26
7. Horvatits T, Pischke S, Proske VM, Outcome and natural course of renal dysfunction in liver transplant recipients with severely impaired kidney function prior to transplantation: United European Gastroenterol J, 2018; 6; 104-11
8. Klaus F, Keitel da Silva C, Meinerz G, Acute kidney injury after liver transplantation: Incidence and mortality: Transplant Proc, 2014; 46; 1819-21
9. Leithead JA, Armstrong MJ, Corbett C, Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation: Transpl Int, 2013; 26; 1116-25
10. Nair S, Verma S, Thuluvath PJ, Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation: Hepatology, 2002; 35; 1179-85
11. O’Riordan A, Wong V, McQuillan R, Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation: Am J Transplant, 2007; 7; 168-76
12. Rahman S, Davidson BR, Mallett SV, Early acute kidney injury after liver transplantation: Predisposing factors and clinical implications: World J Hepatol, 2017; 9; 823-32
13. Zhu M, Li Y, Xia Q, Strong impact of acute kidney injury on survival after liver transplantation: Transplant Proc, 2010; 42; 3634-38
14. Lewandowska L, Malyszko J, Joanna Matuszkiewicz-Rowinska J, Urinary and serum biomarkers for prediction of acute kidney injury in patients undergoing liver transplantation: Ann Transplant, 2019; 24; 291-97
15. Sherman DS, Fish DN, Teitelbaum I, Assessing renal function in cirrhotic patients: Problems and pitfalls: Am J Kidney Dis, 2003; 41; 269-78
16. McGuire BM, Julian BA, Bynon JS, Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation: Ann Intern Med, 2006; 144; 735-41
17. Sharma P, Bari K, Chronic kidney disease and related long-term complications after liver transplantation: Adv Chronic Kidney Dis, 2015; 22; 404-11
18. Weber ML, Ibrahim HN, Lake JR, Renal dysfunction in liver transplant recipients: Evaluation of the critical issues: Liver Transpl, 2012; 18; 1290-301
19. Burra P, Senzolo M, Masier A, Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study: Dig Liver Dis, 2009; 41; 350-56
20. Wong F, O’Leary JG, Reddy KR, Acute kidney injury in cirrhosis: Baseline serum creatinine predicts patient outcomes: Am J Gastroenterol, 2017; 112; 1103-10
21. Wong F, Nadim MK, Kellum JA, Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis: Gut, 2011; 60; 702-9
22. Wong F, Angeli P, New diagnostic criteria and management of acute kidney injury: J Hepatol, 2017; 66; 860-61
23. Bahirwani R, Campbell MS, Siropaides T, Transplantation: Impact of pretransplant renal insufficiency: Liver Transpl, 2008; 14; 665-71
24. Cullaro G, Park M, Lai JC, “Normal” creatinine levels predict persistent kidney injury and waitlist mortality in outpatients with cirrhosis”: Hepatology, 2018; 68; 1953-60
25. Zhang W, Fung J, Limitations of current liver transplant immunosuppressive regimens: renal considerations: Hepatobiliary Pancreat Dis Int, 2017; 16; 27-32
26. Giusto M, Berenguer M, Merkel C, Chronic kidney disease after liver transplantation: Pretransplantation risk factors and predictors during follow-up: Transplantation, 2013; 95; 1148-53
27. Zongyi Y, Baifeng L, Funian Z, Risk factors of acute kidney injury after orthotopic liver transplantation in China: Sci Rep, 2017; 7; 41555
28. Hussaini T, Yoshida EM, Partovi N, Early persistent progressive acute kidney injury and graft failure post liver transplantation: Transplant Direct, 2019; 5; e429
29. Durand F, Francoz C, Asrani SK, Acute kidney injury after liver transplantation: Transplantation, 2018; 102; 1636-49
30. Ojo AO, Held PJ, Port FK, Chronic renal failure after transplantation of a nonrenal organ: N Engl J Med, 2003; 349; 931-40
31. Gojowy D, Adamczak M, Dudzicz S, High frequency of arterial hypertension in patients after liver transplantation: Transplant Proc, 2016; 48; 1721-24
32. Gojowy D, Kubis P, Gorecka M, Chronic kidney disease in patients after liver transplantation: A long-term retrospective analysis from 1 transplantation center: Transplant Proc, 2020; 52; 2492-96
33. Moini M, Schilsky ML, Tichy EM, Review on immunosuppression in liver transplantation: World J Hepatol, 2015; 7; 1355-68
34. Campbell MS, Kotlyar DS, Brensinger CM, Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation: Liver Transpl, 2005; 11; 1048-55
35. O’Leary JG, Levitsky J, Wong F, Protecting the kidney in liver transplant candidates: Practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice: Am J Transplant, 2016; 16; 2516-31
36. Wong F, Jepsen P, Watson H, Vilstrup H, Un-precipitated acute kidney injury is uncommon among stable patients with cirrhosis and ascites: Liver Int, 2018; 38; 1785-92
37. Bassegoda O, Huelin P, Ariza X, Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes: J Hepatol, 2020; 72; 1132-39
38. Chawla LS, Eggers PW, Star RA, Kimmel PL, Acute kidney injury and chronic kidney disease as interconnected syndromes: N Engl J Med, 2014; 371; 58-66
39. Kalisvaart M, Schlegel A, Trivedi PJ, Chronic kidney disease after liver transplantation: impact of extended criteria grafts: Liver Transpl, 2019; 25; 922-33
40. Lekerika N, Gutierrez Rico RM, Arco Vazquez J, Predicting fluid responsiveness in patients undergoing orthotopic liver transplantation: Effects on intraoperative blood transfusion and postoperative complications: Transplant Proc, 2014; 46; 3087-91
41. Abdel-Khalek EE, Alrefaey AK, Yassen AM, Renal dysfunction after living-donor liver transplantation: Experience with 500 cases: J Transplant, 2018; 2018 5910372
42. Mrzljak A, Franusic L, Pavicic-Saric J, Pre- and intraoperative predictors of acute kidney injury after liver transplantation: World J Clin Cases, 2020; 8; 4034-42
43. de Haan JE, Hoorn EJ, de Geus HRH, Acute kidney injury after liver transplantation: Recent insights and future perspectives: Best Pract Res Clin Gastroenterol, 2017; 31; 161-69
44. Karkouti K, Transfusion and risk of acute kidney injury in cardiac surgery: Br J Anaesth, 2012; 109(Suppl 1); i29-38
45. Huang CJ, Cheng KW, Chen CL, Clinical beneficial effects of using crystalloid only in recipients of living donor liver transplantation: Int J Environ Res Public Health, 2017; 14; 1418
46. Tholking G, Gerth HU, Schuette-Nuetgen K, Reuter S, Influence of tacrolimus metabolism rate on renal function after solid organ transplantation: World J Transplant, 2017; 7; 26-33
47. Considine A, Tredger JM, Heneghan M, Performance of modified-release tacrolimus after conversion in liver transplant patients indicates potentially favorable outcomes in selected cohorts: Liver Transpl, 2015; 21; 29-37
48. De Simone P, Nevens F, De Carlis L, Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial: Am J Transplant, 2012; 12; 3008-20
49. Levitsky J, O’Leary JG, Asrani S, Protecting the kidney in liver transplant recipients: Practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice: Am J Transplant, 2016; 16; 2532-44
Tables
 Table 1. Selected clinical features of the study group.
Table 1. Selected clinical features of the study group. Table 2. Clinical differences between liver graft candidates in respect to baseline SCr.
Table 2. Clinical differences between liver graft candidates in respect to baseline SCr. Table 3. Factors associated with the highest SCr during the whole analyzed period.
Table 3. Factors associated with the highest SCr during the whole analyzed period. Table 4. Factors featuring the last recorded SCr during follow-up period.
Table 4. Factors featuring the last recorded SCr during follow-up period. Table 5. Some characteristic impacted increasing or decreasing the highest level of SCr during observation.
Table 5. Some characteristic impacted increasing or decreasing the highest level of SCr during observation. Table 6. The features impacted the changes in the last recorded SCr during follow-up period.
Table 6. The features impacted the changes in the last recorded SCr during follow-up period. Table 1. Selected clinical features of the study group.
Table 1. Selected clinical features of the study group. Table 2. Clinical differences between liver graft candidates in respect to baseline SCr.
Table 2. Clinical differences between liver graft candidates in respect to baseline SCr. Table 3. Factors associated with the highest SCr during the whole analyzed period.
Table 3. Factors associated with the highest SCr during the whole analyzed period. Table 4. Factors featuring the last recorded SCr during follow-up period.
Table 4. Factors featuring the last recorded SCr during follow-up period. Table 5. Some characteristic impacted increasing or decreasing the highest level of SCr during observation.
Table 5. Some characteristic impacted increasing or decreasing the highest level of SCr during observation. Table 6. The features impacted the changes in the last recorded SCr during follow-up period.
Table 6. The features impacted the changes in the last recorded SCr during follow-up period. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860