16 March 2021: Original Paper
Use of Imputation and Decision Modeling to Improve Diagnosis and Management of Patients at Risk for New-Onset Diabetes After Transplantation
Vidit N. Munshi1ACDEF*, Soroush Saghafian2AEG, Curtiss B. Cook3BE, Sumhith Veda Aradhyula2C, Harini A. Chakkera4BEDOI: 10.12659/AOT.928624
Ann Transplant 2021; 26:e928624
Abstract
BACKGROUND: New-onset diabetes after transplantation (NODAT) is a complication of solid organ transplantation. We sought to determine the extent to which NODAT goes undiagnosed over the course of 1 year following transplantation, analyze missed or later-diagnosed cases of NODAT due to poor hemoglobin A1c (HbA1c) and fasting blood glucose (FBG) collection, and to estimate the impact that improved NODAT screening metrics may have on long-term outcomes.
MATERIAL AND METHODS: This was a retrospective study utilizing 3 datasets from a single center on kidney, liver, and heart transplantation patients. Retrospective analysis was supplemented with an imputation procedure to account for missing data and project outcomes under perfect information. In addition, the data were used to inform a simulation model used to estimate life expectancy and cost-effectiveness of a hypothetical intervention.
RESULTS: Estimates of NODAT incidence increased from 27% to 31% in kidney transplantation patients, from 31% to 40% in liver transplantation patients, and from 45% to 67% in heart transplantation patients, when HbA1c and FBG were assumed to be collected perfectly at all points. Perfect screening for kidney transplantation patients was cost-saving, while perfect screening for liver and heart transplantation patients was cost-effective at a willingness-to-pay threshold of $100 000 per life-year.
CONCLUSIONS: Improved collection of HbA1c and FBG is a cost-effective method for detecting many additional cases of NODAT within the first year alone. Additional research into both improved glucometric monitoring as well as effective strategies for mitigating NODAT risk will become increasingly important to improve health in this population.
Keywords: Data Interpretation, Statistical, Diabetes Mellitus, Organ Transplantation, Cost-Benefit Analysis, Decision Support Techniques, Glycated Hemoglobin A, Immunosuppressive Agents, Kidney Transplantation, Risk Factors
Background
New-onset diabetes after transplantation (NODAT) is a potential negative outcome following solid organ transplantation affecting patients without a prior history of a diabetes mellitus (DM) diagnosis. This newly diagnosed DM can lead to decreased graft function, increased risk of cardiovascular disease, and lower survival, resulting in increased downstream healthcare costs [1,2]. Reported estimates of NODAT incidence vary widely: 4–25% in kidney transplantations, 2.5–25% in liver transplants, and 4–40% in hearts transplantations [3–7]. Over 35 000 solid organ transplantations occurred in the United States in 2018, marking the sixth consecutive year in which the number of transplantations increased from the previous year [8]. Despite these numbers, the extent to which NODAT burdens the healthcare system and solutions to improve diagnosis, management, and specialized treatment has not been well-studied.
A 2014 international expert panel released updated recommendations for the management of transplant patients at risk for NODAT [9]. Among their recommendations was the expansion of screening tests for NODAT using hemoglobin A1c (HbA1c) and blood glucose monitoring. There is currently no standardized protocol for regular NODAT screening in the post-transplantation period, and the potential impact of this recommendation is unknown. In previous studies utilizing HbA1c and fasting blood glucose (FBG) as determinants for NODAT diagnosis, collection is inconsistent and unavailable for analysis in many patients across multiple follow-up time points [10–12]. As a result, it is likely that the NODAT incidence is largely underestimated, and that the time course of NODAT development may be different from what is understood in the current literature [12]. In addition, management of diagnosed NODAT remains uncertain, and new guidelines for managing medications and better decision-making are needed [13]. Most guidelines for decision-making follow broad type-2 DM protocols [9]. However, the transplant patient population differs from the broad diabetes population (eg, ability to modify exercise and diet in the immediate post-transplantation period); therefore, standard type 2 DM management methods may need modification for this population [14].
In the face of uncertainty and lack of evidence to inform decision-making, quantitative methods such as data imputation and disease modeling can help to make use of available data and estimate the potential for improved outcomes. Imputation in both clinical trial studies and observational studies, including studies involving transplantation data and imputation of glycemic indicators, have become widely implemented [12,15–19]. In addition, disease and decision models simulate outcomes for a patient population and utilize data on intervention effectiveness from different sources to project outcomes, thereby providing decision-makers with additional evidence to inform decisions. Decision modeling has been previously used, particularly with respect to DM, to estimate life expectancy, costs, and disease progression [20–23].
In this study, we explored a screening strategy for improvement of NODAT diagnosis and potential treatment. First, we used data imputation to estimate the impact of a screening intervention that perfectly monitors HbA1c and FBG in the follow-up period after transplantation. We analyzed data on all major solid organ transplantations (kidney, liver, and heart) to determine (a) the extent to which NODAT goes undiagnosed over the course of 1 year following transplantation, and (b) cases of NODAT that occur later than would have been predicted if HbA1c and FBG had been screened at all follow-ups. Secondly, we develop a disease model of NODAT outcomes to project life expectancy and estimate the impact a screening intervention to better collect glucose metrics may have on long-term outcomes. This study is the first modeling analysis of an intervention tailored to the transplantation population at risk of NODAT and should help decision-makers in determining how effective we might expect such an intervention to be in improving health outcomes.
Material and Methods
STUDY POPULATION:
We utilized 3 previously published datasets on kidney, liver, and heart transplantation patients [10–12,24,25]. The datasets were compiled through a de-identified chart review with IRB approval. The kidney transplantation dataset consisted of 407 patients who underwent transplant between 1999 and 2006. The liver dataset consisted of 346 patients who underwent transplant between 2007 and 2012. Finally, the heart dataset contained 152 patients who underwent transplant between 2010 and 2015. The heart dataset included information on demographics and medical history, as well as HbA1c, FBG, and some lab values at 1-, 2-, 3-, 4-, 6-, 8- and 12-months after transplant. The kidney and liver patients were followed up at 1-, 4-, and 12-months after transplant and data were collected on the same variables as for the heart patients as well as hypoglycemic medication administered to patients at each time point after transplant. Immunosuppression protocols differed by organ and are described in previous studies on these patient populations [10,11].
NODAT DEFINITION AND IMPUTATION:
Patients were classified with NODAT using 2 standard definitions. First, we defined NODAT patients as those who met at least one of the criteria of FBG >126 mg/dL (7.0 mmol/l) or HbA1c ≥6.5% (48 mmol/mol) at one of the follow-up time points. Because collection of HbA1c and blood glucose data was not consistent across all patients and follow-ups, we classified a patient as having NODAT if they were being treated with insulin at a given follow-up visit. While kidney and liver datasets contained 1-, 4-, and 12-month follow-ups, the heart dataset contained many additional follow-ups and inconsistent data collection across them. Therefore, as in previous studies utilizing these datasets, we combined follow-up months 1–3, 4–6, and 8–12 into 3 follow-up “periods” for analysis [11]. To proxy perfect collection of HbA1c and FBG, we used multiple imputation by chained equations (MICE) to estimate missing values for these variables at each follow-up point. MICE has previously been used in the literature using the kidney dataset to replace missing data [12]. We conducted a logistic regression on the probability of missing data points to provide some assurance that the available variables are not associated with missing data.
SIMULATION MODEL OVERVIEW AND STRUCTURE:
We developed a microsimulation model that simulated patients who had undergone transplantation in the United States to estimate aggregated costs and life expectancy after transplant. As long-term outcomes were not available in the data, mortality rates or relative mortality risks for each health state were obtained from relevant literature [1,26–31]. Patients were simulated on an annual cycle and could transition between one of 6 post-transplantation states on the basis of rates calculated from the observed data: (1) a healthy state representing normal glycemic control; (2) an “Untreated NODAT” diabetes state defined as fasting blood glucose (FBG) greater than 125 mg/dL (6.9 mmol/l) or HbA1c greater than 6.4% (47 mmol/mol); (3) a “Treated NODAT” diabetes state representing patients who had been diagnosed with NODAT, but whose glycemic indicators were under control through treatment; (4) a graft rejection state representing patients who had a graft rejection and were either re-transplanted or, in the case of some kidney transplantation patients, assumed to be on dialysis for the remainder of their lifetime; (5) A re-transplanted state representing patients who undergo an additional transplant after graft failure; and (6) death. The Markov chain model representation of these states is provided in Figure 1.
For the screening analysis, we ran 50 cohorts of 100 000 individuals aged 40, 50, and 60 years old at transplant and aggregated life years accrued under both the current and perfect screening scenarios. We conducted a sensitivity analysis on the mortality and diabetes risk reduction due to earlier NODAT detection, a variable on which we currently have no data to inform the model. The model was built and analyses were conducted using TreeAge Pro 2019 software.
Results
KIDNEY:
Table 1 presents descriptive statistics for kidney transplantation patients on HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for both the observed and imputed datasets. We report the number of observations for each variable in both datasets, the number of missing values (with 0 missing for imputed variables), means, and 95% confidence intervals. Means for the imputed dataset were identical to observed data. Mean HbA1c across the 3 follow-ups increased, while FBG decreased over time. The percentage of patients on insulin increased from 11.9% at 1 month to 13.9% at 4 months before dropping to 9.9% at 12 months. The number of missing HbA1c values decreased over time, from 106 at 1 month to 53 at 12 months. FBG collection was perfect at 1 month, but 14 and 29 patients had missing values at 4 and 12 months, respectively.
LIVER:
The 1-, 4-, and 12-month observed and imputed values for liver transplantation patients on HbA1c, FBG, and insulin use are found in Table 2. Similar to the data collected from kidney transplant patients, the number of missing HbA1c values drop from 204 at 1 month to 76 at 4 months, before increasing slightly to 95 at 12 months. In addition, missing FBG values increased from 11 to 14 to 29 going from 1 month to 4 months to 12 months, respectively. Insulin use was much higher in liver patients compared to kidney patients, and also decreased over time, moving from 51.5% at 1 month to 21.5% at 12 months.
HEART:
Table 3 gives descriptive statistics for heart transplantation patients on aggregated HbA1c and FBG across the 1–3 month, 4–6 month, and 8–12 month follow-up periods. FBG data was nearly complete across all 3 time periods in this cohort. However, 82 (81%) of HbA1c values were missing in the 1–3 month period. This improved to just 73 (72%) by 4–6 months and 58 (57%) by 8–12 months. Mean HbA1c and FBG both decreased over time. Compared to the observed dataset, mean imputed HbA1c values were lower and both the 1–3-month (6.6%/49 mmol/mol vs 6.1%/43 mmol/mol) and 4–6-month (6.7%/50 mmol/mol vs 5.8%/40 mmol/mol) periods.
LONG-TERM OUTCOMES:
Table 4 summarizes diagnosis of NODAT by each follow-up time point/period across all organs for both the observed and imputed datasets. Perfect collection of HbA1c and FBG resulted in 12 (15%) more NODAT cases in the kidney cohort, 21 (28%) more cases in the liver cohort, and 23 (51%) more cases in the heart cohort. In terms of time course of NODAT diagnosis, a majority (75%) of extra NODAT diagnoses in the kidney cohort occurred at the 12-month time period. In the liver cohort, however, 16/21 (76%) of extra cases were identified by 1 month, while 16/23 (70%) of extra post-heart transplant cases were identified in the 1–3 month time period. Overall, NODAT incidence using these cohorts increased from 27% to 31% in the kidney group, 31% to 40% in the liver group, and 45% to 68% in the heart group assuming imputation (using MICE) as a proxy for perfect data collection. This suggests that previous studies in which imputation is not used to handle missing values might have underestimated NODAT incidence.
PREDICTORS OF MISSING VALUES:
Odds ratios for the logistic regressions that are used to determine associations between pre-transplant patient characteristics and the probability of missing data values are given in Table 5. In both the kidney and liver datasets, transplant year was a significant predictor of missing HbA1c at 1 month. For each additional year from 2006 to 1999, having a kidney transplant 1 year earlier was associated with a 590% (OR 5.90, 95% CI 3.72, 9.38, P<0.01) increase in the odds of missing data on HbA1c at 1 month. Among liver patients, moving from 2012 to 2007, having a transplant 1 year earlier was associated with a 139% (OR 1.39, 95% CI 1.10–1.75, p<.01) increase in odds of missing 1-month HbA1c data. In addition, White heart transplant patients had a 445% (OR 4.45, 95% CI 1.43–13.86, p<.01) increased odds of having a missing 1-month HA1c. No other factors were significant.
EARLY HYPERGLYCEMIC SCREENING AS A POTENTIAL REMEDY:
Since we found that NODAT incidence might have been higher than previously reported, we now made use of simulation to estimate the impact of early screening as a potential remedy to improve patient outcomes by allowing for earlier control of NODAT and thereby less exposure to downstream risks. Figure 2 shows simulated life expectancy outcomes using base case parameters and results of a sensitivity analysis on the probability of correctly identifying and treating a new patient with NODAT. The intersection of the “Current Practice” and “Perfect Data” graphs reflects a scenario where the decision-maker is unable to correctly identify and treat any of the previously undiagnosed cases. On the other hand, the far right side of the graph indicates the potential differences in life expectancy between current practice and a scenario where every patient who previously had undiagnosed NODAT is now identified and treated. For kidney transplantations (Figure 2A), assuming perfect identification and treatment of NODAT, life expectancy in the perfect data case is 18.97 years compared to 18.84 years in the observed case, which is a difference of 0.13 years, or 1.6 months. While this occurs at a cost-savings, making perfect screening the dominant strategy, screening is still cost-effective under a $100 000 per life-year threshold if the proportion of patients starting in the “Treated” state is increased from 7.3% in the observed group to 8.5% (of a potential 21.1%) in the perfect screening case.
Figure 2B displays impact of early NODAT detection and successful control for liver transplantation recipients. Perfect data collection would result in a 0.10 (9.60 vs 9.50 years) years increase in life expectancy per person at a cost difference of $3700, resulting in an ICER of $37 000 per life-year. Perfect identification and treatment of NODAT assumes 14% of patients being treated, but an increase to just 9% from 7.6% in the current practice case would make the perfect screening strategy cost-effective at the $100 000 threshold. Across 40-year-olds, the perfect screening scenario gave an average life expectancy of 10.74 (10.69–10.81) years compared to 10.64 (10.57–10.70) years in current practice. Fifty-year-olds also gained 0.1 years per person screened from 9.85 years (9.78–9.95) to 9.75 (9.66–9.83). Finally, 60-year-olds under perfect screening gained 0.11 years, from 9.05 years (8.97–9.13) in current screening to 9.16 (9.10–9.23) in full data collection.
Heart transplantation data benefited the most from the perfect data collection, with an absolute increase of 22% in NODAT incidence (Table 4). Much of this increase was seen in the first month, where the estimate of immediate NODAT rose from 38% to 54%. Figure 2C shows the benefit obtained by the earlier detection of these extra NODAT cases in the base case scenario, with a life expectancy of 11.06 years in the perfect data scenario compared to 10.62 in current practice at a cost of $13 500 (ICER $30 800). Virtually any improvement in ability to identify and treat patients with NODAT in the perfect screening scenario was cost-effective. Age-specific life expectancies for 40-year-olds were 11.04 years (10.98–11.15) under current information compared to 11.48 years (11.39–11.56) under perfect information. For 50-year-olds, the difference was 0.45 years with a life expectancy of 10.75 years (10.65–10.82) versus 11.2 years (11.10–11.28). Finally, 60-year-olds lived on average 10.12 years (10.07–10.19) under current practice compared to 10.59 years (10.51–10.65) under perfect data collection.
SENSITIVITY ANALYSIS:
In addition to base case parameters, we also conducted a sensitivity analysis in which data-derived parameter values were varied uniformly across their 95% confidence interval. We ran 50 cohorts of 100 000 patients and output life expectancy for current practice compared to perfect screening for each cohort. Across all 50 runs, the screening scenario resulted in longer life expectancy than current practice. For 40-year-olds, screening resulted in a life expectancy of 23.17 years (95% CI 23.11–23.23) compared to 23.03 years in current practice (22.96–23.12). For 50-year-olds, life expectancy with screening was 19.04 years (18.96–19.10) compared to 18.89 years (18.78–18.94) in current practice. Finally, 60-year-olds had a screening life expectancy of 13.87 years (13.79–13.95) compared to 13.64 years (13.57–13.72) in current practice.
Discussion
Our data from kidney, liver, and heart transplantation patients demonstrate differences in HbA1c and FBG collection across types of organs transplanted as well as inconsistency in collection within a particular cohort at each follow-up time point. Improved collection of these measures can benefit transplant patients in multiple ways. First, improved screening leads to earlier detection and intervention, and potentially to better outcomes. The results of this analysis, at the very least, support design of a study to compare current practice to wide-scale HbA1c and FBG monitoring in transplantation patients to monitor potential development of NODAT and study of patient characteristics that may determine who benefits most for a more targeted intervention. The 2014 Consensus Guidelines on NODAT and other literature describing research in NODAT treatment and management have expressed a need for more prevention strategies and interventions to treat newly diagnosed patients [7,9]. This analysis contributes to this area by providing estimates for how improved collection of glycemic measures after transplant can improve our understanding and consequently our ability to best manage those at risk of NODAT.
Across all 3 organ types, FBG collection was relatively high for all time points across all 3 organs, ranging from 88% to 100% of patients having collected FBG. On the other hand, HbA1c collection was very limited. Around two-thirds of patients had HbA1c collected at 1 month and 4 months in the kidney cohort, while collection in the liver cohort was only 14% at 1 month before rising to 68% at 4 months. Despite combining heart transplant patient data into multi-follow-up time periods, only 19% of HbA1c data was collected in the 1–3-month period, rising to 28% in the 4–6-month period, and 43% in the 8–12-month period.
Estimates for life expectancy gained on average in the perfect screening base case were modest on the individual level (approximately 1.2 months in liver patients to 5.3 months in heart patients). Some patients may not benefit at all from this intervention. However, this value applied to the full population of transplantation patients may represent a significant improvement in health. Furthermore, we showed that the low cost of both HbA1c and FBG screening make monitoring of these values at each follow-up very cost-effective at a $100 000 per life-year threshold.
There are important limitations to note in this study. First, we did not consider immunosuppresion therapy in this study because guidelines recommend prioritization of any therapies related to organ acceptance over NODAT risk. Thus, we only considered factors that would not impact organ health. Second, the reliability and value of the MICE imputation is dependent on the different variables and data points available to inform the regression. All 3 datasets had many missing HbA1c data points. We showed that the imputation did not substantially affect means and standard deviations (Tables 1–3) and that the available variables in these data were not significantly associated with missing data (Table 5). The distribution of the imputed values may differ from the underlying unobserved data, and other unobserved risk factors may have played a role in explaining why some patients had HbA1c and FBG collected at a follow-up while others did not.
There are also limitations with respect to the NODAT simulation model. First, the model itself makes assumptions regarding the limited number of health states and the lack of intermediate levels of glucose tolerance (such as pre-diabetes) which may have impact on mortality and other co-morbidities. In addition, the use of data from multiple sources builds uncertainty in the model results due to the likelihood that populations represented in these data may be inherently different. However, these results still represent the best available information to make a decision and estimate effectiveness of interventions that have not been studied. Finally, data sources for validation of life expectancy outputs were difficult to gather, as NODAT in this populatoin is under-represented in the literature and few studies exist detailing outcomes of a generalizable population.
Conclusions
There are 2 broad implications of this study. First, we found that perfect collection of HbA1c and FBG is a cost-effective method for catching many additional cases of NODAT within the first year alone. This is significant because, as seen in Table 4, it indicates that estimates of NODAT incidence in the published literature may significantly underestimate its burden and time course. Clinical decision-making may benefit from incorporating more widespread screening using HbA1c and FBG in the immediate post-transplant setting. Second, this simulation model is the first model of health outcomes for the post-transplantation population with regards to NODAT. There are important extensions to this modeling work that should be applied, including evaluation of new diagnostic, surveillance, and treatment strategies to mitigate NODAT risk. Additional research into improved glucometric monitoring and safe and effective strategies for managing NODAT risk will become increasingly important to understand the impact of these interventions on our ability to detect and treat early DM cases.
Figures
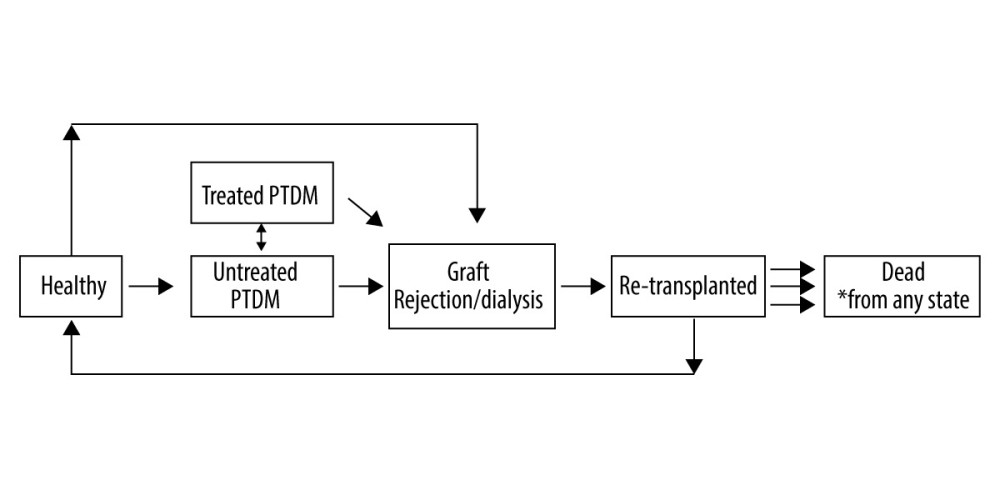 Figure 1. A Markov Chain Model of NODAT. Graft rejection state is possible for all 3 organs; Dialysis only represents kidney transplant recipients; dead state can be reached from any of the other 5 states.
Figure 1. A Markov Chain Model of NODAT. Graft rejection state is possible for all 3 organs; Dialysis only represents kidney transplant recipients; dead state can be reached from any of the other 5 states. 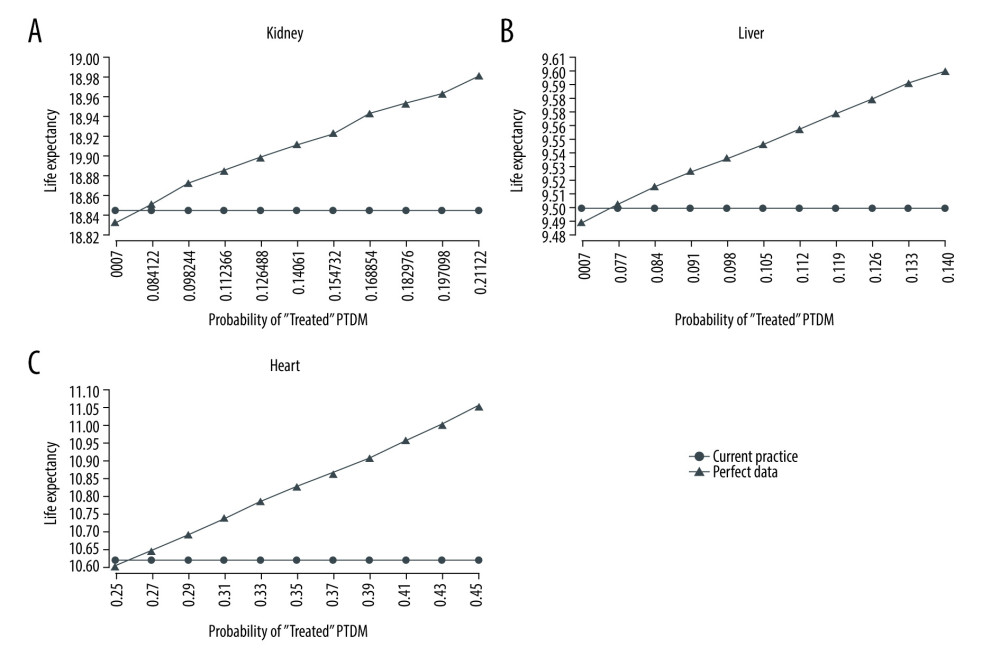 Figure 2. (A–C) Life expectancy for post-transplant liver transplantation patients under observed and perfect data collection scenarios.
Figure 2. (A–C) Life expectancy for post-transplant liver transplantation patients under observed and perfect data collection scenarios. Tables
Table 1. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for kidney transplantation patients who did not have pre-transplant DM.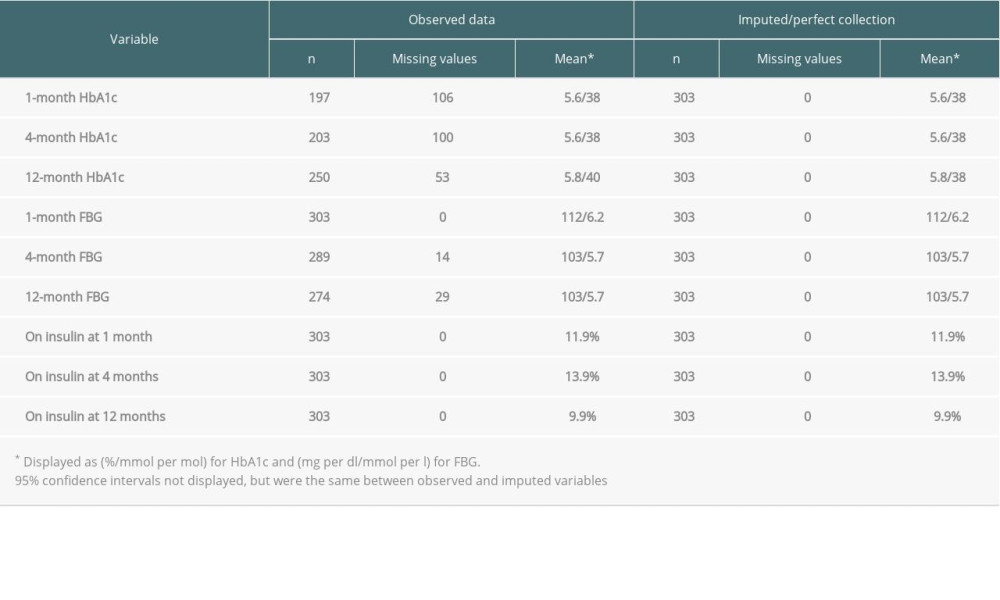 Table 2. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for liver transplantation patients who did not have pre-transplant DM.
Table 2. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for liver transplantation patients who did not have pre-transplant DM.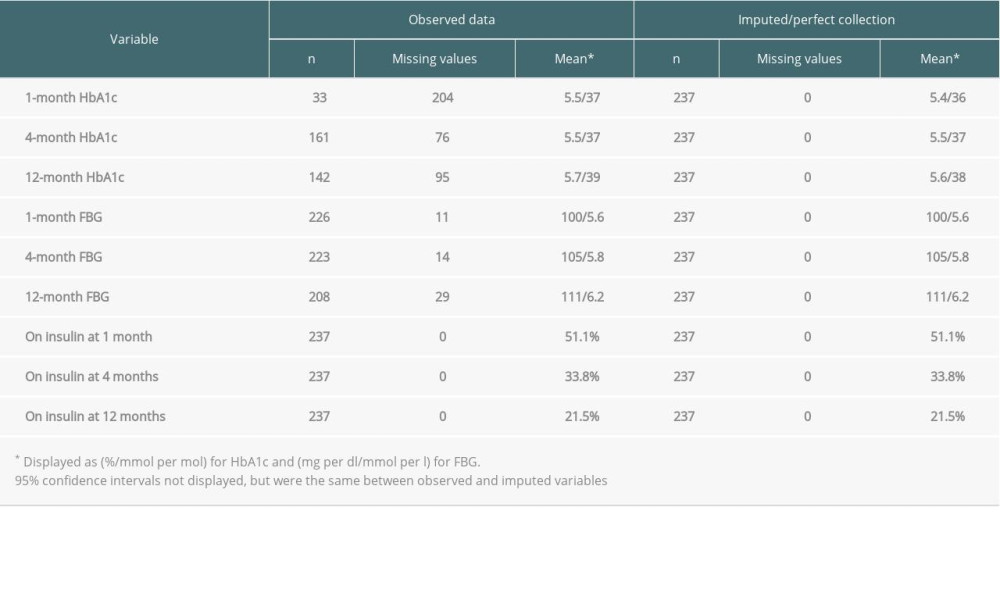 Table 3. Observed and imputed HbA1c and FBG across 1–3-month, 4–6-month, and 8–12-month follow-up periods after transplant for heart transplantation patients who did not have pre-transplant DM.
Table 3. Observed and imputed HbA1c and FBG across 1–3-month, 4–6-month, and 8–12-month follow-up periods after transplant for heart transplantation patients who did not have pre-transplant DM.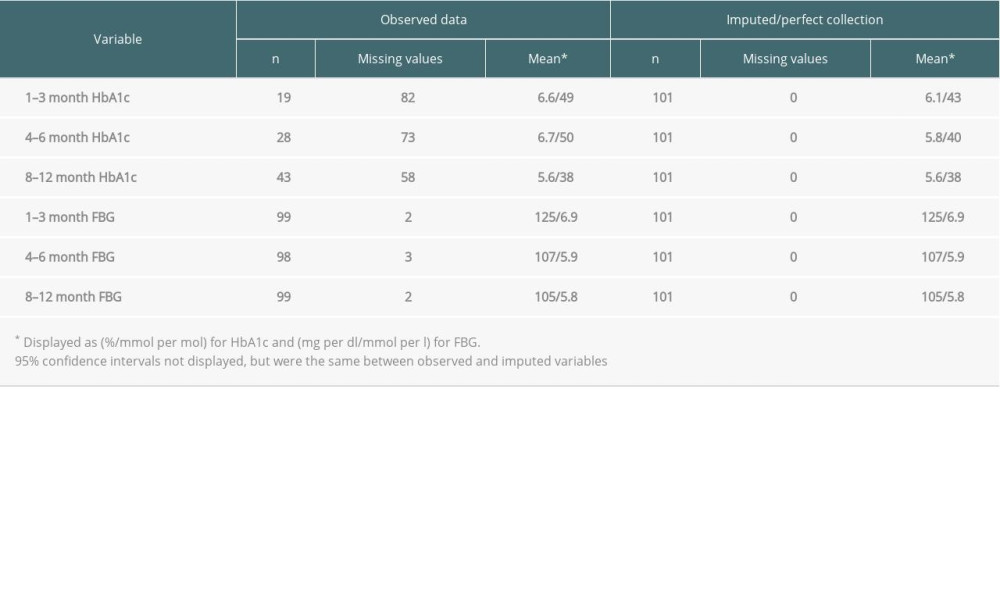 Table 4. NODAT diagnosis by follow-up time period across kidney, liver, and heart patients.
Table 4. NODAT diagnosis by follow-up time period across kidney, liver, and heart patients.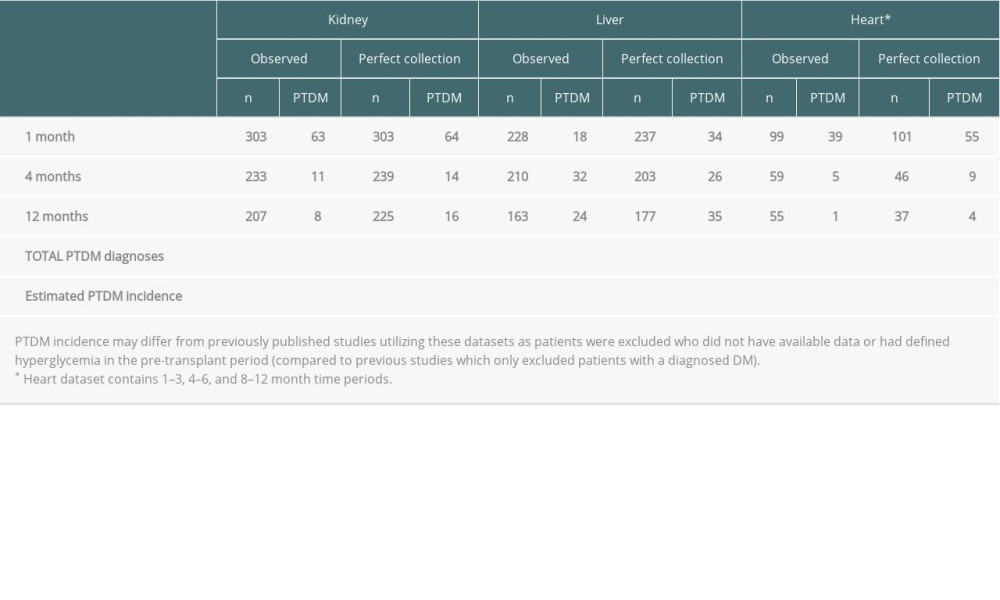 Table 5. Logistic regression odds ratios for association between pre-transplant and inpatient patient characteristics and probability of missing variables.
Table 5. Logistic regression odds ratios for association between pre-transplant and inpatient patient characteristics and probability of missing variables.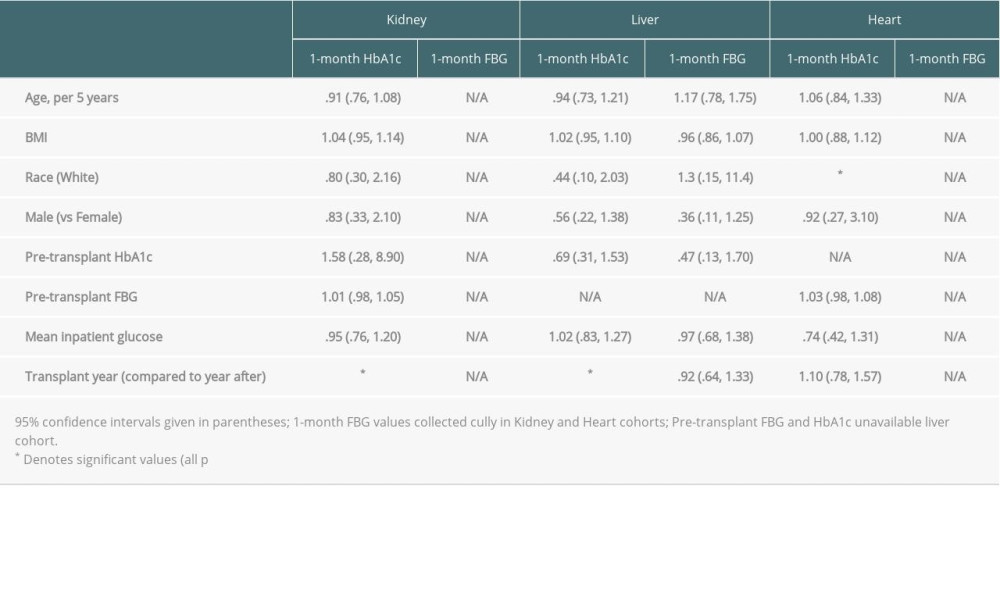
References
1. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ, Diabetes mellitus after kidney transplantation in the United States: Am J Transplant, 2003; 3(2); 178-85
2. Kasiske BL, Chakkera HA, Roel J, Explained and unexplained ischemic heart disease risk after renal transplantation: J Am Soc Nephrol, 2000; 11(9); 1735-43
3. Davidson J, Wilkinson A, Dantal J, New-onset diabetes after transplantation:2003 International consensus guidelines: Transplantation, 2003; 75(10 Suppl); SS3-24
4. Baid S, Cosimi AB, Farrell ML, Posttransplant diabetes mellitus in liver transplant recipients:Risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality: Transplantation, 2001; 72(6); 1066-72
5. Knobler H, Stagnaro-Green A, Wallenstein S, Higher incidence of diabetes in liver transplant recipients with hepatitis C: J Clin Gastroenterol, 1998; 26(1); 30-33
6. Ye X, Kuo HT, Sampaio MS, Risk factors for development of new-onset diabetes mellitus after transplant in adult lung transplant recipients: Clin Transplant, 2011; 25(6); 885-91
7. Pham PT, Pham PM, Pham SV, New onset diabetes after transplantation (NODAT):an overview: Diabetes Metab Syndr Obes, 2011; 4; 175-86
8. United Network for Organ Sharing: Transplant trends https://unos.org/data/transplant-trends/
9. Sharif A, Hecking M, de Vries AP, Proceedings from an international consensus meeting on posttransplantation diabetes mellitus:Recommendations and future directions: Am J Transplant, 2014; 14(9); 1992-2000
10. Munshi VN, Saghafian S, Cook CB, Comparison of post-transplantation diabetes mellitus incidence and risk factors between kidney and liver transplantation patients: PLoS One, 2020; 15(1); e0226873
11. Munshi VN, Saghafian S, Cook CB, Incidence, risk factors, and trends for postheart transplantation diabetes mellitus: Am J Cardiol, 2020; 125(3); 436-40
12. Boloori A, Saghafian S, Chakkera HA, Cook CB, Characterization of remitting and relapsing hyperglycemia in post-renal-transplant recipients: PLoS One, 2015; 10(11); e0142363
13. Boloori A, Saghafian S, Chakkera HA, Cook CB, Data-driven management of post-transplant medications:An ambiguous partially observable markov decision process approach: Manufacturing and Service Operations Management, 2020; 22(5); 1066-87
14. Chakkera HA, Pham PT, Pomeroy J, Response to Comment on:Chakkera et al Can new-onset diabetes after kidney transplant be prevented?. Diabetes Care. 2013;36:1406–12: Diabetes Care, 2013; 36(10); e183
15. Xu S, Schroeder E, Shetterly S, Accuracy of hemoglobin A1c imputation using fasting plasma glucose in diabetes research using electronic health records data: Statistics, Optimization & Information Computing, 2014; 2
16. van der Heijden GJ, Donders AR, Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research:A clinical example: J Clin Epidemiol, 2006; 59(10); 1102-9
17. Janssen KJ, Donders AR, Harrell FE, Missing covariate data in medical research:to impute is better than to ignore: J Clin Epidemiol, 2010; 63(7); 721-27
18. Hung AM, Roumie CL, Greevy RA, Kidney function decline in metformin versus sulfonylurea initiators:Assessment of time-dependent contribution of weight, blood pressure, and glycemic control: Pharmacoepidemiol Drug Saf, 2013; 22(6); 623-31
19. Masica AL, Ewen E, Daoud YA, Comparative effectiveness research using electronic health records:Impacts of oral antidiabetic drugs on the development of chronic kidney disease: Pharmacoepidemiol Drug Saf, 2013; 22(4); 413-22
20. Ramos M, McEwan P, Lamotte M, Foos V, The relationship of predicted benefits in life expectancy and quality adjusted life expectancy for improved glucose control in type 2 diabetes simulation modeling: Value in Health, 2017; 20(9); A747-48
21. Zhou H, Isaman DJM, Messinger S, A computer simulation model of diabetes progression, quality of life, and cost: Diabetes Care, 2005; 28(12); 2856
22. Mount Hood 4 Modeling Group, Computer modeling of diabetes and its complications: A report on the Fourth Mount Hood Challenge Meeting: Diabetes Care, 2007; 30(6); 1638-46
23. Leal J, Gray AM, Clarke PM, Development of life-expectancy tables for people with type 2 diabetes: Eur Heart J, 2009; 30(7); 834-39
24. Chakkera HA, Knowler WC, Devarapalli Y, Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus: Clin J Am Soc Nephrol, 2010; 5(9); 1669-75
25. Chakkera HA, Weil EJ, Castro J, Hyperglycemia during the immediate period after kidney transplantation: Clin J Am Soc Nephrol, 2009; 4(4); 853-59
26. Rao PS, Schaubel DE, Jia X, Survival on dialysis post-kidney transplant failure:Results from the Scientific Registry of Transplant Recipients: Am J Kidney Dis, 2007; 49(2); 294-300
27. Gill JS, Abichandani R, Kausz AT, Pereira BJ, Mortality after kidney transplant failure:The impact of non-immunologic factors: Kidney Int, 2002; 62(5); 1875-83
28. Cosio FG, Pesavento TE, Kim S, Patient survival after renal transplantation:IV. Impact of post-transplant diabetes: Kidney Int, 2002; 62(4); 1440-46
29. Shivaswamy V, Boerner B, Larsen J, Post-transplant diabetes mellitus:Causes, treatment, and impact on outcomes: Endocr Rev, 2016; 37(1); 37-61
30. Arias E, Xu J, United States life tables, 2015: Natl Vital Stat Rep, 2018; 67(7); 1-64
31. Kaballo MA, Canney M, O’Kelly P, A comparative analysis of survival of patients on dialysis and after kidney transplantation: Clin Kidney J, 2018; 11(3); 389-93
Figures
 Figure 1. A Markov Chain Model of NODAT. Graft rejection state is possible for all 3 organs; Dialysis only represents kidney transplant recipients; dead state can be reached from any of the other 5 states.
Figure 1. A Markov Chain Model of NODAT. Graft rejection state is possible for all 3 organs; Dialysis only represents kidney transplant recipients; dead state can be reached from any of the other 5 states. Figure 2. (A–C) Life expectancy for post-transplant liver transplantation patients under observed and perfect data collection scenarios.
Figure 2. (A–C) Life expectancy for post-transplant liver transplantation patients under observed and perfect data collection scenarios. Tables
 Table 1. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for kidney transplantation patients who did not have pre-transplant DM.
Table 1. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for kidney transplantation patients who did not have pre-transplant DM. Table 2. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for liver transplantation patients who did not have pre-transplant DM.
Table 2. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for liver transplantation patients who did not have pre-transplant DM. Table 3. Observed and imputed HbA1c and FBG across 1–3-month, 4–6-month, and 8–12-month follow-up periods after transplant for heart transplantation patients who did not have pre-transplant DM.
Table 3. Observed and imputed HbA1c and FBG across 1–3-month, 4–6-month, and 8–12-month follow-up periods after transplant for heart transplantation patients who did not have pre-transplant DM. Table 4. NODAT diagnosis by follow-up time period across kidney, liver, and heart patients.
Table 4. NODAT diagnosis by follow-up time period across kidney, liver, and heart patients. Table 5. Logistic regression odds ratios for association between pre-transplant and inpatient patient characteristics and probability of missing variables.
Table 5. Logistic regression odds ratios for association between pre-transplant and inpatient patient characteristics and probability of missing variables. Table 1. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for kidney transplantation patients who did not have pre-transplant DM.
Table 1. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for kidney transplantation patients who did not have pre-transplant DM. Table 2. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for liver transplantation patients who did not have pre-transplant DM.
Table 2. Observed and imputed HbA1c, FBG, and insulin use at 1, 4, and 12 months after transplant for liver transplantation patients who did not have pre-transplant DM. Table 3. Observed and imputed HbA1c and FBG across 1–3-month, 4–6-month, and 8–12-month follow-up periods after transplant for heart transplantation patients who did not have pre-transplant DM.
Table 3. Observed and imputed HbA1c and FBG across 1–3-month, 4–6-month, and 8–12-month follow-up periods after transplant for heart transplantation patients who did not have pre-transplant DM. Table 4. NODAT diagnosis by follow-up time period across kidney, liver, and heart patients.
Table 4. NODAT diagnosis by follow-up time period across kidney, liver, and heart patients. Table 5. Logistic regression odds ratios for association between pre-transplant and inpatient patient characteristics and probability of missing variables.
Table 5. Logistic regression odds ratios for association between pre-transplant and inpatient patient characteristics and probability of missing variables. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








