12 July 2022: Original Paper
Effect of Nephrectomy After Allograft Failure on Inflammation, Erythropoiesis, Donor-Specific Antibodies, and Outcome of Re-Transplantation
Panagiota Zgoura1BC*, Adrian Doevelaar1E, Benjamin Rohn1F, Felix S. Seibert1CD, Maximilian Seidel1CD, Falko Markus HeinemannDOI: 10.12659/AOT.935625
Ann Transplant 2022; 27:e935625
Abstract
BACKGROUND: Morbidity and mortality rates are high for patients returning to dialysis after renal graft failure. Keeping failed kidney transplants in situ with concomitant minimization or withdrawal of immunosuppression is standard of care in many transplant centers. It is unclear, however, whether the resulting allospecific immune response can cause a microinflammatory milieu. The present work investigated the impact of allograft nephrectomy on systemic inflammation, erythropoiesis, and donor-specific antibodies (DSA).
MATERIAL AND METHODS: We performed a retrospective analysis evaluating C-reactive protein (CRP), hemoglobin concentration (Hb), ferritin, iron substitution dosages, erythropoietin dosages, and DSA in 92 transplant recipients with allograft failure, of whom 49 did not (Group A) and 43 did undergo transplant nephrectomy (Group B). Blood samples and clinical data were obtained 3-6 months after returning to dialysis. We additionally assessed outcome of kidney re-transplantation in a 10-year follow-up.
RESULTS: There was no significant difference in Hb concentrations, ferritin concentrations, CRP concentrations, iron, and EPO substitution dosages between the 2 groups. Patients undergoing nephrectomy had a significantly higher prevalence of DSA (65.1% vs 38.8%, P<0.0001). In the 10-year follow-up, 3 patients (12%) of Group B and none in Group A had allograft failure after primary successful re-transplantation.
CONCLUSIONS: Keeping a kidney graft in situ after returning to dialysis did not lead to an increase in microinflammation. Although DSA develops in more than 50% of patients after an allograft nephrectomy, the outcome of a renal re-transplantation seems to be unaffected. Thus, both strategies are feasible options in kidney transplant recipients after return to dialysis.
Keywords: Immunization, Inflammation Mediators, Kidney Transplantation, Allografts, Antibodies, Erythropoiesis, Ferritins, Graft Rejection, Graft Survival, Humans, Inflammation, Iron, Nephrectomy, Postoperative Complications
Background
Kidney transplantation is the criterion standard treatment for patients with end-stage renal disease (ESRD). It improves both quality of life and survival [1,2]. Patients who return to dialysis after graft loss (DAGL) have a significantly higher mortality rate compared to those awaiting their first renal transplantation [3]. A second transplantation improves survival among patients with allograft failure, but only 15% of these patients undergo re-transplantation [4].
The reasons underlying adverse outcomes in DAGL are incompletely understood [5,6]. In this context, it remains unclear whether removal of the failed renal transplant improves outcome.
In many transplant centers it is common practice to keep the failed graft in situ with reduced immunosuppression, such as steroid monotherapy, unless there are clinical signs of manifest rejection like pain or fever. It may be hypothesized, however, that keeping the failed graft in situ can induce a chronic inflammatory state leading to elevated C-reactive protein (CRP), erythropoietin-resistant anemia, hypalbuminemia, or elevated serum ferritin concentrations [7,8]. Thus, preliminary data suggest that the presence of a failed allograft is indeed associated with hypalbuminemia [8,9]. Allograft nephrectomy can thus ameliorate inflammation and improve erythropoiesis. Generally, inflammation impairs erythropoiesis by hepcidin-dependent reduction of iron availability and by reducing the production and activity of erythropoietin [10]. To date, however, there are insufficient data to support this hypothesis for patients with DAGL. There are few reports on laboratory parameters of inflammation and on iron and erythropoietin supplementation.
On the other hand, leaving the transplant allograft can improve the occurrence of de-novo donor-specific antibodies (DSA) [11]. One reason might be the removal of the adsorbing donor tissue (the sponge hypothesis), and another reason is the minimization of withdrawal of immunosuppression despite persisting antigen presentation. Moreover, graft nephrectomy is an invasive procedure with a risk for complications. Thus, there is an urgent clinical need to compare the potential benefits regarding inflammation and erythropoiesis to the risks of the surgical procedure and DSA formation.
In this study, we examined the hypothesis that failed kidney allografts that are left in situ are associated with a chronic inflammatory state leading to impaired erythropoiesis [2,6]. To address this issue, we compared parameters of inflammation, erythropoiesis, and prevalence of DSA in patients with persisting failed kidney allografts vs patients who underwent graft nephrectomy after return to dialysis. Moreover, we assessed outcomes of kidney re-transplantation in a 10-year follow in the 2 groups.
Material and Methods
PATIENTS AND DESIGN:
We performed a retrospective observational study of renal transplant recipients in the outpatient clinic of the transplant center of Ruhr-University Bochum, Germany.
We reviewed our electronic patient record data system for renal allograft recipients. We included patients who were age ≥18 years, underwent kidney transplantation, and returned to DAGL between January 1, 2000 and December 31, 2019. Exclusion criteria were active malignancy and death within 90 days after renal allograft failure. According to the center’s standard, patients underwent graft nephrectomy if allograft failure occurred <3 months after transplant or later in case of clinical signs of rejection (eg, pain, fever), transplant vascular thrombosis, or biopsy-proven necrosis of the transplant.
We divided our cohort retrospectively into 2 groups. Group A comprised transplant recipients with the allograft remaining in situ after return to dialysis. Group B comprised patients who underwent transplant nephrectomy in this situation. Group A contained 49 patients and Group B contained 43. Forty-three patients underwent re-transplantation after return to dialysis. As a secondary endpoint, we assessed outcome of kidney re-transplantation in a 10-year follow-up in this cohort.
COLLECTION OF LABORATORY AND CLINICAL DATA:
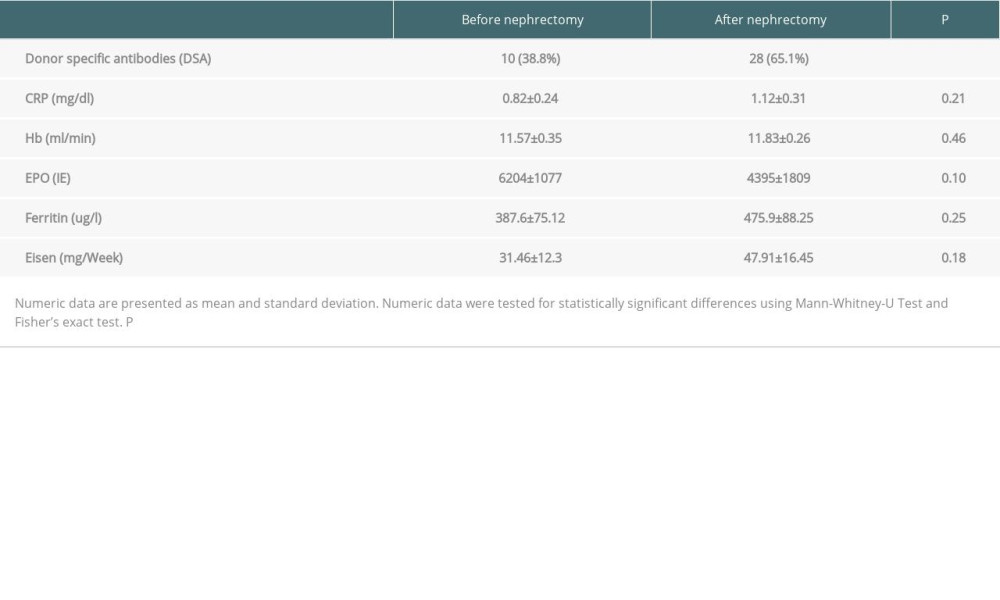
Laboratory data were collected 3–6 months after returning to dialysis or after nephrectomy. Median time to nephrectomy was 3 months after return to dialysis. Laboratory data included the following: serum CRP, hemoglobin (Hb) levels, ferritin, erythropoietin dose, and iron supplementation. All laboratory measurements except HLA analyses were performed in the central laboratory of Knappschaftskrankenhaus Bochum according to manufacturer’s instructions. In those subjects undergoing allograft nephrectomy, an additional laboratory assessment was conducted prior to the surgical procedure.
ASSESSMENT AND QUANTIFICATION OF HLA ANTIBODIES AND ASSIGNMENT AS DSA:
All patient sera were analyzed for the presence of antibodies using the standard lymphocytotoxicity test (complement-dependent cytotoxicity, CDC) in combination with Luminex™-based antibody screening for HLA class I and class II. For the detection of cytotoxic anti-HLA antibodies, the CDC test according to Terasaki [12] was performed using an in-house panel of freshly prepared lymphocytes. Dithiothreitol (DTT)-resistant positive reactions were attributed to IgG antibodies. In a broadly accepted step-by-step analysis, all sera were first analyzed for the presence of anti-HLA class I and II IgG antibodies using the LABScreen™ Mixed beads assay (One Lambda/Thermo Fisher). Only in case of LABScreen™ Mixed positivity for class I and/or class II, the sera were subsequently analyzed using LABScreen™ SAB (One Lambda/Thermo Fisher). The SAB assay uses beads coated with single HLA specificities and enables the identification of IgG alloantibody specificities against HLA-A, -B, -C, -DR, -DQ, and -DP antigens. Both assays were performed according to the manufacturer’s instructions, with no modification of the protocol. Samples were measured on Luminex™ 100 or 200 machines and analyzed using HLA Fusion software (One Lambda/Thermo Fisher). The positive cutoff value for mean fluorescence intensity (MFI) in the SAB assay was set as higher than 1000. To address a potential effect of interfering antibodies or prozone effects on our MFI analysis, all sera were tested after EDTA treatment [13].
STATISTICS:
Continuous variables were tested for Gaussian distribution using the Kolmogorov-Smirnov test. Our data had non-normal distribution, so we used the Wilcoxon test to examine differences between groups. Dichotomous parameters were analyzed using the chi-squared test (Fisher’s exact test). Transplant survival in case of re-transplantation in Group A and B was compared by the Kaplan-Meier estimator. All statistical analyses were done using SPSS Statistics 25 (SPSS Inc, Chicago, Illinois, USA) and Prism 5 (GraphPad Software, La Jolla, California, USA).
ESTIMATION OF NECESSARY STUDY SIZE:
CRP was used as a primary marker of inflammation. Based on laboratory data of our transplant center, we expected a mean of 1.0 mg/dl with a standard deviation of 0.5 mg/dl in the group undergoing allograft nephrectomy. Assuming a 50% higher CPR concentration (1.5 mg/dl) in those subjects with the allograft remaining in situ, 36 subjects per group were required to detect this difference with a statistical power of 80% and an alpha significance level of
Results
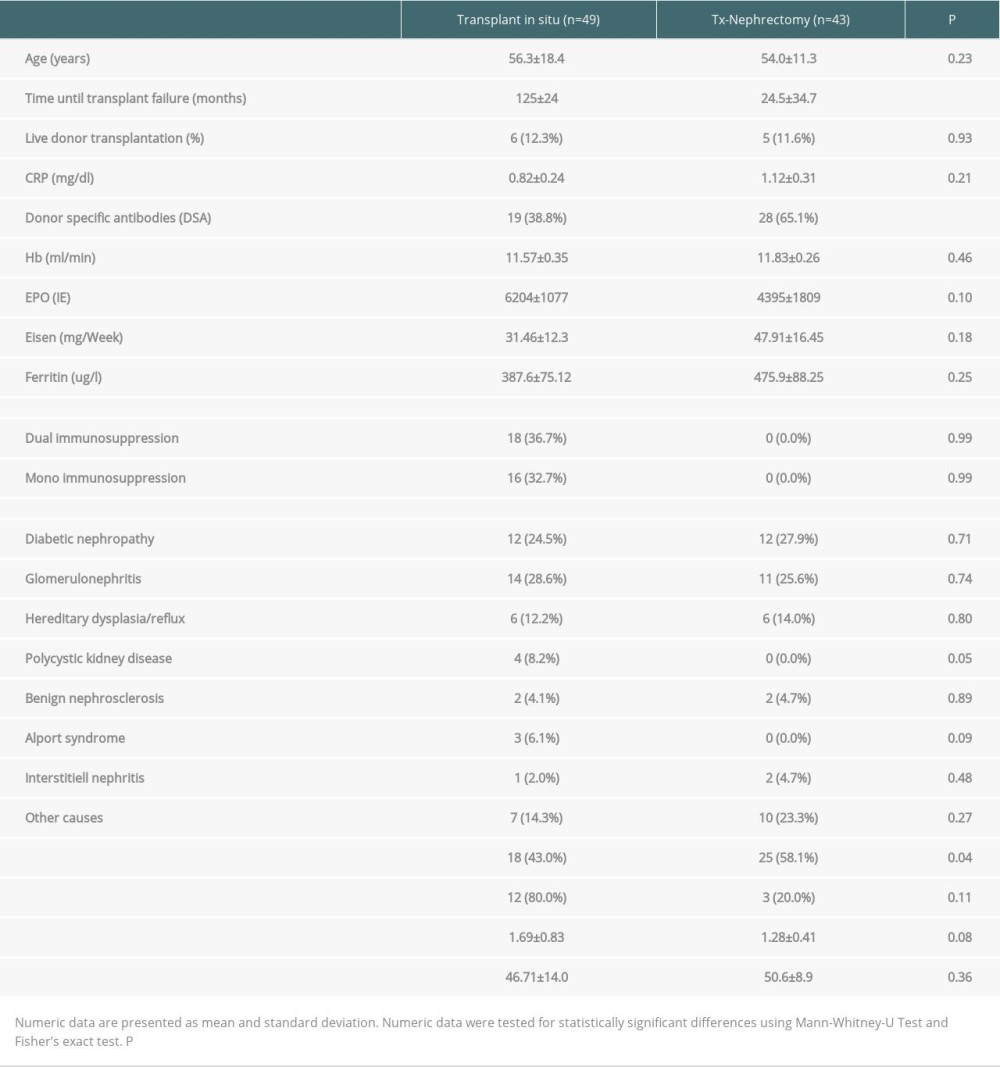
We included 92 patients who returned to dialysis after renal transplant failure. In 49 patients, transplant allograft was left in situ (Group A). Forty-three patients underwent transplant nephrectomy for the above-mentioned indications (Group B). In 43 patients, nephrectomy was performed for a mean period of 55 months after transplant: in 16 (37.2%) for clinical signs of rejection, in 15 (34.9%) for vascular thrombosis, and in 11 (25.6%) for other reasons, including biopsy-proven allograft necrosis. Groups did not differ in terms of sex or age. Table 1 summarizes the characteristics of Group A and Group B, including epidemiological data, transplant data, immunosuppression, and inflammatory and immunological parameters. Laboratory values were obtained 3–6 months after return to dialysis in Group A and 3–6 months after return to dialysis and nephrectomy in Group B. In Group B, additional laboratory data were retrieved before nephrectomy (Table 2).
Immunosuppression was completely withdrawn after nephrectomy and reduced in all recipients with allografts remaining in situ. Thus, 16 patients were on monotherapy and 18 patients (36.7%) were on dual immunosuppressive therapy. Immunosuppression with 5 mg prednisolone was prescribed in 35 patients and low-dose CNI in 16 patients.
Figure 1 describes CRP concentrations and DSA in patients with and without allograft nephrectomy. CRP concentrations did not statistically differ in these groups (
Moreover, there was no significant effect of allograft nephrectomy on erythropoiesis. Thus, neither hemoglobin concentrations nor erythropoietin dosages differed significantly in the 2 groups (Figure 2A, 2B). Erythropoietin dosages, however, tended to be higher in Group A. Iron substitution dosages and ferritin concentrations were comparable in the 2 groups (Figure 2C, 2D). Table 1 provides the numerical data of all these parameters.
In summary, there was no statistically significant difference in markers of inflammation and erythropoiesis. Patients with allograft remaining in situ, however, needed more doses of erythropoietin, without reaching statistical significance.
An additional longitudinal analysis in Group B investigated potential changes of the laboratory parameters before and after nephrectomy. CRP, hemoglobin, and ferritin concentrations as well as erythropoietin and iron doses did not significantly change from baseline to follow-up (
In Group B, 25 (58.1%) of the patients underwent re-transplantation, and in Group A there were 18 (36.7%) such patients. All transplantations were primarily successful. Kaplan-Meier analyses revealed a comparable 10-year allograft survival with 3 patients of Group B and none in Group A having allograft failure.
One of the 3 patients lost his allograft by interstitial nephritis (the first transplant was lost by rejection), the other 2 patients lost both the first and the second allograft by biopsy-proven rejection episodes.
Discussion
The present work provides the first systematic analysis on the effects of nephrectomy after allograft failure regarding parameters of microinflammation, renal anemia, and the development of DSA.
Our findings do not support the hypothesized induction of an inflammatory state due to the presence of alloantigens in patients with substantially reduced or withdrawn immunosuppression [6,8]. Chronic inflammation yields an increase in ferritin and impairs iron resorption and mobilization from iron storages in the liver and reticulo-histiocytic system. Thereby, it affects erythropoiesis and leads to anemia, with increased ferritin and reduced transferrin serum concentrations [14]. Our results show no differences in ferritin concentrations or hemoglobin concentrations. Of note, hemoglobin and ferritin concentrations are substantially affected by iron and erythropoietin supplementation in transplant patients after return to dialysis. Therefore, substitution dosages were analyzed as well. Both dosages were not significantly different in the 2 groups. Thus, an in-situ allograft did not evoke a significant impairment of erythropoiesis in our study as hypothesized [9]. The trend to numerically lower erythropoietin dosages in patients who underwent allograft nephrectomy should be reinvestigated in larger study populations. It appears possible that in situ transplants have a minor inhibitory effect on erythropoiesis that did not reach statistical significance due to the small study size.
Thus, the present analysis could not confirm the postulation that patients with a failed renal allograft in situ are at a higher risk for developing a chronic inflammatory syndrome. The higher prevalence of DSA, however, could constitute another argument for transplant nephrectomy for those patients undergoing a further transplantation. In this context, our study confirms previous reports on a higher prevalence of DSA in case of allograft nephrectomy [11]. This finding was observed in both the cross-sectional primary investigation and the longitudinal observation of those subjects undergoing nephrectomy. The most probable explanations for this finding are that an in-situ transplant provides binding capacity for circulating antibodies and the withdrawal of immunosuppression after nephrectomy.
In a secondary analysis, we analyzed allograft survival in those 43 patients with re-transplantation after allograft failure. Despite a higher risk of allosensitization, patients in Group B were more likely to be re-transplanted. Transplant survival was comparable in patients with and without prior nephrectomy of the first allograft in a 10-year follow-up. Hence, keeping a failed allograft in situ did not induce a microinflammatory state leading to impaired erythropoiesis, nor did it impair the success of re-transplantation. The present study thereby indicates that both strategies are feasible options for patients who return to dialysis after transplant failure, independent of a potential re-transplantation.
The present analysis is limited by several aspects. First, the present work is limited by its retrospective character. Thus, a bias by indication cannot be excluded and the findings have to be interpreted with caution. The well-matched groups, however, reduced this bias as far as possible. Secondly, the sample size was small. Even in a large transplant center, however, it is difficult to define 2 well-matched cohorts with comparable age and comorbidities. The present findings should therefore be confirmed in a larger cohort in a multi-center design.
In light of the findings of this analysis, we decided to define minimization of immunosuppression to 5 mg prednisolone after allograft failure as standard of care in our center. Those patients who present clinical signs of rejection like pain or fever undergo nephrectomy. If there are no signs of clinical rejection, the allograft remains in situ. Patients welcome this approach, since they benefit from the remaining diuresis and do not have to undergo a further surgical procedure.
Conclusions
Keeping a kidney graft in situ after returning to dialysis did not lead to an increase in microinflammation or impairment of erythropoiesis. DSA, however, occurred more often after transplant nephrectomy. With the limitations of its retrospective character and small sample size, this study implies that both keeping the transplant in situ and transplant nephrectomy are feasible options in kidney transplant recipients after return to dialysis.
Figures
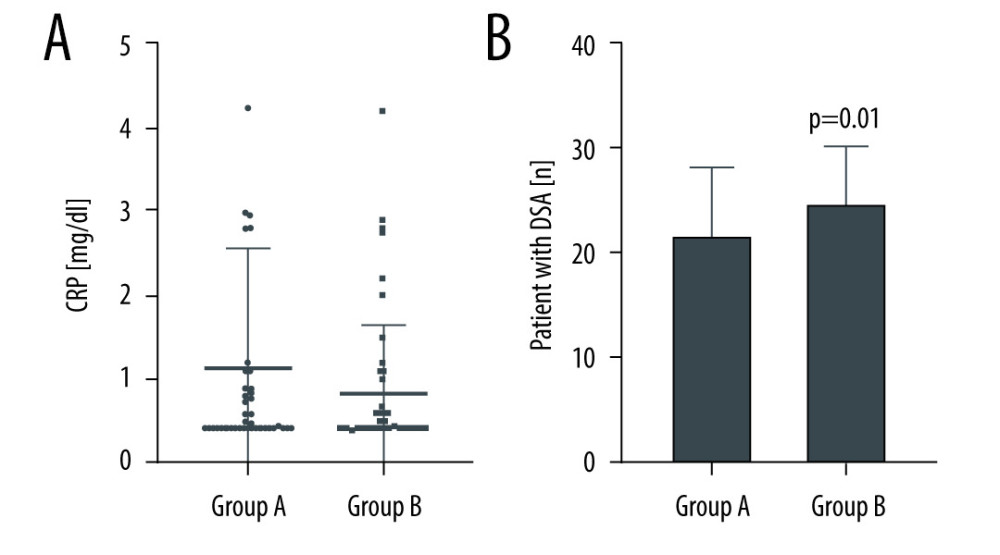 Figure 1. (A, B) Differences in C-reactive protein (CRP) and donor-specific antibodies (DSA) in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). CRP-levels are presented as dot plots and DSA data as a bar chart. Horizontal lines indicate median and interquartile range. GraphPad Prism 8.4.3. GraphPad Software, Inc.
Figure 1. (A, B) Differences in C-reactive protein (CRP) and donor-specific antibodies (DSA) in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). CRP-levels are presented as dot plots and DSA data as a bar chart. Horizontal lines indicate median and interquartile range. GraphPad Prism 8.4.3. GraphPad Software, Inc. 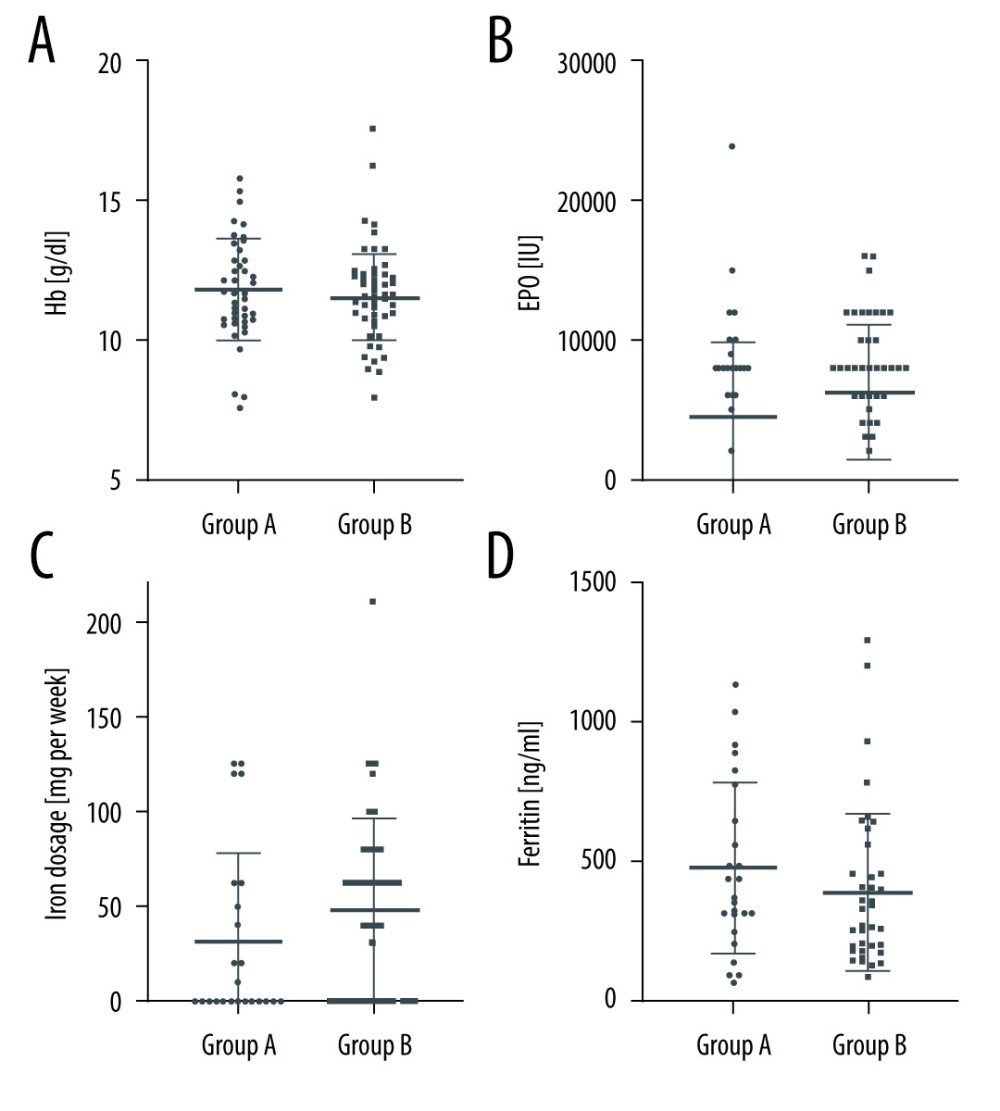 Figure 2. (A–D) Erythropoietin (EPO) dosages, hemoglobin (Hb) concentration, ferritin concentration, and iron substitution dosages in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). Dosages and concentrations are presented as dot plots. Horizontal lines indicate median and interquartile range. * P<0.05 was regarded as significant. GraphPad Prism 8.4.3. GraphPad Software, Inc.
Figure 2. (A–D) Erythropoietin (EPO) dosages, hemoglobin (Hb) concentration, ferritin concentration, and iron substitution dosages in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). Dosages and concentrations are presented as dot plots. Horizontal lines indicate median and interquartile range. * P<0.05 was regarded as significant. GraphPad Prism 8.4.3. GraphPad Software, Inc. References
1. Schnuelle P, Lorenz D, Trede M, Van der Woude FJ, Impact of renal cadaveric transplantation on survival in end-stage renal failure: Evidence for reduced mortality risk compared with hemodialysis during long-term follow-up: J Am Soc Nephrol, 1998; 9; 2135-41
2. Wolfe RA, Ashby VB, Milford EL, Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant: N Engl J Med, 1999; 341; 1725-30
3. Rao PS, Schaubel DE, Jia X, Li S, Survival on dialysis post-kidney transplant failure: Results from the Scientific Registry of Transplant Recipients: Am J Kidney Dis, 2007; 49; 294-300
4. Ojo A, Wolfe RA, Agodoa LY, Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System: Transplantation, 1998; 66; 1651-59
5. Gill JS, Abichanandi R, Khan S, Opportunities to improve the care of patients with kidney transplant failure: Kidney Int, 2002; 61; 2193-200
6. Gill JS, Abichanandi R, Kausz AT, Pereira BJ, The impact of non-immunologic factors: Kidney Int, 2002; 62; 1875-83
7. Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS, Transplant nephrectomy improves survival following a failed renal allograft: J Am Soc Nephrol, 2010; 21(2); 374-80
8. Lopez-Gomez JM, Perez-Flores I, Jofre R, Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance: J Am Soc Nephrol, 2004; 15; 2494-501
9. Almond MK, Tailor D, Marsh FP, Increased erythropoietin requirements in patients with failed renal transplants returning to a dialysis program: Nephrol Dial Transplant, 1994; 9; 270-73
10. Lanser L, Fuchs D, Kurz K, Weiss G, Physiology and inflammation driven pathophysiology of iron homeostasis – mechanistic insights into anemia of inflammation and its treatment: Nutrients, 2021; 13(11); 3732
11. Del Bello A, Congy N, Sallusto F, Anti-human leukocyte antigen immunization after early allograft nephrectomy: Transplantation, 2012; 93; 936-41
12. Terasaki PI, Mandell M, Vandewater J, Edgington TS, Human blood lymphocyte cytotoxicity reactions with allogenic antisera: Ann N Y Acad Sci, 1964; 120; 322-34
13. Susal C, Seidl C, Schonemann C, Heinemann FM, Determination of unacceptable HLA antigen mismatches in kidney transplant recipients: Recommendations of the German Society for Immunogenetics: Tissue Antigens, 2015; 86(5); 317-23
14. Babitt JL, Lin HY, Mechanisms of anemia in CKD: J Am Soc Nephrol, 2012; 23(10); 1631-34
Figures
 Figure 1. (A, B) Differences in C-reactive protein (CRP) and donor-specific antibodies (DSA) in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). CRP-levels are presented as dot plots and DSA data as a bar chart. Horizontal lines indicate median and interquartile range. GraphPad Prism 8.4.3. GraphPad Software, Inc.
Figure 1. (A, B) Differences in C-reactive protein (CRP) and donor-specific antibodies (DSA) in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). CRP-levels are presented as dot plots and DSA data as a bar chart. Horizontal lines indicate median and interquartile range. GraphPad Prism 8.4.3. GraphPad Software, Inc. Figure 2. (A–D) Erythropoietin (EPO) dosages, hemoglobin (Hb) concentration, ferritin concentration, and iron substitution dosages in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). Dosages and concentrations are presented as dot plots. Horizontal lines indicate median and interquartile range. * P<0.05 was regarded as significant. GraphPad Prism 8.4.3. GraphPad Software, Inc.
Figure 2. (A–D) Erythropoietin (EPO) dosages, hemoglobin (Hb) concentration, ferritin concentration, and iron substitution dosages in Group A (failed renal allograft in situ) and Group B (allograft nephrectomy). Dosages and concentrations are presented as dot plots. Horizontal lines indicate median and interquartile range. * P<0.05 was regarded as significant. GraphPad Prism 8.4.3. GraphPad Software, Inc. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860