15 December 2020: Original Paper
The Impact of Hypothermic Pulsatile Machine Perfusion Versus Static Cold Storage: A Donor-Matched Paired Analysis in a Scenario of High Incidence of Delayed Kidney Graft Function
Tainá Veras de Sandes-Freitas12ABCDEF*, Silvana Daher Costa12ABDEF, Luis Gustavo Modelli de Andrade3CDEF, Celi Melo Girão2BCEF, Paula Frassinetti Castelo Branco Camurça Fernandes4DEF, Claudia Maria Costa de Oliveira25DEF, Ronaldo de Matos Esmeraldo2ADEFDOI: 10.12659/AOT.927010
Ann Transplant 2020; 25:e927010
Abstract
BACKGROUND: The present study analyzed the impact of hypothermic pulsatile machine perfusion (MP) following a long period of static cold (SC) storage in the peculiar Brazilian scenario of high incidence of delayed graft function (DGF), despite good donor characteristics.
MATERIAL AND METHODS: A retrospective analysis, with a 1-year follow-up, of 206 recipients of donor-matched paired kidneys was performed. Of the 206 donor kidneys, 103 were maintained exclusively in static cold storage (SC group) and 103 were kept on machine perfusion after a period of SC preservation (MP group). All donors were brain dead.
RESULTS: Only 4.9% of the kidneys were from expanded-criteria donors. Static cold ischemia time (CIT) in the SC group was 20.8±4.1 hours vs. 15.8±6.2 hours in the MP group (P<0.001). Dynamic CIT in the MP group was 12.3±5.7 hours. MP significantly reduced DGF incidence (29.1% vs. 55.3%, P<0.001), and this effect was confirmed in multivariable analysis (OR, 1.115; 95% CI, 1.033–1.204, P=0.001). No differences were observed between the groups with regard to DGF duration, length of hospital stay, incidence of primary nonfunction and acute rejection, graft loss, death, or renal function.
CONCLUSIONS: In this Brazilian setting, MP following a long period of SC preservation was associated with reduced DGF incidence in comparison with SC storage without MP.
Keywords: Delayed Graft Function, Organ Preservation, Pulsatile Flow, Brazil, Graft Survival, Incidence, Kidney, Kidney Transplantation, Perfusion, Tissue Donors, young adult
Background
Brazil has the second-highest kidney transplantation (KT) program in the world, performing about 6,000 kidney transplants per year [1,2]. Brazilian studies have reported delayed kidney allograft function (DGF) incidences varying between 50% and 82%. These rates are 2- to 3-fold higher than those described by European and USA cohorts, without obvious explanation based on recipient and donor demographics [3–8]. Poor donor maintenance before and during the organ procurement process and longer cold ischemia time (CIT) should explain these results [9]. In this scenario, measures to reduce DGF are warranted.
Previous studies showed that machine perfusion (MP) reduces the incidence of DGF [10,11]. However, MP is not widely used in Brazil and there is scarce evidence on the benefits of MP in Brazilian patients.
A Brazilian multicenter, prospective, randomized, controlled study showed a significant reduction in DGF incidence (61%
Another Brazilian study reported MP results in a scenario closer to that of most kidney transplantation scenarios in Brazil: kidneys were kept on pulsatile perfusion after a long time in SC storage. About 20% were ECD. As a result, MP was associated with lower DGF incidence (79.2
Brazil is a large country, with significant regional disparities [13]. In our region (Ceará, in the northeast area of the country), standard criteria deceased kidney donor (SCD) transplants are predominant, and Custodiol HTK is the main perfusion solution; nevertheless, DGF incidence remains high [16]. There are 2 main transplant centers. In 1 of these 2, MP became available in 2012. The present study aimed to analyze the impact of pulsatile perfusion following a long period of SC storage due to this peculiar scenario.
Material and Methods
STUDY DESIGN AND POPULATION:
This retrospective cohort analysis included 206 donor-matched recipients of 103 pairs of deceased-donor kidneys, in which 1 kidney was maintained exclusively on SC storage preservation (SC group), and the contralateral organ was placed on MP following an initial period of SC storage (MP group). Transplants were performed at 2 transplant centers located in northeastern Brazil, between July 2013 and December 2017. Donors whose recipients lost the graft or died within 7 days after KT were excluded. All donors were brain dead (DBD).
The study was performed following the ethical standards of National Health Council Resolution 466/12 and the Declaration of Helsinki, and was approved by the local Institutional Review Board (IRB) (number 3.660.383). Data were retrospectively collected by a systematic review of medical charts and the electronic database after obtaining informed consent from patients.
OBJECTIVES:
The main objective was to analyze the impact of MP on the incidence and duration of DGF. Secondary objectives included analysis of the incidence of PNF, length of hospital stay, renal function at 1 year post-transplantation, and incidence of acute rejection, graft loss, and death.
LOGISTICS AND DEFINITIONS:
Kidneys transplanted in the 2 main centers of the Ceará region of Brazil were included in the study. At Site 1, kidneys were maintained in SC storage. At Site 2, kidneys meeting 1 of the following criteria were preserved by MP: donor age ≥50 years; final serum creatinine (sCr) >1.5 mg/dL, estimated CIT ≥20 h, severe hemodynamically unstable donors, small children, and immunologically high-risk recipients. These kidneys were kept on MP (LifePort Kidney Transporter, Organ Recovery Systems, Chicago, IL USA) for at least 6 hours using Kidney Preservation Solution-1 (KPS-1). Intra-renal resistance and flow were closely monitored and no kidney was discarded based only on hemodynamic parameters.
DGF was defined as the need for at least 1 dialysis session in the week after KT [17]. DGF duration was assessed by 2 measures: the time to the last dialysis session (days) and the number of dialysis sessions performed during this period. ECD were defined using the United Network for Organ Sharing (UNOS) definition: a) donors >60 years, or b) donors 50–59 years with at least 2 of the following: sCr >1.5 mg/dL, history of hypertension, or cardiovascular death [18]. Kidney Donor Profile Index was assessed using the Organ Procurement and Transplantation Network (OPTN) online calculator [19]:
STATISTICAL ANALYSIS:
Nominal variables are presented as absolute frequency and percentage and compared using Chi-square or Fisher tests. Normally distributed continuous variables are expressed as mean and standard deviation and compared using the
Results
DEMOGRAPHICS:
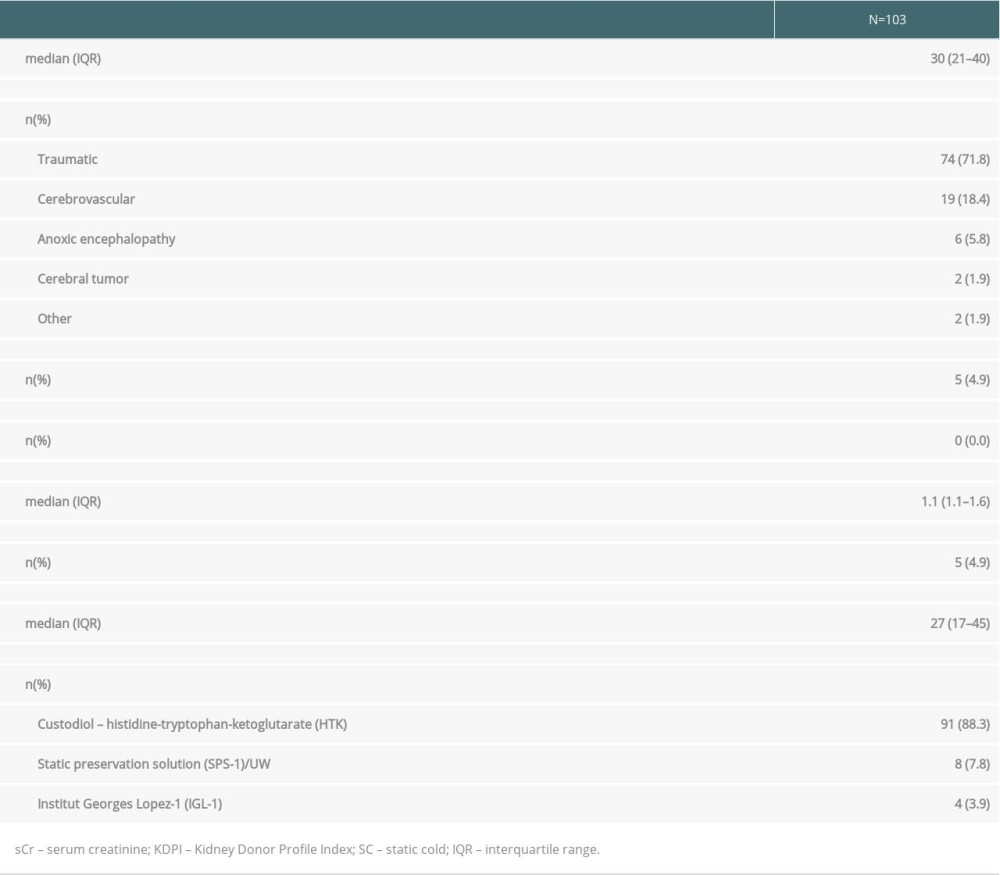
Donors were predominantly young adults (median age, 30 years) who died from trauma. Only 4.9% were ECD and the median KDPI was 27%. During SC storage, the main perfusion solution was Custodiol HTK (Table 1).
Ninety-eight (95.1%) kidneys from the MP group were transplanted at Site 2. Five (4.9%) of these MP kidneys were implanted in patients from Site 1 due to clinical/immunological problems with Site 2 candidates. Eighty (77.7%) kidneys from the SC group were transplanted at Site 1.
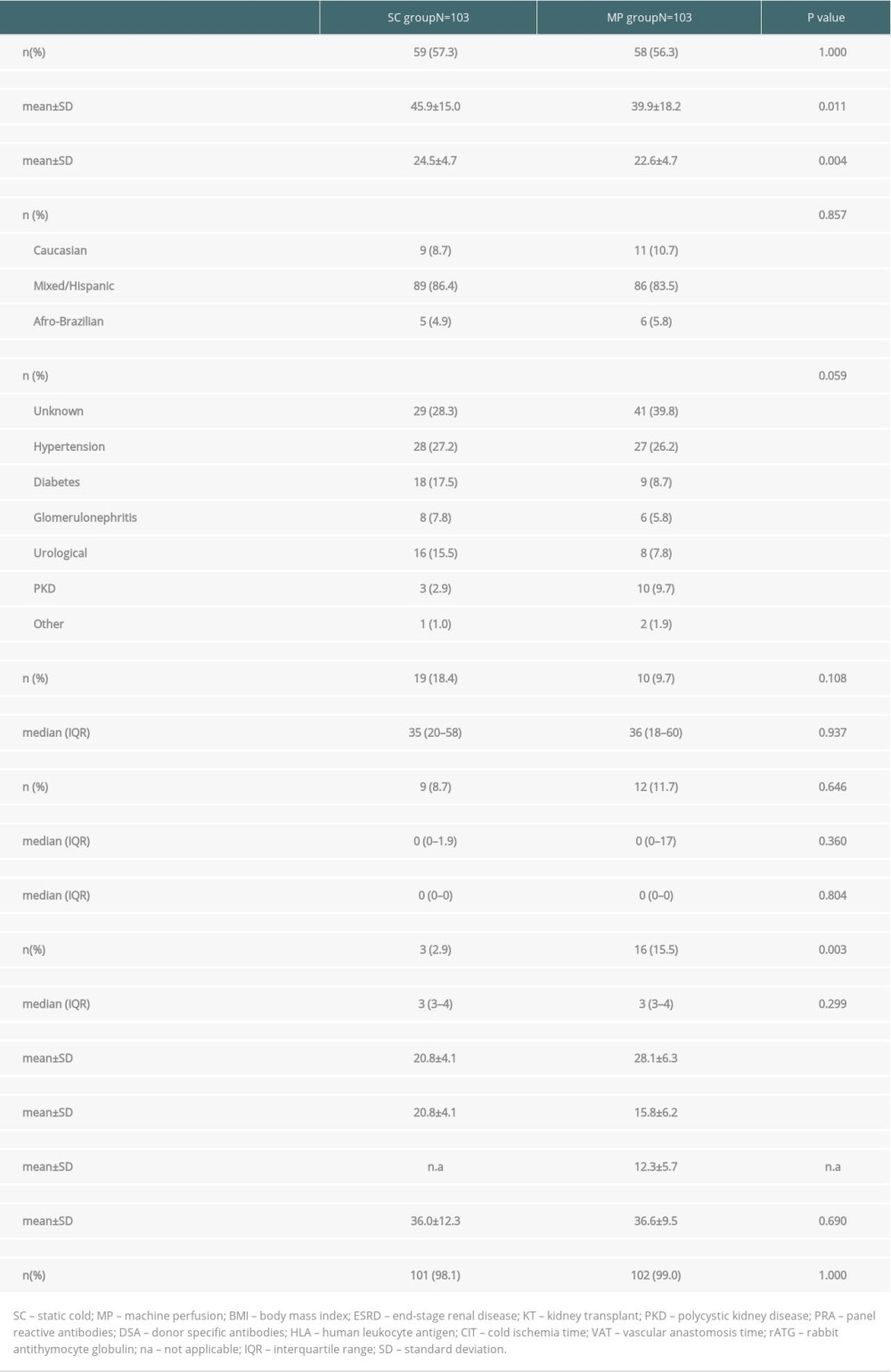
Due to the clinical protocol for MP use, patients in the MP group were younger (39.9±18.2 vs. 45.9±15.0 years, P=0.011) and a higher percentage presented preformed donor-specific antibodies (15.5% vs. 2.9%, P=0.003). In addition, this group had lower body-mass index than the SC group (22.6±4.7 vs. 24.5±4.7 kg/m2, P=0.004). The mean static CIT was 20.8±4.1 h in the SC group versus 15.8±6.2 h in the MP group (P<0.001) and dynamic CIT was 12.3±5.7 h in the MP group. Induction therapy with rabbit antithymocyte globulin was used in 98.5% of the patients, without any significant differences between the groups. More detailed information on recipient demographics and clinical characteristics is available in Table 2.
MACHINE PERFUSION HEMODYNAMIC PARAMETERS:
As expected, a significant reduction in intra-renal resistance [0.44 (0.30–0.60) mmHg/mL/min reduced to 0.22 (0.18–0.28) mmHg/mL/min,
OUTCOMES:
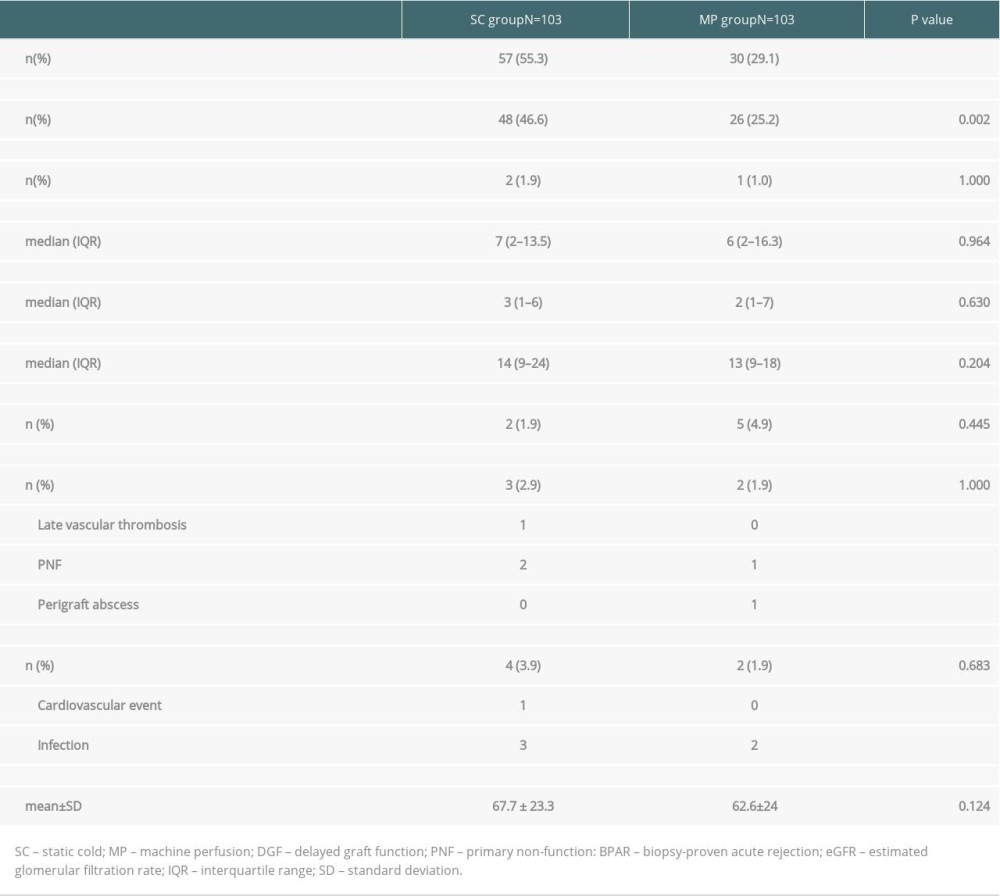
There was a significant reduction in DGF incidence in the MP group (55.3 vs. 29.1%, P<0.001). When we excluded patients who underwent a single dialysis session on the immediate postoperative day, motivated by hyperkalemia or hypervolemia, the incidence of DGF was 46.6% in the SC group and 25.2% in the MP group (P =0.002). There were no differences in duration of DGF, as measured by the time until the last dialysis session [7 (2–13.5) days vs. 6 (2–16.3) days, P=0.964] or by the number of required dialysis sessions [3 (1–6) vs. 2 (1–7), P=0.630]. Only 2 patients in the SC group and 1 in the MP group had PNF (P=1.000). There were also no differences in the length of hospital stay, acute rejection incidence, graft loss, death, or renal function (Table 3).
RISK FACTORS FOR DGF:
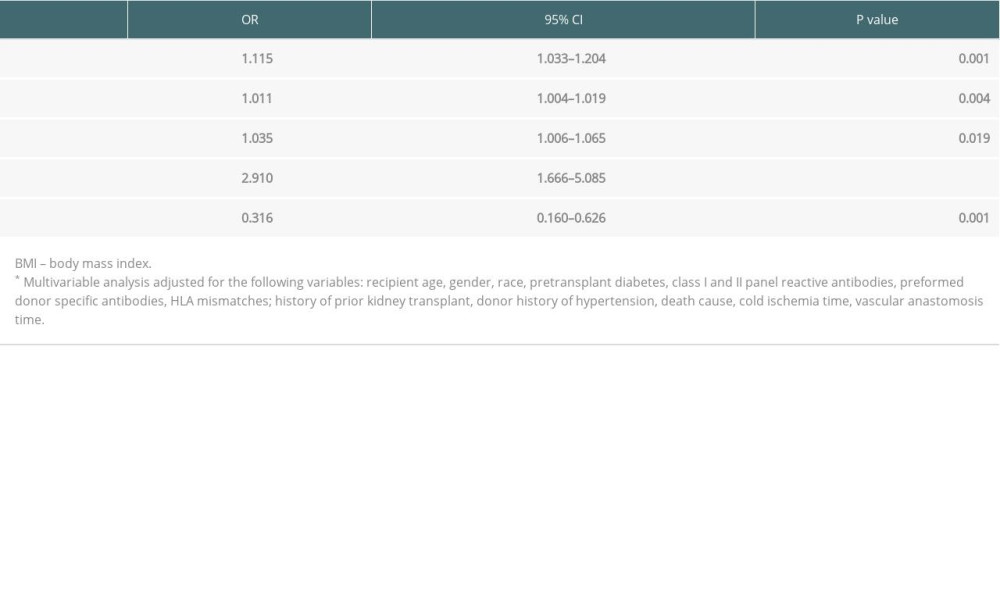
Multivariable analysis demonstrated that MP was associated with DGF reduction (OR, 0.316; 95% CI, 0.160–0.626; P=0.001). Risk factors for DGF occurrence were recipient BMI, time on dialysis, donor age, and donor final creatinine (Table 4).
Discussion
This study demonstrated that pulsatile machine perfusion reduced DGF incidence in KT in which the donor kidneys had been previously maintained in SC storage. Interestingly, we observed a 47% reduction in DGF incidence, a markedly greater impact than those observed in previous studies [10,12,14].
The benefit of kidney reconditioning with hypothermic pulsatile perfusion after a period of SC storage was previously demonstrated in experimental models and clinical settings [20–23]. The strategy usually adopted in Brazil is to only place donor kidneys with high predicted risk of DGF on MP, following an initial period of SC storage. Besides the hemodynamic issues involved in this strategy, using different perfusion solutions may be another concern, since the impact of electrolytic and osmotic environment changes on tubular renal cells is unclear.
Our results are aligned with robust and consistent evidence showing that MP reduces DGF incidence, regardless of KT modality (kidneys from SCD or ECD; donation after brain death or circulatory/cardiac death) [11,24–27]. However, we observed no impact on other transplant outcomes, despite previous results showing an inhomogeneous impact on DGF duration, PNF incidence, acute rejection incidence, graft survival, patient survival, and long-term renal function [24,25,28]. Of note, available studies list heterogeneous donor and recipient characteristics, static and dynamic ischemia times, and immunosuppressive regimens as affecting long-term outcome, in addition to short-term follow-up results.
Despite its impact being limited to DGF incidence (and not DGF duration), MP may be cost effective in our scenario of high DGF incidence. Using data from the Brazilian multicenter trial [12], Tedesco-Silva et al. evaluated the cost-effectiveness of MP in the context of public health assistance. The authors concluded that MP is a cost-effective alternative to SC preservation, with an incremental cost-effectiveness ratio of USD $22 117, adjusted for quality-adjusted life years [29]. Cost-effectiveness studies in distinct Brazilian scenarios are required. In addition, considering the scarcity of resources, a major challenge is to determine those patients who will most benefit from this strategy.
Other potential uses of MP not explored in this study are: logistic benefits; allowing transplantation with longer CIT without increasing DGF [30]; and better evaluation and improvement of hemodynamic parameters of intra-renal vasculature, reducing discard rates [21,31].
The present study has some limitations inherent in any retrospective study design in a limited number of patients. On the other hand, this study complements the existing set of clinical studies, providing evidence of the benefits of MP in the scenario of high DGF incidence despite KT with ideal donors. In addition, this study was performed in a Brazilian real-life scenario, in which MP occurred after a long period of SC ischemia. In our study, kidneys in the SC group were perfused with Custodiol HTK, a solution associated with similar outcomes when compared with KPS-1 [15], which served to minimize the influence of perfusion solution on outcomes. Another strength of the study was that it was a paired-kidney analysis, reducing donor-related biases.
Conclusions
In conclusion, MP use after a long period of SC preservation was associated with reduced DGF incidence. This result supports the benefit of this strategy in countries where machine perfusion equipment is not available at the time of the retrieval surgery. Studies with larger sample sizes are needed to define which patient subgroups are most likely to benefit from this strategy.
References
1. Garcia VD, Abbud-Filho M, Felipe C, Pestana JM, An overview of the current status of organ donation and transplantation in Brazil: Transplantation, 2015; 99; 1535-37
2. Pego-Fernandes PM, Pestana JOM, Garcia VD, Transplants in Brazil: Where are we?: Clinics (Sao Paulo), 2019; 74; e832
3. Azevedo LS, Castro MC, Monteiro de Carvalho DB, Incidence of delayed graft function in cadaveric kidney transplants in Brazil: A multicenter analysis: Transplant Proc, 2005; 37; 2746-47
4. Bronzatto EJ, da Silva Quadros KR, Santos RL, Delayed graft function in renal transplant recipients: Risk factors and impact on 1-year graft function: A single center analysis: Transplant Proc, 2009; 41; 849-51
5. de Sandes-Freitas TV, Felipe CR, Aguiar WF, Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals: PLoS One, 2015; 10; e0144188
6. Helfer MS, Vicari AR, Spuldaro F, Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation in a Brazilian center: Transplant Proc, 2014; 46; 1727-29
7. Quintella A, Lasmar MF, Fabreti-Oliveira RA, Nascimento E, Delayed graft function, predictive factors, and 7-year outcome of deceased donor kidney transplant recipients with different immunologic profiles: Transplant Proc, 2018; 50; 737-42
8. Gorayeb-Polacchini FS, Caldas HC, Gauch CR, Factors that influence delayed graft function in kidney transplants: A single-center paired kidney analysis: Transplant Proc, 2019; 51; 1568-70
9. Costa SD, de Andrade LGM, Barroso FVC, The impact of deceased donor maintenance on delayed kidney allograft function: A machine learning analysis: PLoS One, 2020; 15; e0228597
10. Moers C, Smits JM, Maathuis MH, Machine perfusion or cold storage in deceased-donor kidney transplantation: N Engl J Med, 2009; 360; 7-19
11. O’Callaghan JM, Morgan RD, Knight SR, Morris PJ, Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes: Br J Surg, 2013; 100; 991-1001
12. Tedesco-Silva HJ, Mello Offerni JC, Ayres Carneiro V, Randomized trial of machine perfusion versus cold storage in recipients of deceased donor kidney transplants with high incidence of delayed graft function: Transplant Direct, 2017; 3; e155
13. Medina-Pestana JO, Galante NZ, Tedesco-Silva H, Kidney transplantation in Brazil and its geographic disparity: J Bras Nefrol, 2011; 33; 472-84
14. Matos ACC, Requiao Moura LR, Borrelli M, Impact of machine perfusion after long static cold storage on delayed graft function incidence and duration and time to hospital discharge: Clin Transplant, 2018; 32; 13130
15. O’Callaghan JM, Knight SR, Morgan RD, Morris PJ, Preservation solutions for static cold storage of kidney allografts: A systematic review and meta-analysis: Am J Transplant, 2012; 12; 896-906
16. Mota LS, Oliveira CM, Pinheiro FMJ, Comparative study between kidney transplantation with deceased donor expanded criteria and donor standard criteria in a single center in Brazil: J Bras Nefrol, 2016; 38; 334-43
17. Mallon DH, Summers DM, Bradley JA, Pettigrew GJ, Defining delayed graft function after renal transplantation: Simplest is best: Transplantation, 2013; 96; 885-89
18. Port FK, Bragg-Gresham JL, Metzger RA, Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors: Transplantation, 2002; 74; 1281-86
19. Rao PS, Schaubel DE, Guidinger MK, A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index: Transplantation, 2009; 88; 231-36
20. Gallinat A, Paul A, Efferz P, Hypothermic reconditioning of porcine kidney grafts by short-term preimplantation machine perfusion: Transplantation, 2012; 93; 787-93
21. Gallinat A, Amrillaeva V, Hoyer DP, Reconditioning by end-ischemic hypothermic in-house machine perfusion: A promising strategy to improve outcome in expanded criteria donors kidney transplantation: Clin Transplant, 2017; 31; 12904
22. Guy A, McGrogan D, Inston N, Ready A, Hypothermic machine perfusion permits extended cold ischemia times with improved early graft function: Exp Clin Transplant, 2015; 13(2); 130-37
23. Koetting M, Frotscher C, Minor T, Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys: Transpl Int, 2010; 23; 538-42
24. Jiao B, Liu S, Liu H, Cheng D, Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: A meta-analysis: PLoS One, 2013; 8; e81826
25. Tingle SJ, Figueiredo RS, Moir JA, Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation: Cochrane Database Syst Rev, 2019; 3; CD011671
26. Treckmann J, Moers C, Smits JM, Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death: Transpl Int, 2011; 24; 548-54
27. Watson CJ, Wells AC, Roberts RJ, Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: A UK multicenter randomized controlled trial: Am J Transplant, 2010; 10; 1991-99
28. Sandal S, Luo X, Massie AB, Machine perfusion and long-term kidney transplant recipient outcomes across allograft risk strata: Nephrol Dial Transplant, 2018; 33; 1251-59
29. Tedesco Silva H, Evans RW, Gavaghan MB, Vazquez VC, A cost-effectiveness analysis of organ preservation methods for deceased donor kidneys at high risk for delayed graft function in Brazil: Transplant Proc, 2018; 50; 3121-27
30. Ciancio G, Gaynor JJ, Sageshima J, Machine perfusion following static cold storage preservation in kidney transplantation: Donor-matched pair analysis of the prognostic impact of longer pump time: Transpl Int, 2012; 25; 34-40
31. Paredes-Zapata D, Ruiz-Arranz A, Rodriguez-Villar C, Does the pulsatile preservation machine have any impact in the discard rate of kidneys from older donors after brain death?: Transplant Proc, 2015; 47; 2324-27
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860