30 April 2021: Original Paper
Organ Donation from Donors with Hepatitis B or C in South Korea: A 2013–2017 Nationwide Data Analysis
Hoonsung Park1CEF, Eun-sil Jung2BE, Myoung Hwa Lee3BC, Jae-Myeong Lee4ACDEF*DOI: 10.12659/AOT.928947
Ann Transplant 2021; 26:e928947
Abstract
BACKGROUND: The number of organ donations from hepatitis B virus (HBV) or hepatitis C virus (HCV)-positive donors is gradually increasing; however, the current status of organ donation from brain-dead donors with hepatitis in South Korea has not been analyzed. This study aimed to analyze this.
MATERIAL AND METHODS: In total, 9210 potential brain deaths were reported in South Korea from January 2013 to December 2017, of which 333 were hepatitis carriers (HBV, n=246; HCV, n=87). Based on the data from the Korean Network for Organ Sharing and Korea Organ Donation Agency, 2460 completion of transplantations from brain-dead donors have been performed, of which 71 were hepatitis carriers (HBV, n=60; HCV, n=11).
RESULTS: There were 60 and 11 transplantations from HBV- and HCV-positive brain-dead donors, respectively. The main reasons for organ transplantation failure were recipient’s refusal (n=90), unsuitability as donors (n=80), non-brain death (n=45), and cardiac death (n=20). There were 71 and 31 kidney and liver donations, respectively; the average number of organs donated by HBV-positive donors was higher than that donated by HCV-positive donors. HBV-positive donors donated more hearts and livers than HCV-positive donors.
CONCLUSIONS: There are few organ donations from brain-dead donors with hepatitis B or C which led to transplantation completion in South Korea, and the main reasons for failure are recipient’s refusal to receive organs from donors with hepatitis and unsuitability for donation due to active viral conditions. To promote organ transplantations from donors with hepatitis B and C virus, we could consider 3 strategies: 1) reducing recipient’s refusal rates by educating recipients and their families on the outcomes of organ donation from hepatitis carriers, 2) establishing treatment protocols for infection management after organ transplantations from HBV/HCV brain-dead donors, and 3) increasing the relevant experience of medical staff.
Keywords: Brain Death, Hepatitis B, Hepatitis C, Tissue Donors, Transplantation, data analysis, Organ Transplantation, Republic of Korea, Tissue and Organ Procurement
Background
The demand for transplantations has been increasing with the increase in the number of patients with organ failure, such as kidney, liver, heart, and lung failures. However, the supply of donated organs is lower than the demand [1]. Each year, approximately 3000 patients in the U.S. die waiting for an organ transplantation, and approximately 100 000 potential candidates for transplantation die before they are placed on a waiting list [2]. Donations from marginal- or expanded-criteria donors have been considered to solve organ shortage and the number of donations from such donors has been increasing [3]. These include elderly donors, pediatric donors, diabetic donors, donors with an extended cold ischemic time, donation after circulatory death, split-liver transplantation, and donors with infection. Liver transplantations from HBV- and HCV-infected donors are also being considered [4].
According to the Korean Network for Organ Sharing (KONOS) guidelines, HBV-positive donors can donate organs only to HBV-positive recipients, and anti-HCV-positive donors can donate organs only to anti-HCV-positive recipients. Heart and lung transplantations using organs from HBV-positive and anti-HCV-positive donors are allowed for HBV-negative and anti-HCV-negative recipients if they are in critical condition [5]. Conversely, liver and kidney transplantations from HBV/HCV-positive donors to negative recipients have been increasingly performed recently with the development of therapeutic agents to treat hepatitis.
In cases of organ transplantations from anti-HBc-positive donors to negative donors among HBV serologic types, hepatitis B immunoglobulin and nucleos(t)ide analogs, such as lamivudine, entecavir, and tenofovir, are used to prevent de novo hepatitis, and their safety has been proven in multiple studies [6]. In cases of liver transplantations from HCV-infected donors to uninfected recipients, the recipients’ RNA titer is monitored, and treatment with direct-acting antivirals (DAAs) is administered [7]. Between 2008 and 2017, there were 2635 liver transplantations using livers of HCV-seropositive donors performed in the U.S., of which 2378 cases were for HCV-seropositive recipients. The number of transplantations from HCV-seropositive donors to negative recipients increased from 7 cases in 2008 to 107 in 2017; 55 cases were in the pre-DAA era and 202 cases were in the post-DAA era [8].
Although organ donations from HBV- and HCV-infected donors have been increasing in South Korea, most of the donations are limited to HBV/HCV-positive recipients, and there is no report on their specific status. Therefore, this study aimed to analyze the current status of transplantations from brain-dead donors with HBV or HCV infections from 2013 to 2017 in South Korea.
Material and Methods
The Korea Organ Donation Agency (KODA) provided data for 9210 potential brain deaths in South Korea from January 2013 to December 2017, of which 333 were hepatitis carriers (HBV, n=246; HCV, n=87). The data of 2460 cases of transplantations completed from 9210 potential brain deaths reported by the KONOS were analyzed. Of the 2460 brain-dead organ donors, a total of 71 had hepatitis (HBV, n=60; HCV, n=11) (Figure 1).
All continuous data are presented as medians±standard deviations and ranges. All categorical data are presented as numbers and percentages/frequency. IBM SPSS Statistics version 23.0 for Windows (IBM Corp., Illinois, USA) was used for all statistical analyses. The Kolmogorov-Smirnov test and the
This study was approved by the Institutional Review Board of Korea University Medical Center (approval number: 2018AN0211), which waived the requirement for informed consent.
Results
Table 1 shows the details related to the donations by hepatitis-infected donors among the potential brain deaths reported by the KODA between 2013 and 2017.
Among the 246 cases of HBV-positive potential brain deaths, 60 (24.4%) led to transplantation completion, while 186 (75.6%) led to failures. The main reasons for transplantation failures were recipient’s refusal (n=66), followed by unsuitability as donors (n=56), not-brain death (n=33), and cardiac death (n=16). Among the 87 cases of HCV-positive potential brain deaths, 11 (12.6%) led to transplantation completion and 76 led to failures. The main reasons for transplantation failures were recipient’s refusal (n=24), unsuitability as donors (n=24), not-brain death (n=12), no recipient matched (n=6), cardiac death (n=4), no legal guardians (n=2), consent retrieval (n=2), legal issues (n=1), and accident problem (n=1).
Although brain-dead organ donor management began with HBV and HCV carriers, a total of 89 cases (the sum of not suitable as donors and no recipient matched; 22 cases in 2013, 23 in 2014, 15 in 2015, 13 in 2016, and 16 in 2017) led to donation failures owing to the lack of donation organizations or incompatible recipients (HBV, n=59; HCV, n=30).
Table 2 shows the general characteristics of 60 HBV-positive organ donors and 11 HCV-positive organ donors with completion of transplantation. The average number of organs donated by HBV-positive donors was 1.9, which was higher than the number of organs donated by HCV-positive donors (1.5); however, there was no significant difference (
In terms of HAV-positive brain-dead donors, 4 transplantations were performed from 2013 to 2017. The donors’ mean age was 36.8±10.8 years, and 3 (75%) donors were men. The causes of brain death were cerebral hemorrhage, traumatic brain injury, hypoxia, cerebral infarction, and acute hepatic failure. The mean number of organs donated was 3.0±1.2. Kidneys were donated in all the cases; hearts, lungs, and livers were donated in 2 cases; and corneas were donated in 2 cases.
During the management of HBV-infected brain-dead donors between 2013 and 2017, there were 12 cases of donation failures even after completing the first brain death assessment and recipient selection process (Table 3). The reasons for failure included not suitable as donor (n=5: active viral conditions; n=4 and unsuitability for donation during organ procurement; n=1), absence of recipients (n=3), abdominal tuberculosis infection during procurement (n=1), non-brain death due to seizure activity during the electroencephalogram test (n=1), unsuitability for donation owing to renal cell carcinoma on abdominal CT (n=1), and cardiac death from hemodynamic instability (n=1) (Table 3).
There were 9 cases of donation failures from HCV-infected brain-dead donors (Table 4). The main reasons were no recipients (n=5), not suitable as donor owing to a high RNA titer (n=1), not suitable as donor owing to HCV positivity confirmation after the first brain death assessment (n=1), non-brain death (n=1), and renal cell carcinoma during organ procurement (n=1). For case no. 1 (Table 4), 12 eligible recipients with the same AB blood type refused to receive organ donations.
Discussion
Between 2013 and 2017, there were 60 and 11 cases of donations from HBV or HCV-positive brain-dead donors, respectively. There were 71 kidney donations and 31 liver donations from HBV or HCV-positive brain-dead donors, which accounted for 1.6% (71/4421) of the total number of brain-dead kidney transplantations and 1.4% (31/2185) of the total number of liver transplantations during the study period in South Korea [9]. Based on U.S. Scientific Registry of Transplant Recipients data, there were 2131 cases of HCV-positive liver grafts between 2005 and 2015 (HCV-negative liver grafts, n=23 435). The proportion of patients receiving HCV-positive liver grafts from 2005 to 2010 was 6.2%, which increased to 9.1% in 2013 during the early DAA/interferon (IFN) era, 12.2% in 2014 (the IFN-free DAA era), and 16.9% in 2015. In addition, the number of HCV-positive liver grafts discarded every year showed a decreasing trend from 28% between 2005 and 2010 and to 22% in 2011 and 11% in 2015 [10]. In South Korea, limited liver/kidney transplantations from HBV/HCV-positive donors to HBV/HCV-positive recipients have been performed for a long time; however, transplantations to virus-negative recipients have just recently begun. The number of transplantations from HBV-positive donors has been gradually increasing, from 6 cases in 2013 to 14 cases in 2017. However, there were only 3 cases of transplantations from HCV-positive donors in 2017; thus, donations from hepatitis carriers are not active in South Korea [5].
While the number of donations from HBV- and HCV-positive donors is important, the function of the donated organs and the medical outcome of the recipients are more important. Using the UNOS data, a study on patients who underwent liver transplantations owing to HCV-related liver cirrhosis showed that the patient and graft survival rates were similar between 96 patients who underwent organ transplantations from HCV-positive donors and 2827 patients who underwent organ transplantations from HCV-negative donors; further, the group that underwent organ transplantations from HCV-positive donors showed better patient and graft survival rates than the group that underwent organ transplantations from HCV-negative donors (2-year survival rate: 90% vs 77%,
HBV infection is not endemic in the U.S. Although it is endemic in South Korea, transplantations from HBV-positive donors to negative recipients are limited in South Korea. Therefore, few studies have been conducted on HBV infection compared to those on HCV infection. According to a single-center retrospective study in the U.S. comparing 25 recipients who underwent liver transplantations from anti-HBc-positive donors with 843 recipients who underwent liver transplantations from HBV-negative donors, the 1-month, 1-year, and 5-year survival rates for recipients who underwent liver transplantations from anti-HBc-positive donors were 92%, 74.3%, and 74.3%, while those for recipients who underwent liver transplantations from HBV-negative donors were 96%, 89.1%, and 76.6% (
For kidney transplantation, only 2402 (37%) of 6546 kidneys were transplanted from 3273 anti-HCV-positive donors in the U.S. between 2005 and 2014 [14]. Thus far, only 2 open-label nonrandomized single-center trials and 1 single-center observational study have assessed the outcomes of kidney transplantations from anti-HCV-positive donors to negative recipients. In the study by Reese et al (the THINKER trial), 20 HCV-negative recipients who received kidneys from HCV-positive donors received HCV treatment 5 days after transplantation; all 20 patients reached a sustained virologic response 12 weeks after treatment initiation; the median estimated glomerular filtration rate (eGFR) after 6 and 12 months was 67.5 and 72.8 mL/min/1.73 m2, respectively [15]. In the study by Durand et al (the EXPANDER trial), HCV prophylaxis was performed on 10 HCV-negative recipients who received kidneys from HCV-positive donors before and up to 12 weeks after surgery; there was no virologic or clinical evidence of HCV infection after 12 weeks in all cases. The median eGFR after 12 weeks was 63.5 mL/min/1.73 m2 [16]. In their study, Molnar et al initiated HCV treatment (DAA) when HCV RNA was detected in 53 HCV-negative recipients who received kidneys from HCV-positive donors. All 53 recipients were HCV RNA-negative and reached sustained virologic response after 12 weeks; the median eGFR after 12 weeks was 67 mL/min/1.73 m2 [17]. However, no relevant multicenter studies have been conducted thus far.
According to the 2018 OPTN/STSR annual data report, the willingness to accept HCV-positive organ transplants increased for candidates for kidneys, pancreas, heart, and lungs from 2006 to 2018. In terms of kidneys, this rise began in 2014, which is consistent with the expanded use of second-generation DAAs for HCV infection treatment in 2014. However, the willingness to accept small intestines showed a decreasing trend, and that for livers showed a rebound trend after decreasing until 2016. The reasons for such trends are unclear, but the trends could be attributed to the fact that the waiting period for small intestine transplantations is shorter than that for other organ transplantations and to concerns of potential infection by receiving livers from HCV-positive donors [18].
In the present study, the main reasons for organ transplantation failures from brain-dead donors with viral hepatitis (HBV or HCV) were recipient’s refusal (n=90), unsuitability as donors (n=80), non-brain death (n=45), and cardiac death (n=20). These results show that recipients often refuse to receive organs from hepatitis-infected donors, even if they are hepatitis carriers. Therefore, it is necessary to promote current therapeutic agents to treat hepatitis and help recipients and their families to understand the outcomes of transplantation to encourage organ transplantations from donors with viral hepatitis in South Korea.
Further, it is also important to establish a treatment protocol for infection management after organ transplantations from HBV/HCV-positive brain-dead donors and to increase the relevant experience of medical staff. Doctors who manage recipients after organ transplantations should also gain sufficient knowledge and experience in treating hepatitis. Objective data on the results after transplantation should also be presented.
The significance of this study is there has been no analysis on the current status of organ transplantations from brain-dead donors with hepatitis in South Korea. Although the number of cases is small, the number of donations from HBV- and HCV-positive donors is gradually increasing. However, there is no data collection system in South Korea that can be used to analyze the transplantation outcomes of recipients who received organ donations from hepatitis carriers; thus, the relevant results cannot be presented together. In 2012, Korean Organ Transplantation Registry (KOTRY) was established and has published annual reports since 2014. It is a government-driven project and collects data from involved institutions. However, it is not connected with the KODA/KONOS database, so there exists a need to integrate databases. Therefore, follow-up studies are necessary to evaluate the outcomes of treating recipients who received organ donations from hepatitis carriers in South Korea. If the follow-up study results are positive, organ transplantations from hepatitis carriers will become more active; this will then help to narrow the gap between the supply and demand for organ transplantations.
Conclusions
Although the number of donations from HBV- and HCV-positive donors is gradually increasing, in South Korea there have been few organ donations from brain-dead donors with hepatitis that led to transplantation completion. The main reasons for failure are recipient’s refusal to receive organs from donors with hepatitis and unsuitability for donation concerning active viral conditions. To promote organ transplantations from donors with hepatitis B and C virus, we could consider 3 strategies: 1) reducing recipient’s refusal rates by educating recipients and their families on the outcomes of organ donation from hepatitis carriers, 2) establishing treatment protocols for infection management after organ transplantations from HBV/HCV brain-dead donors, and 3) increasing the relevant experience of medical staff.
Figures
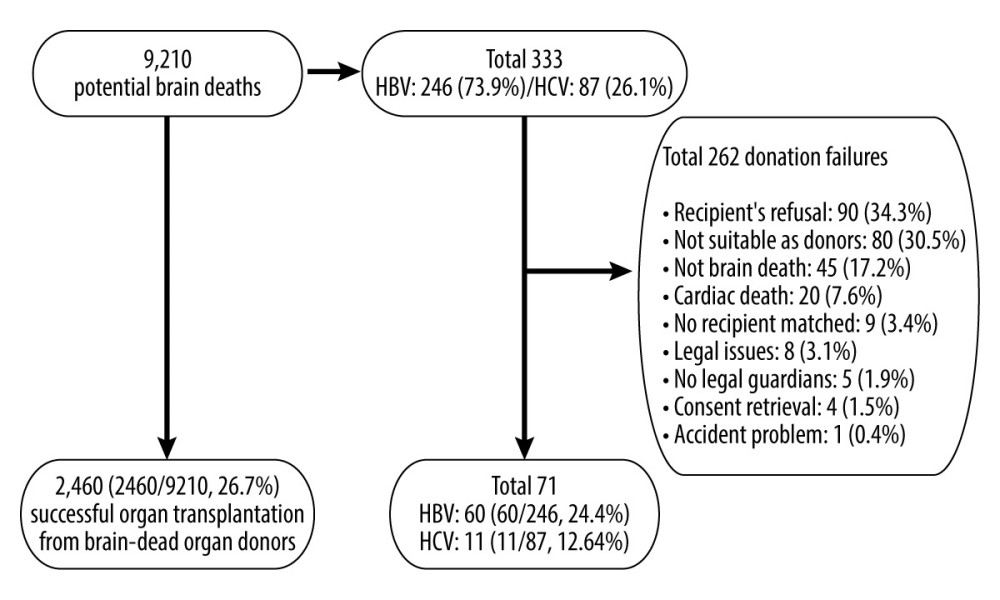 Figure 1. Study population.
Figure 1. Study population. Tables
Table 1. Summary of viral hepatitis cases among potential brain-dead donors according to the year between 2013 and 2017 in South Korea.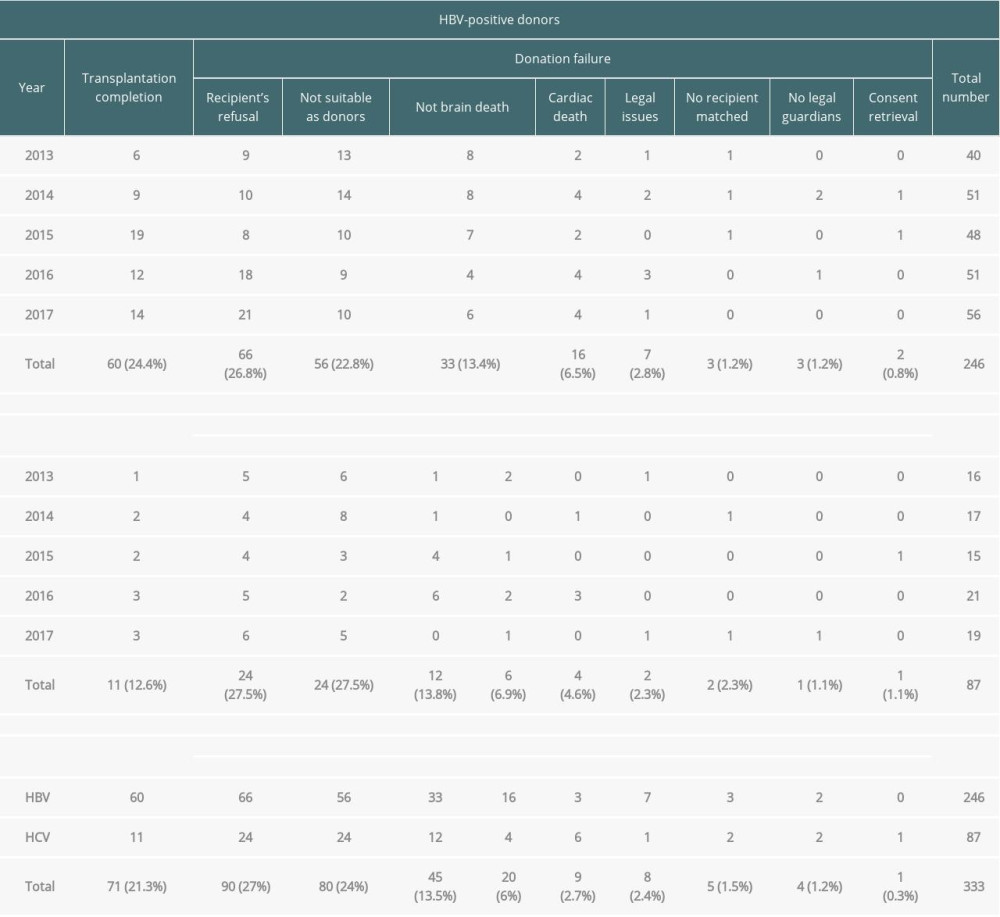 Table 2. General characteristics of brain-dead organ donors with hepatitis B or C with transplantation completion.
Table 2. General characteristics of brain-dead organ donors with hepatitis B or C with transplantation completion.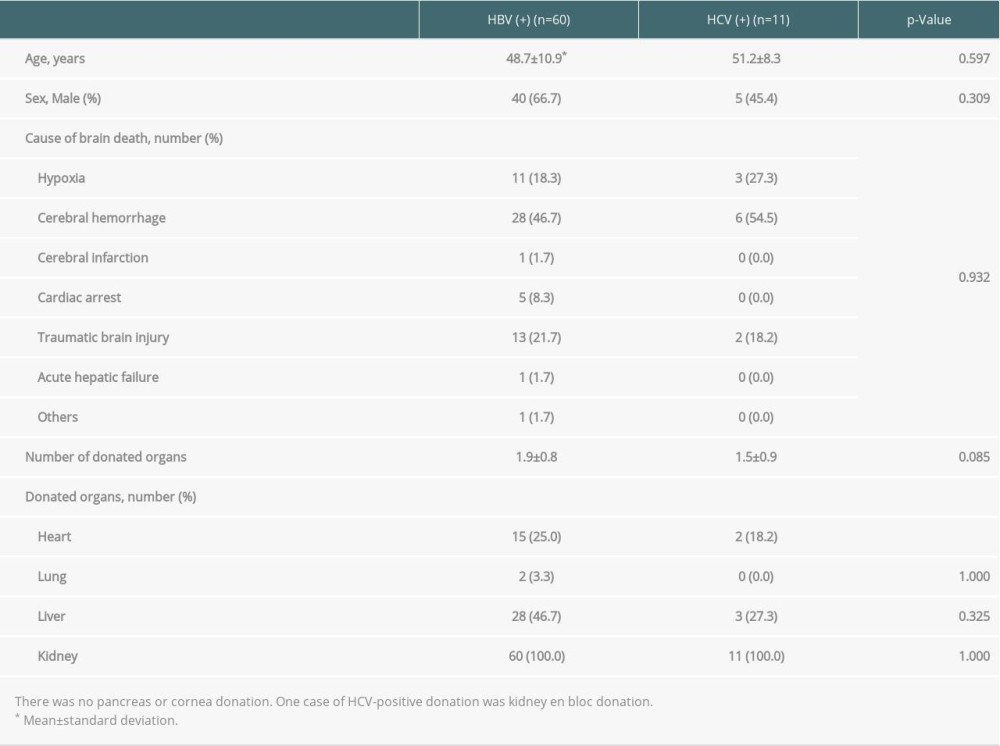 Table 3. Donation failure cases after obtaining donation consent and determining brain death owing to HBV infection between 2013 and 2017 in South Korea.
Table 3. Donation failure cases after obtaining donation consent and determining brain death owing to HBV infection between 2013 and 2017 in South Korea.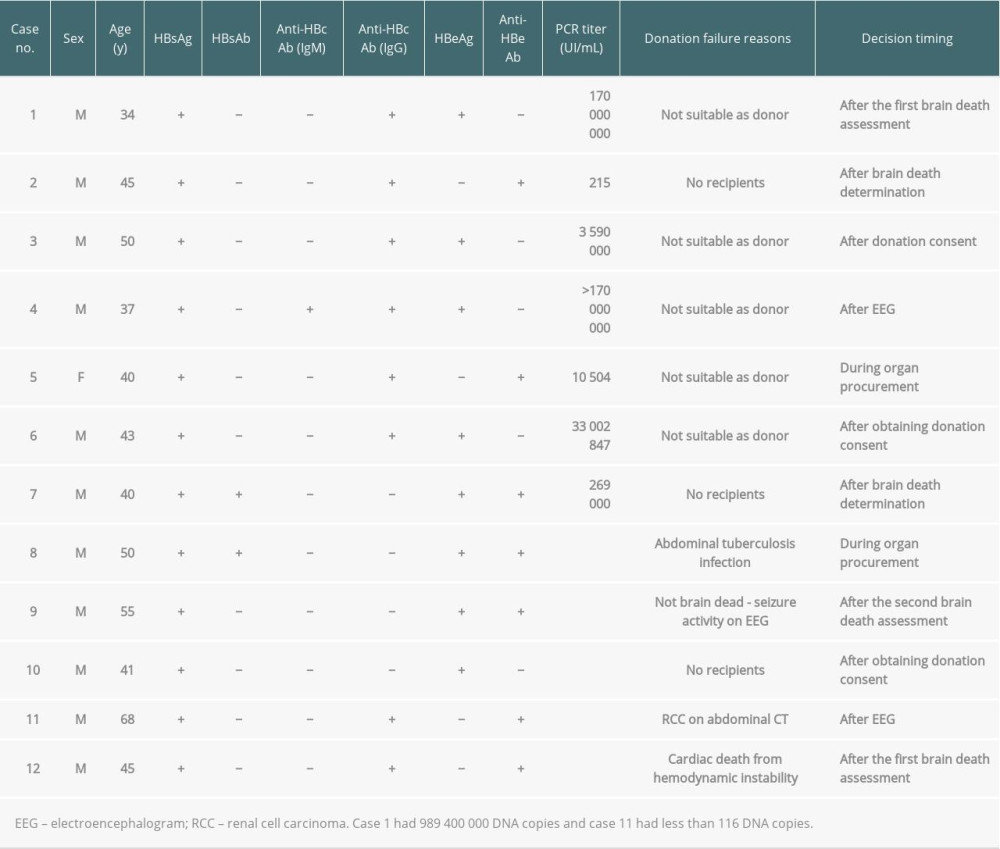 Table 4. Donation failure cases after obtaining donation consent owing to HCV infection between 2013 and 2017 in South Korea.
Table 4. Donation failure cases after obtaining donation consent owing to HCV infection between 2013 and 2017 in South Korea.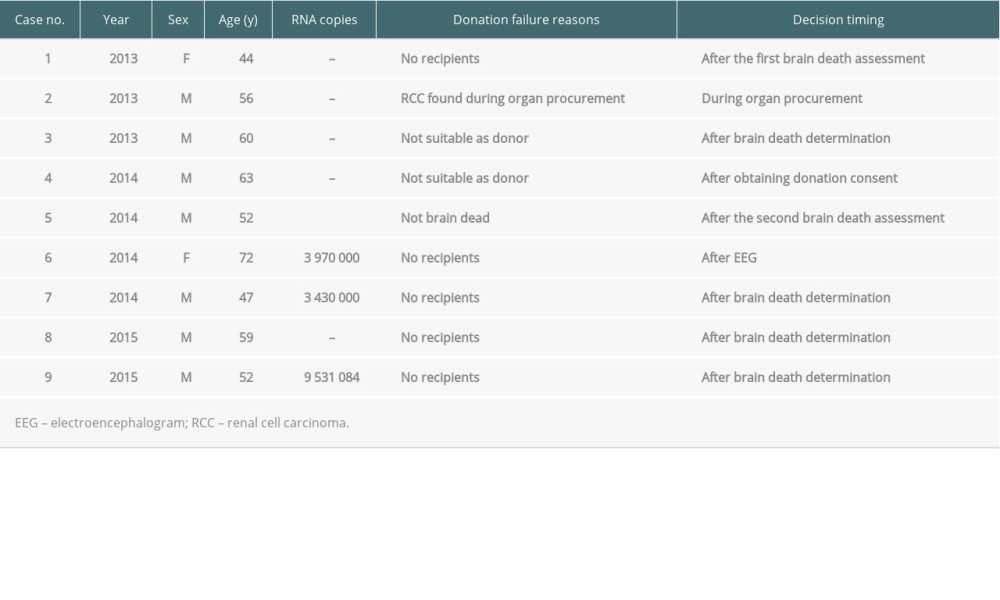
References
1. Kim WR, Lake JR, Smith JM, OPTN/SRTR 2016 annual data report: Liver: Am J Transplant, 2018; 18(Suppl 1); 172-253
2. Gridelli B, Remuzzi G, Strategies for making more organs available for transplantation: N Engl J Med, 2000; 343(6); 404-10
3. Abouna GM, Organ shortage crisis: Problems and possible solutions: Transplant Proc, 2008; 40(1); 34-38
4. Matesanz R, Marazuela R, Domínguez-Gil B, The 40 donors per million population plan: An action plan for improvement of organ donation and transplantation in Spain: Transplant Proc, 2009; 41(8); 3453-56
5. Korean Network for Organ Sharing (KONOS): Guidelines for organ transplantation management, 2016 Available from: http://www.konos.go.kr
6. Anwar N, Sherman KE, Transplanting organs from hepatitis B positive donors: Is it safe? Is it ethical?: J Viral Hepat, 2018; 25(10); 1110-15
7. Selzner N, Berenguer M, Should organs from hepatitis C-positive donors be used in hepatitis C-negative recipients for liver transplantation? [published correction appears in Liver Transpl 2018;24(8): 1152]: Liver Transpl, 2018; 24(6); 831-40
8. Cotter TG, Paul S, Sandıkçı B, Increasing utilization and excellent initial outcomes following liver transplant of hepatitis C virus (HCV)-viremic donors into HCV-negative recipients: Outcomes following liver transplant of HCV-viremic donors: Hepatology, 2019; 69(6); 2381-95
9. Korean Network for Organ Sharing (KONOS): Annual data report, 2017 https://www.konos.go.kr/konosis/common/bizlogic.jsp
10. Bowring MG, Kucirka LM, Massie AB, Changes in utilization and discard of hepatitis C-infected donor livers in the recent era: Am J Transplant, 2017; 17(2); 519-27
11. Marroquin CE, Marino G, Kuo PC, Transplantation of hepatitis C-positive livers in hepatitis C-positive patients is equivalent to transplanting hepatitis C-negative livers: Liver Transpl, 2001; 7(9); 762-68
12. Ballarin R, Cucchetti A, Spaggiari M, Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: A European multicentric case-control study: Transplantation, 2011; 91(11); 1265-72
13. MacConmara MP, Vachharajani N, Wellen JR, Utilization of hepatitis B core antibody-positive donor liver grafts: HPB (Oxford), 2012; 14(1); 42-48
14. Reese PP, Abt PL, Blumberg EA, Goldberg DS, Transplanting hepatitis C-positive kidneys: N Engl J Med, 2015; 373(4); 303-5
15. Reese PP, Abt PL, Blumberg EA, Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: A single-group trial: Ann Intern Med, 2018; 169(5); 273-81
16. Durand CM, Bowring MG, Brown DM, Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: An open-label nonrandomized trial: Ann Intern Med, 2018; 168(8); 533-40
17. Molnar MZ, Nair S, Cseprekal O, Transplantation of kidneys from hepatitis C-infected donors to hepatitis C-negative recipients: Single center experience: Am J Transplant, 2019; 19(11); 3046-57
18. Wang JH, Gustafson SK, Skeans MA, OPTN/SRTR 2018 annual data report: hepatitis C: Am J Transplant, 2020; 20(Suppl 1); 542-68
Figures
Tables
 Table 1. Summary of viral hepatitis cases among potential brain-dead donors according to the year between 2013 and 2017 in South Korea.
Table 1. Summary of viral hepatitis cases among potential brain-dead donors according to the year between 2013 and 2017 in South Korea. Table 2. General characteristics of brain-dead organ donors with hepatitis B or C with transplantation completion.
Table 2. General characteristics of brain-dead organ donors with hepatitis B or C with transplantation completion. Table 3. Donation failure cases after obtaining donation consent and determining brain death owing to HBV infection between 2013 and 2017 in South Korea.
Table 3. Donation failure cases after obtaining donation consent and determining brain death owing to HBV infection between 2013 and 2017 in South Korea. Table 4. Donation failure cases after obtaining donation consent owing to HCV infection between 2013 and 2017 in South Korea.
Table 4. Donation failure cases after obtaining donation consent owing to HCV infection between 2013 and 2017 in South Korea. Table 1. Summary of viral hepatitis cases among potential brain-dead donors according to the year between 2013 and 2017 in South Korea.
Table 1. Summary of viral hepatitis cases among potential brain-dead donors according to the year between 2013 and 2017 in South Korea. Table 2. General characteristics of brain-dead organ donors with hepatitis B or C with transplantation completion.
Table 2. General characteristics of brain-dead organ donors with hepatitis B or C with transplantation completion. Table 3. Donation failure cases after obtaining donation consent and determining brain death owing to HBV infection between 2013 and 2017 in South Korea.
Table 3. Donation failure cases after obtaining donation consent and determining brain death owing to HBV infection between 2013 and 2017 in South Korea. Table 4. Donation failure cases after obtaining donation consent owing to HCV infection between 2013 and 2017 in South Korea.
Table 4. Donation failure cases after obtaining donation consent owing to HCV infection between 2013 and 2017 in South Korea. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








