19 March 2021: Original Paper
Klotho Regulates Epithelial-to-Mesenchymal Transition In Vitro via Wnt/β-Catenin Pathway and Attenuates Chronic Allograft Dysfunction in a Rat Renal Transplant Model
Xiaojun Li12ABEF, Pei Lu3BCDEF, Xue-Feng Shao2BDF, Ting Jiang2CD, Feng Liu2CD, Gang Li1EFG*DOI: 10.12659/AOT.930066
Ann Transplant 2021; 26:e930066
Abstract
BACKGROUND: Klotho deficiency has been implicated in various kidney diseases and has been associated with renal fibrosis. However, the role of Klotho in renal allograft fibrosis still remains undetermined.
MATERIAL AND METHODS: A 24-week-old rat renal transplant model with chronic allograft dysfunction (CAD) was carried out by orthotopic kidney transplantation using F344 donor rats to Lewis recipient rats. Successful establishment of the model was verified by HE and Masson staining and renal allograft function assessment. HK-2 cells were cultured and treated with TGF-β1 and/or siRNA-Klotho at various time points. Total proteins and RNA were extracted from the cultured cells and kidney tissues. Western blot assay and quantitative RT-PCR were used to analyze the expression of Klotho, fibronectin, and β-catenin pathways.
RESULTS: We successfully established and identified a 24-week-old rat renal transplant model with CAD. Loss of Klotho was identified to be associated with epithelial-to-mesenchymal transition (EMT), renal allograft fibrosis, and CAD. In HK-2 cells, a significant decrease of Klotho protein was observed in the renal fibrosis induced by TGF-β1 in a time-dependent manner. Moreover, intervention of siRNA-Klotho remarkably promoted the progression of renal fibrosis and activation of the Wnt/β-catenin signaling pathway.
CONCLUSIONS: Our results show that Klotho has a significant protective role against EMT, renal allograft fibrosis, and CAD following kidney transplantation, which is mediated by inhibition of the Wnt/β-catenin signaling pathway.
Keywords: beta Catenin, Kidney Failure, Chronic, Kidney Transplantation, Allografts, Fibrosis, Glucuronidase, Kidney, Klotho Proteins, Rats, Inbred F344, Rats, Inbred Lew, Transforming Growth Factor beta1
Background
With the development of surgical technology and novel immunosuppressive agents, the short-term survival of allograft has increased dramatically, whereas the long-term survival still remains relatively low [1]. Chronic allograft dysfunction (CAD) was reported to be the crucial factor influencing the long-term prognosis of renal transplant recipients [2]. CAD was pathologically characterized as interstitial fibrosis and tubular atrophy (IF/TA), glomerular sclerosis, and monocytes infiltration, whereas there is still a lack of substantial understanding of the cellular mechanism involved in CAD [3].
Originating from the kidneys, Klotho is a membrane-bound protein which has been extensively studied as a molecule responsible for spontaneous mutation in mice that express a variety of aging symptoms [4]. In recent decades, a decreased expression of Klotho protein which accompanies reduced renal function has been observed, indicating that Klotho has a protective effect on renal function [5]. Furthermore, studies have reported that the protective role of Klotho partly occurs by attenuating renal fibrosis by inhibiting transforming growth factor-β1 (TGF-β1) signaling [6,7]. In renal transplant recipients, Bleskestad et al reported a trend toward lower expression of serum levels of Klotho compared with those from healthy volunteers matched for estimated glomerular filtration rate (eGFR), suggesting a potential link between Klotho and renal allograft function [8].
In this study, we designed, established, and verified a 24-week-old rat renal transplant model with significant CAD. Based on this, we explored the expression of Klotho in renal allograft fibrosis and CAD, as well as the cellular mechanism involved.
Material and Methods
ETHICS STATEMENT:
All animals were kept in a pathogen-free environment with free access to food and water. The procedures for care and use of animals were approved by the local Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2019-SRFA-091) and all applicable institutional and governmental regulations concerning the ethical use of animals were followed.
ANIMALS AND EXPERIMENTAL DESIGN:
Adult male Lewis and F344 rats weighted ranging from 200 to 250 g were purchased from Charles River Laboratories Company (Yokohama, Japan). All rats were raised in the Animal Feeding Center of Nanjing Medical University.
Orthotopic left kidney transplantation surgery was performed using previously described methods, and we strictly followed the detailed steps [9]. The mean warm and cold ischemia times for each surgery were 30.5 min and 10.4 min, respectively. We performed right kidney nephrectomy surgery of recipient rats on the 3rd post-transplant day. Cyclosporine A (5 mg/kg per body weight) was administered once a day intraperitoneally for 14 post-transplant days. We assigned these rats into the CAD group (n=5) and Sham group (n=5). For the CAD group, F344 recipient rats were transplanted with the donor kidney from Lewis rats, whereas F344 recipient rats only had general anesthesia without any transplant surgery in the Sham group. Kidney tissues were harvested after 24 post-transplant weeks and divided into 2 parts, which were either stored in liquid nitrogen or fixed in paraffin.
RENAL FUNCTION ASSESSMENT:
Renal allograft function of each recipient in the Sham and CAD groups was evaluated by blood creatinine and blood urea nitrogen using a kit purchased from Changchun HUILI BioTech, Changchun, China, according to the manufacturer’s instructions.
CELL CULTURE:
Human proximal tubular (HK-2) cells were purchased from American Type Culture Collection (CRL-2190) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium which was contained 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in a humidified atmosphere with 5% CO2 at 37°C. For TGF-β1 stimulation, cells were treated with human recombinant TGF-β1 (5ng/mL; Santa Cruz, CA, USA) after being starved overnight, for 0 h, 24 h, 48 h, or 72 h. For siRNA-
HISTOLOGY AND IMMUNOHISTOCHEMISTRY:
Kidney tissues from rats in the renal transplant CAD model group were fixed in paraffin and stained with hematoxylin and eosin (HE) and Masson’s trichrome according to the manufacturer’s instructions. The severity of renal allograft fibrosis, including IF/TA, glomerulitis, and monocyte infiltration, were semi-quantitatively evaluated using a color image analyzer. The extent of interstitial fibrosis was assessed by viewing 10 independent fields on each slide stained with Masson’s trichrome.
Then, fixed kidney tissues were incubated with primary antibodies against Klotho (1: 100, CST technology, USA) and Fibronectin (FN; 1: 100, CST Technology, USA). The protocol of immunohistochemistry staining was carried out according to the instructions. The expression and distribution of Klotho and FN were quantitatively assessed using Image Pro plus 6.0 software (Media Cybernetics, MA, USA).
WESTERN BLOT ANALYSIS:
Total proteins were obtained from kidney tissues and cultured cells, and concentrations were assessed using the BCA protein kit (Beyotime, Shanghai, China). NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (ThermoFisher Scientific, MA, USA) was used to isolate proteins from cytoplasm or nucleus according to the manufacturer’s instructions. Western blotting analysis was performed strictly according to previously published studies [2]. Briefly, proteins were electro-transferred by PVDF membrane. The membrane was blocked using 5% skim milk and then incubated overnight with primary antibodies against Klotho (1: 1000, Abcam, USA), FN (1: 1000, Abcam, USA), E-Cadherin (1: 1000, Abcam, USA), phosphor-β-catenin (1: 1000, CST Technology, USA), and β-catenin (1: 1000, CST Technology, USA), GAPDH (1: 1000, Abcam, USA). GAPDH was used to assess the relative abundance of proteins, which were considered as an internal reference. The intensity of the protein signals was explored using the Odyssey infrared imaging system (LI-COR Biotechnology, Lincoln, NE, USA). The individual experiments were performed at least 3 times.
QUANTITATIVE RT-PCR:
Total RNA was extracted from kidney tissues and cultured cells using Trizol Reagent (Pufei, Shanghai, China). cDNA was synthesized with a PrimeScriptTM RT reagent kit (TaKaRa Biotechnology, Shiga, Japan). Amplification and detection of cDNA were carried out using the ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The sequences of primers used were:
Expression of each mRNA was normalized according to β-actin expression. Each experiment described above was repeated at least 3 times.
STATISTICAL ANALYSIS:
Data are shown as the mean ± standard deviations (SD). The
Results
ESTABLISHMENT AND IDENTIFICATION OF THE RAT RENAL TRANSPLANT CAD MODEL:
We established a 24-week-old rat renal transplant model with significant CAD, and performed the modeling identification by HE and Masson staining. For the CAD group, renal transplant was performed in 8 recipients, 5 of which survived for 24 weeks (3 recipients died from surgical complications); while in Sham group, all 5 recipients survived for 24 weeks. The renal allograft function in the 2 groups was evaluated, and concentrations of blood creatinine and blood urea nitrogen in the CAD group were significantly higher than those in the Sham group (P<0.05, Supplementary Figure 1). As illustrated in Figure 1, remarkable interstitial fibrosis and tubular atrophy (IF/TA) was observed in the CAD group when compared to the control group through HE staining (Figure 1A) and Masson staining (Figure 1B). In addition, the mono-nuclear cells were highly infiltrated in the allograft interstitium and atrophic tubules (Figure 1A). The quantitative analysis showed the proportion of fibrosis area in the renal allograft tissue was significantly higher than those in the Sham group (Figure 1C). Therefore, significant morphologic changes suggested that the rat renal transplant CAD model was successfully established and identified.
LOSS OF KLOTHO WAS ASSOCIATED WITH CHRONIC ALLOGRAFT DYSFUNCTION:
Moreover, we explored the protein expressions of Klotho and Fibronectin (FN) in the renal allograft tissues. The results showed that loss of Klotho distributed in the fibrosis area (Figure 1D, 1E) and highly expressed FN (Figure 1F, 1G) were potentially associated with the IF/TA and CAD progression. The total protein and transcript expression of Klotho was also significantly reduced in the CAD group, which was consistent with the results of IHC staining (Figure 1H, 1I). Furthermore, highly expressed FN protein and mRNA were found in the allograft tissue with significant IF/TA (Figure 1H, 1J), suggesting the potential link of loss of Klotho with the progression of CAD.
INHIBITION OF KLOTHO IN VITRO PROMOTES PROGRESSION OF EPITHELIAL-TO-MESENCHYMAL TRANSITION (EMT) AND RENAL FIBROSIS:
Then, we assessed the effect of Klotho on the progression of EMT induced by TGF-β1 during fibrosis. As illustrated in Figure 2A and 2B, in HK-2 cells in a time-dependent manner, TGF-β1 suppressed the protein expression of Klotho and E-Cadherin (epithelial cells biomarker), and stimulated the expression of FN and α-SMA, which were considered as biomarkers of EMT. Levels of Klotho mRNA and E-Cadherin were also rapidly reduced by the stimulation of TGF-β1 (Figure 2C, 2F), and, similarly, FN and α-SMA mRNA were remarkably highly expressed in a time-dependent manner (Figure 2D, 2E).
LOSS OF KLOTHO PROMOTED DEVELOPMENT OF EMT AND CAD VIA INHIBITING ACTIVATION OF WNT/β-CATENIN SIGNALING PATHWAY:
Consequently, we investigated the potential cellular mechanism of inhibition of Klotho in the progression of fibrosis. We transfected the HK-2 cells with the siRNA-kl to silence the expression of Klotho and observed that the FN protein expression was remarkably increased after the inhibition of Klotho, along with the increase of EMT in HK-2 cells (Figure 3A, 3B). Our results showed that Klotho deficiency promoted the development of renal fibrosis. Furthermore, the opposite expression of β-catenin in the cytoplasm and nucleus of HK2 cells was observed to be induced by siRNA-kl treatment (Figure 3C, 3D). Combined with the increase of Wnt target gene – Matrix Metalloprotease-2 (MMP-2) and MMP-9, activation of the Wnt/β-catenin signaling pathway by the loss of Klotho was examined (Figure 3E). To identify the role of the Wnt/β-catenin signaling pathway in the loss of Klotho, HK2 cells were treated with ICG-001, the specific inhibitor of β-catenin, and no change in expression of Klotho was found, along with the significant decrease of α-SMA (Figure 3F, 3G). Finally, the effect of EMT and activation of β-catenin was tested in the rat CAD model, which was consistent with in vitro results (Figure 3H, 3I).
Discussion
In this study, we explored the protective role of Klotho in the progression of renal allograft fibrosis and CAD for the first time. The results showed that Klotho has a remarkably protective role against progression of EMT, renal allograft fibrosis, and CAD following kidney transplantation by inhibition of the Wnt/β-catenin signaling pathway.
Numerous studies have explored the cause of and mechanisms leading to the CAD, and reported that immunological and non-immunological factors are involved in the renal allograft fibrosis and CAD, such as acute rejection, immunosuppressive agents-related nephrotoxicity, delayed graft function, and virus infections [10–12]. Our previous studies have already shown that endothelial-to-mesenchymal transition (EndMT) induced by TGF-β1 was remarkably activated during the pathogenesis of CAD [2]. EMT has also been found to significantly contribute to the progression of CAD [13]. In this study, we found that Klotho is a promising target for protecting renal allografts from fibrosis and CAD. A recent study has suggested the positive correlation of Klotho with renal function and eGFR in chronic kidney disease by an evidence-based method, suggesting that Klotho is a promising early biomarker for renal injury and repair [14]. Furthermore, soluble Klotho in renal transplant recipients with acute ischemia kidney injury has also been explored, and a similar trend was found in renal allograft function [15]. Considering the occurrence of renal allograft fibrosis and CAD in the late stage of renal transplant, early and persistent monitoring of renal allograft function early identification of allograft injury appears to be crucial for improvement of renal allograft fibrosis and prevention of CAD. Because the kidneys are the primary source of Klotho production, dynamic monitoring of soluble Klotho becomes more practical for renal transplant recipients, which is consistent with our outcomes. Therefore, our results supported the hypothetical benefit of soluble Klotho in the monitoring of allograft function.
Our results also showed therapeutic effects on renal allograft fibrosis and CAD. The silencing of Klotho substantially increased the extent of renal fibrosis in cultured HK-2 cells, indicating the protective role of Klotho in renal fibrosis and the therapeutic effect of soluble Klotho in kidney diseases. Qi-feng Liu et al [16] observed that treatment with Klotho remarkably attenuates the cyclosporine A-induced epithelial-to-mesenchymal transition (EMT) and renal fibrosis in rats, which was consistent with our results. Moreover, the Klotho expression has been found to decrease after stimulation of inflammatory cytokines such as TGF-β1 [17,18]. In addition to the correlation of Klotho with renal damage, this also suggests that an epigenetic mechanism may be involved in Klotho intervention, which could help develop drugs regulating epigenetics as treatments for renal disease [5]. Also, for renal transplant recipients, the complexity of immunosuppressive protocols not only promotes the drug-related nephrotoxicity in a time-dependent manner, but also limits the administration of novel agents to protect against renal allograft fibrosis. Epigenetics regulation allows us to view the disease from a novel perspective as enhanced or depressed transcription activity, which may help explain the diversity of renal allograft fibrosis, as well as the treatment of CAD [19].
TGF-β/Smad signaling has been indicated to be crucial among the downstream signaling pathways participating in renal interstitial fibrosis and CAD [2]. However, certain pathways are drawing more and more attention in renal fibrosis, including β-catenin pathways. Substantial studies have suggested that Klotho acts as a tumor suppressor and an inhibitor of the Wnt/β-catenin pathway, which is considered as the key β-catenin pathway in human hepatocellular carcinoma (HCC), providing a novel downstream signal for Klotho treatment [20]. Consistent with these findings, our data showed the potential relationship between the Klotho and its downstream signaling of β-catenin in renal allograft fibrosis, which also could be considered as the therapeutic molecule for CAD.
Importantly, our results should be interpreted with great caution due to several limitations in this study. Firstly, a mouse renal transplant CAD model based on the knockout of Klotho should be established. Based on this model, a direct relationship between Klotho and CAD could be demonstrated in vivo instead of the hypothesis in this study. Moreover, studies on the expression of Klotho and EMT, cell culture of various cell types (fibroblast, endothelial cells, and macrophages), and extensive signaling pathway exploration may provide solid evidence of detailed molecular mechanisms.
Conclusions
In conclusion, we revealed for the first time that Klotho is an anti-fibrotic molecule in the pathogenesis of EMT, renal allograft fibrosis, and CAD by inhibiting activation of Wnt/β-catenin pathways. Elucidation of the underlying mechanism may provide therapeutic targets for Klotho in CAD and renal transplant patients.
Figures
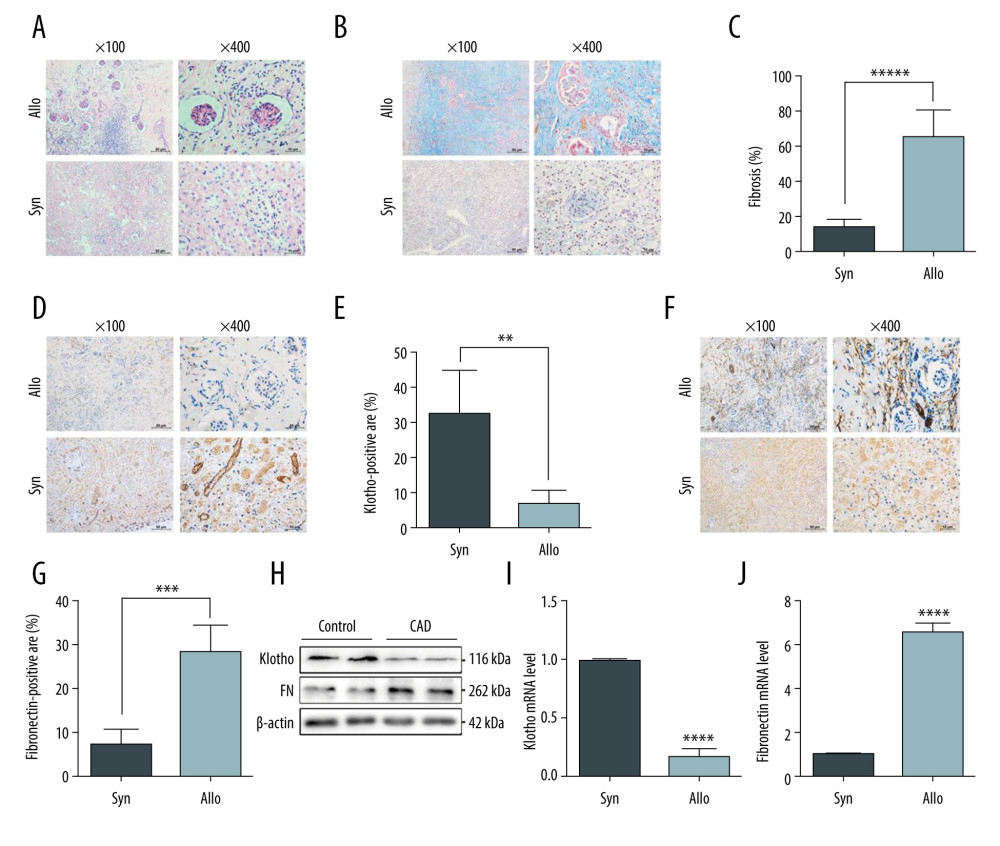 Figure 1. Reduced Klotho expression was strongly associated with the progression of chronic allograft dysfunction. Renal allografts derived from the rat renal transplant model were harvested and identified by HE staining (A) and Masson trichrome staining (B). The quantitative analysis of fibrosis area is shown in Figure C. Furthermore, distribution and expression of Klotho were examined by immunohistochemistry staining (D), and quantitative analysis showed significantly reduced Klotho expression in the renal tubular area and the interstitial area (E). Fibronectin expression was tested (F) and quantitative analysis indicated the remarkable expression of fibronectin in the Allo group (G). Finally, the total protein and RNA was extracted and we performed western blotting and RT-PCR to examine the protein and mRNA levels of Klotho (H, I) and fibronectin (H, J). *** P<0.001 compared to control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times.
Figure 1. Reduced Klotho expression was strongly associated with the progression of chronic allograft dysfunction. Renal allografts derived from the rat renal transplant model were harvested and identified by HE staining (A) and Masson trichrome staining (B). The quantitative analysis of fibrosis area is shown in Figure C. Furthermore, distribution and expression of Klotho were examined by immunohistochemistry staining (D), and quantitative analysis showed significantly reduced Klotho expression in the renal tubular area and the interstitial area (E). Fibronectin expression was tested (F) and quantitative analysis indicated the remarkable expression of fibronectin in the Allo group (G). Finally, the total protein and RNA was extracted and we performed western blotting and RT-PCR to examine the protein and mRNA levels of Klotho (H, I) and fibronectin (H, J). *** P<0.001 compared to control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times. 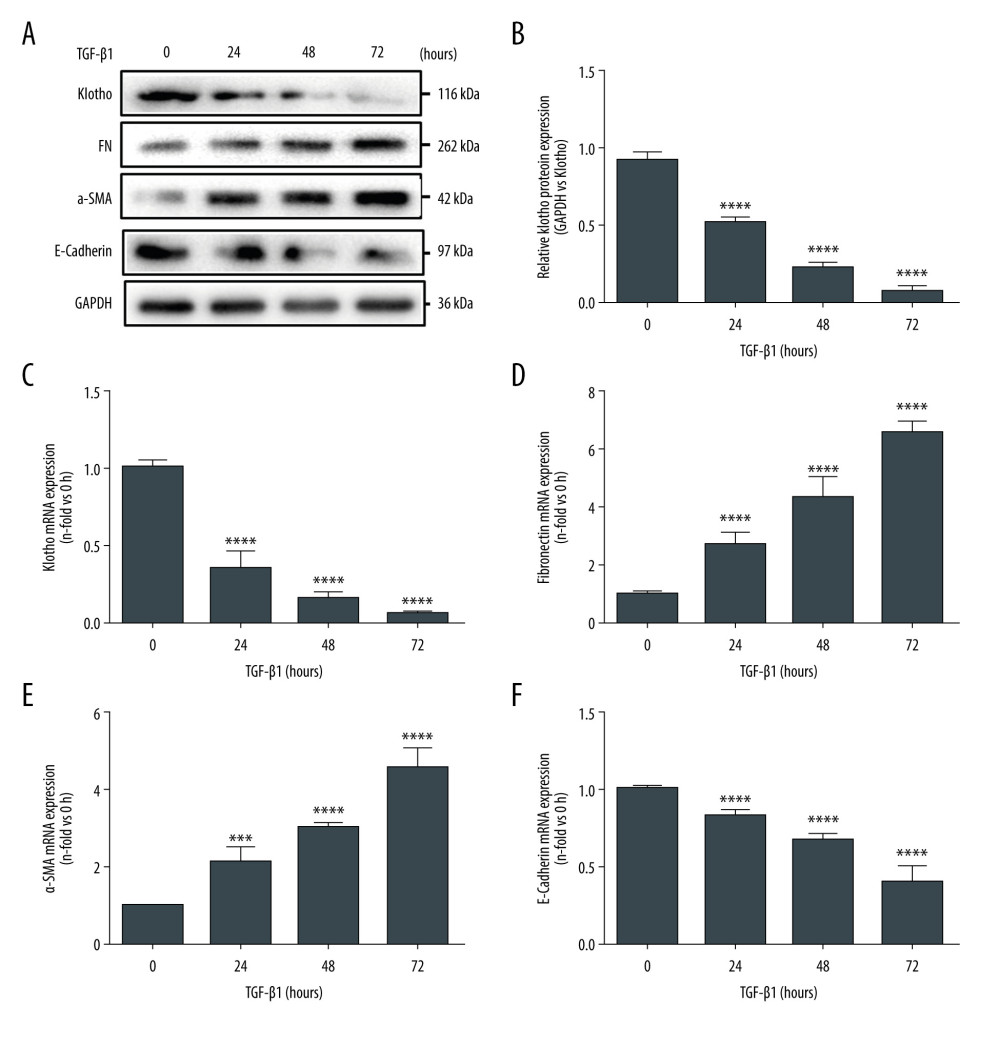 Figure 2. Inhibition of Klotho in vitro promotes the progression of epithelial-to-mesenchymal transition (EMT) and renal fibrosis. We stimulated the HK-2 cells by TGF-β1 (5 ng/ml) at various time points (0–72 h). Western Blot assay and RT-PCR were performed to investigate the protein and mRNA expression levels of Klotho in HK-2 cells (A, C), as well as the expression of EMT-related biomarkers: Fibronectin (A, D), α-SMA (A, E), and E-Cadherin (A, F). The quantitative analysis of western blotting (B) and PCR (C-F) indicated the potential relationship between Klotho and the progression of EMT. *** P<0.001 compared with control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times.
Figure 2. Inhibition of Klotho in vitro promotes the progression of epithelial-to-mesenchymal transition (EMT) and renal fibrosis. We stimulated the HK-2 cells by TGF-β1 (5 ng/ml) at various time points (0–72 h). Western Blot assay and RT-PCR were performed to investigate the protein and mRNA expression levels of Klotho in HK-2 cells (A, C), as well as the expression of EMT-related biomarkers: Fibronectin (A, D), α-SMA (A, E), and E-Cadherin (A, F). The quantitative analysis of western blotting (B) and PCR (C-F) indicated the potential relationship between Klotho and the progression of EMT. *** P<0.001 compared with control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times. 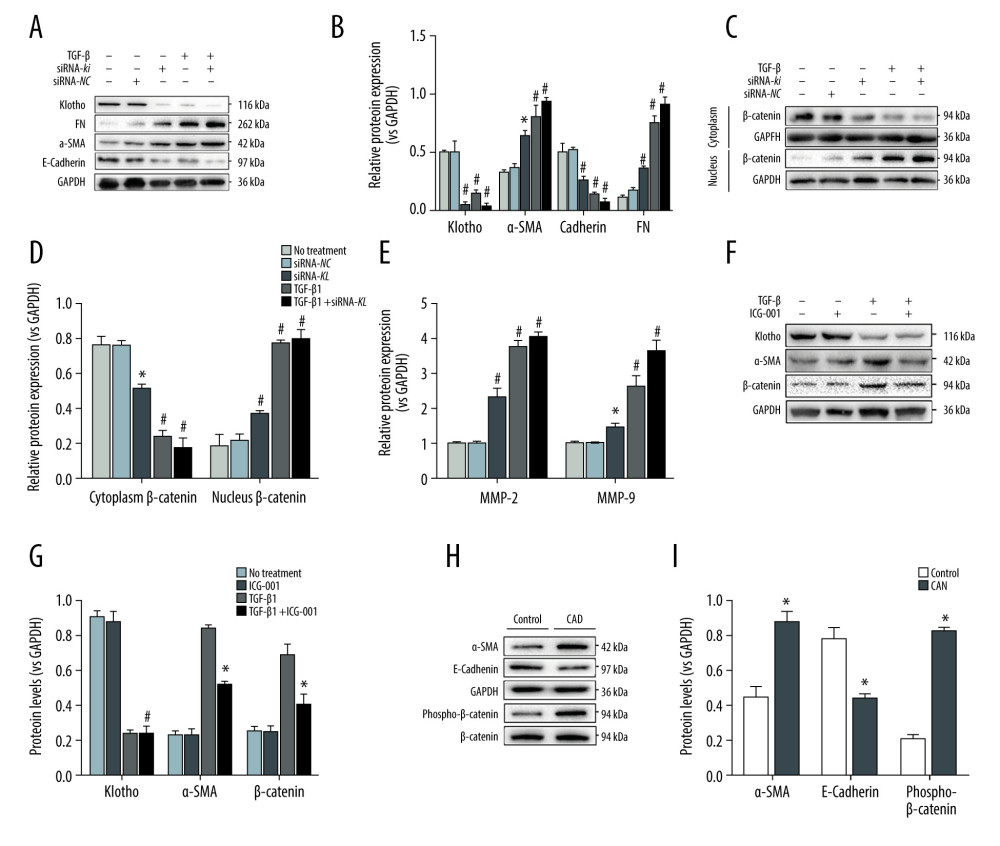 Figure 3. Loss of Klotho promoted the development of EMT and CAD by inhibiting activation of the Wnt/β-catenin signaling pathway. To confirm the role of loss of Klotho in the development of EMT and CAD, we transfected the HK-2 cells with siRNA-kl to silence the expression of Klotho and observed that the FN protein expression was remarkably increased after the inhibition of Klotho, along with the increase of EMT procedure in HK-2 cells (A, B). Furthermore, the contradictory expression of β-catenin in the cytoplasm and nucleus of HK2 cells was observed to be induced by the treatment of siRNA-kl (C, D). Combined with the increase of Wnt target gene – Matrix Metalloprotease-2 (MMP-2) and MMP-9, activation of the Wnt/β-catenin signaling pathway by the loss of Klotho was examined (E). HK2 cells were treated with ICG-001, the specific inhibitor of β-catenin, and no change of expression of Klotho was found, along with the significant decrease of α-SMA (F, G). Finally, the effect of EMT and activation of β-catenin were tested in the rat CAD model, which was consistent with the in vitro results (H, I). * P<0.001 compared with control group; # P<0.0001 compared to control group. Each experiment was performed at least 3 times.
Figure 3. Loss of Klotho promoted the development of EMT and CAD by inhibiting activation of the Wnt/β-catenin signaling pathway. To confirm the role of loss of Klotho in the development of EMT and CAD, we transfected the HK-2 cells with siRNA-kl to silence the expression of Klotho and observed that the FN protein expression was remarkably increased after the inhibition of Klotho, along with the increase of EMT procedure in HK-2 cells (A, B). Furthermore, the contradictory expression of β-catenin in the cytoplasm and nucleus of HK2 cells was observed to be induced by the treatment of siRNA-kl (C, D). Combined with the increase of Wnt target gene – Matrix Metalloprotease-2 (MMP-2) and MMP-9, activation of the Wnt/β-catenin signaling pathway by the loss of Klotho was examined (E). HK2 cells were treated with ICG-001, the specific inhibitor of β-catenin, and no change of expression of Klotho was found, along with the significant decrease of α-SMA (F, G). Finally, the effect of EMT and activation of β-catenin were tested in the rat CAD model, which was consistent with the in vitro results (H, I). * P<0.001 compared with control group; # P<0.0001 compared to control group. Each experiment was performed at least 3 times. References
1. Hart A, Smith JM, Skeans MA, OPTN/SRTR 2018 annual data report: Kidney: Am J Transplant, 2020; 20(Suppl s1); 20-130
2. Wang Z, Han Z, Tao J, Role of endothelial-to-mesenchymal transition induced by TGF-beta1 in transplant kidney interstitial fibrosis: J Cell Mol Med, 2017; 21(10); 2359-69
3. Zhou J, Cheng H, Wang Z, Bortezomib attenuates renal interstitial fibrosis in kidney transplantation via regulating the EMT induced by TNF-alpha-Smurf1-Akt-mTOR-P70S6K pathway: J Cell Mol Med, 2019; 23(8); 5390-402
4. Kuro-o M, Matsumura Y, Aizawa H, Mutation of the mouse klotho gene leads to a syndrome resembling ageing: Nature, 1997; 390(6655); 45-51
5. Doi S, Masaki T, Klotho as a therapeutic target during the development of renal fibrosis: Contrib Nephrol, 2017; 189; 178-83
6. Sugiura H, Yoshida T, Shiohira S, Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis: Am J Physiol Renal Physiol, 2012; 302(10); F1252-64
7. Doi S, Zou Y, Togao O, Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice: J Biol Chem, 2011; 286(10); 8655-65
8. Bleskestad IH, Thorsen IS, Jonsson G, Soluble Klotho and intact fibroblast growth factor 23 in long-term kidney transplant patients: Eur J Endocrinol, 2015; 172(4); 343-50
9. Hardinger KL, Alford K, Murillo D, Bortezomib for acute humoral rejection in two repeat transplant recipients: Clin Transpl, 2009; 479-83
10. Wang Z, Yang H, Suo C, Application of ultrasound elastography for chronic allograft dysfunction in kidney transplantation: J Ultrasound Med, 2017; 36(9); 1759-69
11. Zununi Vahed S, Samadi N, Mostafidi E, Genetics and epigenetics of chronic allograft dysfunction in kidney transplants: Iran J Kidney Dis, 2016; 10(1); 1-9
12. Yang C, Qi R, Yang B, Pathogenesis of chronic allograft dysfunction progress to renal fibrosis: Adv Exp Med Biol, 2019; 1165; 101-16
13. Zhao C, Xu Z, Wang Z, Role of tumor necrosis factor-alpha in epithelial-to-mesenchymal transition in transplanted kidney cells in recipients with chronic allograft dysfunction: Gene, 2018; 642; 483-90
14. Wang Q, Su W, Shen Z, Correlation between Soluble alpha-Klotho and renal function in patients with chronic kidney disease: A review and meta-analysis: Biomed Res Int, 2018; 2018; 9481475
15. Panah F, Ghorbanihaghjo A, Argani H, Ischemic acute kidney injury and klotho in renal transplantation: Clin Biochem, 2018; 55; 3-8
16. Liu QF, Ye JM, Yu LX, Klotho mitigates cyclosporine A (CsA)-induced epithelial-mesenchymal transition (EMT) and renal fibrosis in rats: Int Urol Nephrol, 2017; 49(2); 345-52
17. Li SS, He AL, Deng ZY, Ginsenoside-Rg1 protects against renal fibrosis by regulating the Klotho/TGF-beta1/Smad signaling pathway in rats with obstructive nephropathy: Biol Pharm Bull, 2018; 41(4); 585-91
18. Mencke R, Olauson H, Hillebrands JL, Effects of Klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies: Adv Drug Deliv Rev, 2017; 121; 85-100
19. Katoh M, Multilayered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/betacatenin signaling activation (Review): Int J Mol Med, 2018; 42(2); 713-25
20. Tang X, Wang Y, Fan Z, Klotho: A tumor suppressor and modulator of the Wnt/beta-catenin pathway in human hepatocellular carcinoma: Lab Invest, 2016; 96(2); 197-205
Figures
 Figure 1. Reduced Klotho expression was strongly associated with the progression of chronic allograft dysfunction. Renal allografts derived from the rat renal transplant model were harvested and identified by HE staining (A) and Masson trichrome staining (B). The quantitative analysis of fibrosis area is shown in Figure C. Furthermore, distribution and expression of Klotho were examined by immunohistochemistry staining (D), and quantitative analysis showed significantly reduced Klotho expression in the renal tubular area and the interstitial area (E). Fibronectin expression was tested (F) and quantitative analysis indicated the remarkable expression of fibronectin in the Allo group (G). Finally, the total protein and RNA was extracted and we performed western blotting and RT-PCR to examine the protein and mRNA levels of Klotho (H, I) and fibronectin (H, J). *** P<0.001 compared to control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times.
Figure 1. Reduced Klotho expression was strongly associated with the progression of chronic allograft dysfunction. Renal allografts derived from the rat renal transplant model were harvested and identified by HE staining (A) and Masson trichrome staining (B). The quantitative analysis of fibrosis area is shown in Figure C. Furthermore, distribution and expression of Klotho were examined by immunohistochemistry staining (D), and quantitative analysis showed significantly reduced Klotho expression in the renal tubular area and the interstitial area (E). Fibronectin expression was tested (F) and quantitative analysis indicated the remarkable expression of fibronectin in the Allo group (G). Finally, the total protein and RNA was extracted and we performed western blotting and RT-PCR to examine the protein and mRNA levels of Klotho (H, I) and fibronectin (H, J). *** P<0.001 compared to control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times. Figure 2. Inhibition of Klotho in vitro promotes the progression of epithelial-to-mesenchymal transition (EMT) and renal fibrosis. We stimulated the HK-2 cells by TGF-β1 (5 ng/ml) at various time points (0–72 h). Western Blot assay and RT-PCR were performed to investigate the protein and mRNA expression levels of Klotho in HK-2 cells (A, C), as well as the expression of EMT-related biomarkers: Fibronectin (A, D), α-SMA (A, E), and E-Cadherin (A, F). The quantitative analysis of western blotting (B) and PCR (C-F) indicated the potential relationship between Klotho and the progression of EMT. *** P<0.001 compared with control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times.
Figure 2. Inhibition of Klotho in vitro promotes the progression of epithelial-to-mesenchymal transition (EMT) and renal fibrosis. We stimulated the HK-2 cells by TGF-β1 (5 ng/ml) at various time points (0–72 h). Western Blot assay and RT-PCR were performed to investigate the protein and mRNA expression levels of Klotho in HK-2 cells (A, C), as well as the expression of EMT-related biomarkers: Fibronectin (A, D), α-SMA (A, E), and E-Cadherin (A, F). The quantitative analysis of western blotting (B) and PCR (C-F) indicated the potential relationship between Klotho and the progression of EMT. *** P<0.001 compared with control group; **** P<0.0001 compared to control group. Each experiment was performed at least 3 times. Figure 3. Loss of Klotho promoted the development of EMT and CAD by inhibiting activation of the Wnt/β-catenin signaling pathway. To confirm the role of loss of Klotho in the development of EMT and CAD, we transfected the HK-2 cells with siRNA-kl to silence the expression of Klotho and observed that the FN protein expression was remarkably increased after the inhibition of Klotho, along with the increase of EMT procedure in HK-2 cells (A, B). Furthermore, the contradictory expression of β-catenin in the cytoplasm and nucleus of HK2 cells was observed to be induced by the treatment of siRNA-kl (C, D). Combined with the increase of Wnt target gene – Matrix Metalloprotease-2 (MMP-2) and MMP-9, activation of the Wnt/β-catenin signaling pathway by the loss of Klotho was examined (E). HK2 cells were treated with ICG-001, the specific inhibitor of β-catenin, and no change of expression of Klotho was found, along with the significant decrease of α-SMA (F, G). Finally, the effect of EMT and activation of β-catenin were tested in the rat CAD model, which was consistent with the in vitro results (H, I). * P<0.001 compared with control group; # P<0.0001 compared to control group. Each experiment was performed at least 3 times.
Figure 3. Loss of Klotho promoted the development of EMT and CAD by inhibiting activation of the Wnt/β-catenin signaling pathway. To confirm the role of loss of Klotho in the development of EMT and CAD, we transfected the HK-2 cells with siRNA-kl to silence the expression of Klotho and observed that the FN protein expression was remarkably increased after the inhibition of Klotho, along with the increase of EMT procedure in HK-2 cells (A, B). Furthermore, the contradictory expression of β-catenin in the cytoplasm and nucleus of HK2 cells was observed to be induced by the treatment of siRNA-kl (C, D). Combined with the increase of Wnt target gene – Matrix Metalloprotease-2 (MMP-2) and MMP-9, activation of the Wnt/β-catenin signaling pathway by the loss of Klotho was examined (E). HK2 cells were treated with ICG-001, the specific inhibitor of β-catenin, and no change of expression of Klotho was found, along with the significant decrease of α-SMA (F, G). Finally, the effect of EMT and activation of β-catenin were tested in the rat CAD model, which was consistent with the in vitro results (H, I). * P<0.001 compared with control group; # P<0.0001 compared to control group. Each experiment was performed at least 3 times. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








