08 March 2022: Original Paper
The Role of Cavoportal and Renoportal Hemitransposition in Liver Transplantation
Betty Maillot1ABCDEF*, Guillaume Bouzille2C, Jean-Yves Mabrut3B, Edouard GirardDOI: 10.12659/AOT.935892
Ann Transplant 2022; 27:e935892
Abstract
BACKGROUND: Few series of cavoportal (CPA) or renoportal (RPA) anastomosis have been published and their survival rates have never been compared. The objective of this study was to evaluate perioperative and long-term outcomes of CPA and RPA in a nationwide multicentric series and to compare hemitranspositions (HT) to paired orthotopic liver transplantations (OLT).
MATERIAL AND METHODS: HT performed in France up to April 2019 were analyzed. Endpoints were the incidence of severe (Clavien-Dindo>IIIa) 90-day perioperative complications and long-term patient and graft survival.
RESULTS: Sixty-four HT (13 CPA, 51 RPA) were performed in 59 patients. The rates of perioperative CD>IIIa complications were 64% and 49% in patients with CPA and RPA, respectively (P=0.59), and the rates of portal thrombosis and ascites were 38.5% and 9.8% (p=0.023) and 53.8% and 21.6% (p=0.049) in patients with CPA and RPA, respectively. The patient and graft perioperative survival rates were 54.4% and 83.3% (HR=3.2; CI 95 [1.1-9.9]; p=0.039) and 54.4% and 77.1% (HR=2.2; CI 95 [0.77-6.4]; P=0.14) in the CPA and RPA groups, respectively. Five-year patient survival was 36.4% and 61.8% in the CPA and RPA groups, respectively (HR=2.5; CI95 [1-6.1]; P=0.039). Compared with OLT grafts, long-term HT graft survival rates were not different (HR=1.7; CI 95 [0.96-3.1]; P=0.066), while patient survival rates were lower in the HT group (HR=4.6; CI 95 [2-11]; P<0.001).
CONCLUSIONS: Compared to OLT, HT significantly reduces patient survival. Given the poor survival results of CPA, the indication deserves to be limited in the context of organ shortage and RPA should be preferred when HT is needed.
Keywords: Graft Survival, Liver Transplantation, Portacaval Shunt, Surgical, Venous Thrombosis, Anastomosis, Surgical, Humans, Portal Vein
Background
Liver transplantation (LT) is the most effective treatment for end-stage liver diseases. In France, the overall 5-year survival rate of patients who underwent transplantation between 2007 and 2017 was 74.5% [1]. Unfortunately, the need for LT far exceeds the availability of grafts. In 2018, the ratio of liver transplantation candidates/available grafts was 2.5 [1].
Splanchnic venous system obstruction (ie, complete obstruction of the portal vein and superior mesenteric vein (Yerdel grade IV) [2]) is rarely present in transplant candidates [3,4]. It was considered an absolute contraindication to liver transplantation until the late 1990s when Sheil et al [5] and Tzakis et al [6] first described the hemitransposition technique (HT). HT consists of connecting the graft portal vein either into the left renal vein (renoportal anastomosis, RPA) or into the infrahepatic inferior vena cava (cavoportal anastomosis, CPA) (Figure 1). HT provides venous inflow to the graft, which allows for the restoration of normal metabolic function. However, HT does not correct venous hypertension in the splanchnic bed, which may increase postoperative complications such as variceal bleeding and ascites [7,8].
Few studies have analyzed the outcomes of HT and most of them were case reports or short series (up to 10 cases, see Table 1 [6,9–29]). In 2007, Selvaggi et al [8] reported a series of 23 HTs with CPA. The 1- and 5-year survival rates were 60% and 38%, respectively. In 2011 Bhangui et al [3] reported the outcome of 20 HTs, of which 17 (85%) had an RPA. The 1- and 5-year survival rates were 83% and 68%, respectively. Recently, Azoulay et al [30] reported the outcomes of 57 RPAs from 5 selected Western expert centers and showed that the presence of a spontaneous or surgical large splenorenal shunt prior to transplantation was associated with excellent long-term patient and graft survival.
These results encouraged us to suggest that RPA, when feasible, should be preferred to CPA. However, owing to the limited number of patients, comparisons between RPA and CPA or even between HT and conventional orthotopic liver transplantation (OLT) could not be performed.
The question of which type of HT should be preferred in cases of diffuse thrombosis of the splanchnic venous system therefore remains unanswered. Moreover, in an organ shortage, whether hemitransposition remains an acceptable procedure is debatable. Using a nationwide database of HTs, the aim of our study was to analyze the outcome of patients receiving HTs, compare CPA with RPA, and assess the therapeutic value of HTs compared with matched OLTs.
Material and Methods
PATIENTS AND STUDY DESIGN:
This research protocol was reviewed and approved by the Research Ethics Committee of the University of Rennes 1 (No. 20.99). This study was retrospective and noninterventional, and the collected data were anonymous. The committee waived the requirement for patient consent.
The base population consisted of all patients who had HTs performed in France from January 1, 1998 to April 1, 2019. A questionnaire was sent to all French transplant centers to collect pre-, peri-, and postoperative data, which were compiled using a local prospectively maintained database.
The endpoints were i) perioperative complications; ie, complications occurring during surgery or within 90 days of transplantation or at any time during the first hospitalization, and ii) patient and graft survival. Complications were reported using the Clavien-Dindo classification (CD) [31]. Serious complications were defined as type IIIb, IVa, IVb, and V. (Type V corresponded to patient death). The date of the end of follow-up was set for April 1, 2019.
STATISTICAL ANALYSIS:
Quantitative variables were represented as the average and standard deviation if their distribution was considered normal by the Shapiro-Wilk test or by the median and interquartile range [IQR] if not. Qualitative variables were represented as numbers and percentages. To respect the conditions of validity, comparisons between groups were based on Student’s
The indications of HT were elective and decided prior to LT. Patients who received CPA or RPA were compared with a matched group of patients who received standard OLT performed in the center of the first author between 1998 and 2018. Matching was carried out in a semiautomated manner using the data mining software eHOP® [31,32]. For each patient treated with HT, 3 patients in the control group of the same gender and with the closest age, body mass index and model for end-stage liver disease (MELD) score were selected. A review of these patients who received OLT was performed to ultimately retain only the recipient whose indication for LT and duration of cold ischemia of the graft were identical to those of the patient treated with HT. The comparison was eventually made with a 1: 1 match.
Survival was calculated using the Kaplan-Meier method. The date of graft loss corresponded to the date of recipient death or retransplantation. Comparisons between groups were performed using the log-rank test.
We used Cox regression analyses to identify the cofactors potentially associated with survival, apart from the type of surgical technique. A univariate analysis was performed to identify covariables with a P value <0.05, which were retained for the multivariate model (Table 2). This restrictive threshold was chosen because of the limited size of our sample. Quantitative variables were dichotomized beforehand and the threshold retained was the one that maximized the Youden index (sensitivity+specificity-1) regarding the loss of the graft. These threshold choices were made by bootstrap resampling with 100 replications at the end of which the median threshold was retained. Missing data were accounted for by the multiple imputation method with 5 replications using the R ‘mice’ library [33].
Statistical analyses were performed using SPSS software (SPSS version 21; IBM Corp) and the R statistical programming environment (version 3.4.0). Two-sided P<.05 were considered statistically significant.
Statistical analyses were performed using SPSS software (SPSS version 21; IBM Corp) and the R statistical programming environment (version 3.4.0). Two-sided P<.05 were considered statistically significant. Analyses were performed using the R statistical programming environment (version 3.6.0). Two-sided p<0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS:
Eleven French LT centers had performed at least one HT at the time of data collection. Only 9 centers were included because 2 centers did not provide patient data. The first HT was performed in 1998 and at the time of our study, 64 HTs had been performed on 59 patients. Thirteen (20.3%) involved CPA and 51 (79.7%) involved RPA. Two patients first transplanted with RPA were retransplanted with CPA, one patient first transplanted with RPA was retransplanted with RPA, and one patient transplanted with RPA was retransplanted twice with RPA. All grafts were total liver except for one right hemiliver transplant.
Patient, graft and surgical characteristics are reported in Table 3. A history of variceal bleeding, ascites or hepatic encephalopathy was found in 90% of the patients. Seven (11.9%) patients had already received a standard liver transplant before HT. Six (10.2%) patients had a patent surgical mesocaval (n=4) or splenorenal (n=2) shunt at the time of the first HT.
PERIOPERATIVE AND LONG-TERM COMPLICATION RATES:
A comparison of the perioperative complications that occurred in patients who received CPA and RPA is presented in Table 4. A perioperative complication was observed in 87.5% of the patients who received HT. The rates of portal thrombosis and ascites were 38.5% and 9.8% (P=0.023) and 53.8% and 21.6% (P=0.049) in patients who received HT with CPA and RPA, respectively. The rate of CD type ≥III complications was high and not significantly different between the CPA and RPA groups (64% vs 49%, respectively, P=0.59). Perioperative CPA and RPA related death rates were 45.6% and 16.7%, respectively (HR=3.2; IC95 [1.1–9.9]; P=0.039).
Fourteen patients had long-term (>3 months) complications related to PHT, i.e., ascites (n=6) and/or recurrent hemorrhage (n=8).
Among the patients who experienced recurrent hemorrhage, 6 were still alive and the end of follow-up. Two patients died at 10 and 7 months after LT owing to recurrent hemorrhage and liver failure, respectively.
LONG-TERM GRAFTS, PATIENT SURVIVAL RATES, AND PROGNOSTIC FACTORS:
As of April 1, 2019, the mean follow-up was 57 months. In the CPA group, median graft and patient survival time was 3.2 months (CI 95 [0.17-Inf]) and 3.2 months (CI 95 [0.17-Inf]), respectively. In the RPA group, median graft and patient survival time was 10.9 years (CI 95 [3.39-Inf]) and 11.6 years (CI 95 [4.18-Inf]), respectively.
Figure 2 shows the Kaplan-Meier survival curves of patients and grafts for the CPA and RPA groups. Patient survival was significantly better when RPA was performed (HR=2.5; CI 95 [1–6.1]; P=0.039). Multivariate analysis found no independent factor that affected patient survival.
COMPARISON OF PATIENTS WHO RECEIVED HT WITH PAIRED PATIENTS WHO RECEIVED OLT:
The characteristics of the paired patients who received OLT are reported in Table 5. Patients who received OLT were comparable to patients who received HT except for the duration of the surgery, the rate of grafts retrieved from extended-criteria donors, and the performance status of the patients prior to transplantation.
A comparison of the perioperative complications that occurred in patients who received HT and OLT is presented in Table 6. The rate of complications related to portal hypertension was significantly higher after HT, particularly the rate of ascites (28.1% vs 7.8%, P=0.006) and the rate of gastrointestinal bleeding episodes (17.2% vs 4.7%, P=0.047). Compared with OLT, HT was associated with a higher rate of perioperative graft loss and patient death (27.1% vs 11.9%, P=0.033 and 21.9% vs 0%, P=0.0001, respectively).
Median graft survival after HT and OLT was 6.5 years and 11.1 years, respectively. Figure 3 shows the Kaplan-Meier survival curves of patients and grafts for the HT and OLT groups. HT graft survival rates tended to be lower than those of OLT grafts but the difference did not reach a significant threshold (HR=1.7; CI 95 [0.96–3.1]; P=0.066). Patient survival rates were significantly lower after HT than after OLT (HR=4.6; CI 95 [2–11]; P<0.001). On multivariate analysis, hemitransposition and a donor’s age higher than 61 years were the only independent prognostic factors that negatively impacted patient survival (HR=4.2; CI 95 [1.4–13]; P=0.01 and HR=2.8; CI 95 [1.1–1.7]; P=0.02, respectively) (Table 7).
Discussion
When the recipient splanchnic venous bed is not suitable for graft portal implantation, the alternative to conventional OLT is to perform hemitransposition, either renoportal or cavoportal. In this study, we analyzed the outcome of all HTs performed in France until April 2019. We found that, compared with a matched group of patients who received OLT, HT was significantly associated with patient survival and that among patients who received HT, those who received renoportal HT did better than those who received cavoportal HT.
The reason for the superiority of RPA over CPA is poorly understood. It has been suggested that RPA allows for the drainage of the venous splanchnic system via the development of spontaneous renoportal shunts. However, this explanation is not entirely satisfactory since CPA also carries the venous return from the left renal vein and may mimic a reno-portal derivation in case of spontaneous splenorenal shunts. Indeed, CPA includes stapling or ligation of the vena cava downstream from the implantation of the graft portal vein and therefore collects both vena cava and left renal flows (Figure 1). The poor results of CPA compared with RPA could instead be explained by the absence of congruence between the recipient inferior vena cava (IVC) and donor portal vein and the difficulty of adjusting the length of the portal vein to avoid kinking at the time of abdomen closure. Indeed, all CPAs were of the side-to-end type, which could explain why the rate of perioperative portal vein thromboses was significantly higher in the CPA group. In other series, postoperative portal vein thrombosis occurred after CPA in 16% to 29% of patients [7,12,16] and was associated with a dismal prognosis. When a cavoportal anastomosis is unavoidable, we recommend adopting the technique described by Tzakis et al to separate the IVC to align the IVC and portal axes. The use of postoperative anticoagulation could be a means to prevent thrombosis. However, its administration in patients whose early allograft function remains precarious is still debated.
In our series, 6 patients had a patent splenorenal or a mesocaval shunt at the time of first transplantation. All 6 patients were still alive at the time of data collection. This suggests that the disappearance of hypertension in the venous splanchnic territory plays a major role in the occurrence of posttransplant complications. Moreover, we believe that performing an RPA or CPA when a surgical mesocaval or splenorenal shunt is still patent is the equivalent of orthotopic transplantation since neither portal hypertension nor ascites persists after transplantation. Indeed, Azoulay et al [30] showed that the presence of a large patent spontaneous or surgical splenorenal shunt was associated with excellent long-term graft and patient survival.
In this French series, the rate of portal thrombosis in the perioperative period was unexpectedly high. Portal vein thrombosis may be explained by nonanatomical reconstruction or low renal venous flow after RPA transplantations (due to the absence of surgical or spontaneous splenorenal shunts). The state of hypercoagulability associated with cirrhosis in these patients could be an explanation and would justify the recommendation that all patients who receive HT receive long-term postoperative anticoagulation.
The study period was 20 years and the mean survival time was only 57 months. This was related to higher postoperative mortality at the early time of the study. Moreover, the median survival of CPAs was very low, which reflected unacceptable early postoperative mortality. Our study also showed that the survival of patients who received HT was significantly lower than that of a matched control group of patients receiving standard OLT, although graft survival was not different between the HT and OLT groups. Analysis of the survival curve profiles shows that most of the graft loss occurred during the first year after transplantation and that the rate of retransplantation was reduced in patients who received HT patients compared with those who received OLT. The collected data did not enable us to explain this discrepancy. However, 2 hypotheses can be put forward. The first is that the patients who received HT were in too poor a condition to be retransplanted or to wait the necessary time to obtain a new graft. The second is that the decision not to retransplant was related to the poor relevance of performing HT again at a time when the availability of grafts was far below demand and the dropout rate of these seriously ill patients from the waiting list before transplantation was 30% [1].
An important question that arises from these results is whether a long-term patient survival rate of 50% is acceptable to the community of transplant recipients and patients awaiting transplants in the context of organ shortage. In terms of profitability and public health, this survival rate is low and the transplantation of these patients could be considered futile. It would therefore appear reasonable not to consider thrombosis of the splanchnic venous system as a factor that should accelerate the indication of transplantation, but rather continue to consider the severity of the initial disease based on the MELD score. In France, the perioperative mortality rate of CPA is markedly high (45.6%), and this percentage could justify the definitive abandonment of CPA. Portal arterialization and multivisceral transplantation (including or not the pancreas) have been proposed as alternatives to HT.
Although Cheng et al [34] reported a case of portal arterialization with survival greater than 10 years, this technique usually led to high portal flow, sinusoid injury, and, eventually, fibrosis. Moreover, this arteriovenous fistula induces rapid right heart failure. We suggest that multivisceral transplantation should be offered as an alternative to HT for patients who have received multiple operations to postpone difficult dissection and a significant risk of bleeding or for when the IVC is occluded. Multivisceral transplantation has the main advantage of treating both liver disease and portal hypertension. Although very rare, en-bloc liver-small bowel transplantations have shown significant improvement over time [35]. Overall 5-year survival rates now reach 53% to 72% [36,37], and these results compare favorably with the survivals obtained with HT.
Of course, our study has the same bias as all retrospective studies, including those related to the collection of data unrelated to standardized attitudes. The first HT was performed more than 20 years ago at a time when the transplantation technique, immunosuppression, and postoperative management were less efficient than their recent counterparts. The reason for using CPA or RPA was not mentioned in patient files. It is assumed that the choice of the HT technique was dictated by difficulty accessing the left renal vein or the infrahepatic vena cava. Moreover, the rate of early retransplantation in the OLT group was unexpectedly high and this was related to the pairing procedure, which selected the most severe case and the frequent use of marginal grafts. Since it is not realistic to evaluate rarely-applied HTs through a prospective study, comparing the HT group with the paired OLT group remains the only way to estimate the performance and utility of HT. Finally, the HT group was collected in a multicenter manner, unlike the OLT group, in which all the LT were performed at the Rennes University Hospital. We were aware that this method of data collection could lead to bias; however, the difficulty accessing patient data and matching did not enable us to create an OLT group in a multicenter manner.
Conclusions
In patients with cirrhosis with extended splanchnic thrombosis associated with chronic liver disease, HT can be an efficient solution. Due to the current shortage of organs, the individual benefit of LT in patients with extended splanchnic thrombosis should be balanced in regard to the low survival rate, especially in those needing CPA.
Figures
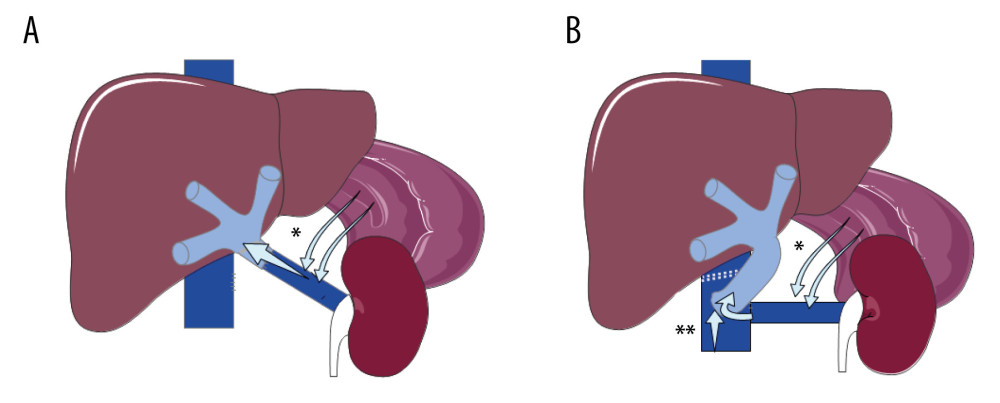 Figure 1. Hemitransposition anastomosis. (A) Renoportal anastomosis; (B). Cavoportal anastomosis. * Splenoral spontaneous or surgical shunt. ** Staplle line on the IVC downstream to the cavoportal anastomosis.
Figure 1. Hemitransposition anastomosis. (A) Renoportal anastomosis; (B). Cavoportal anastomosis. * Splenoral spontaneous or surgical shunt. ** Staplle line on the IVC downstream to the cavoportal anastomosis. 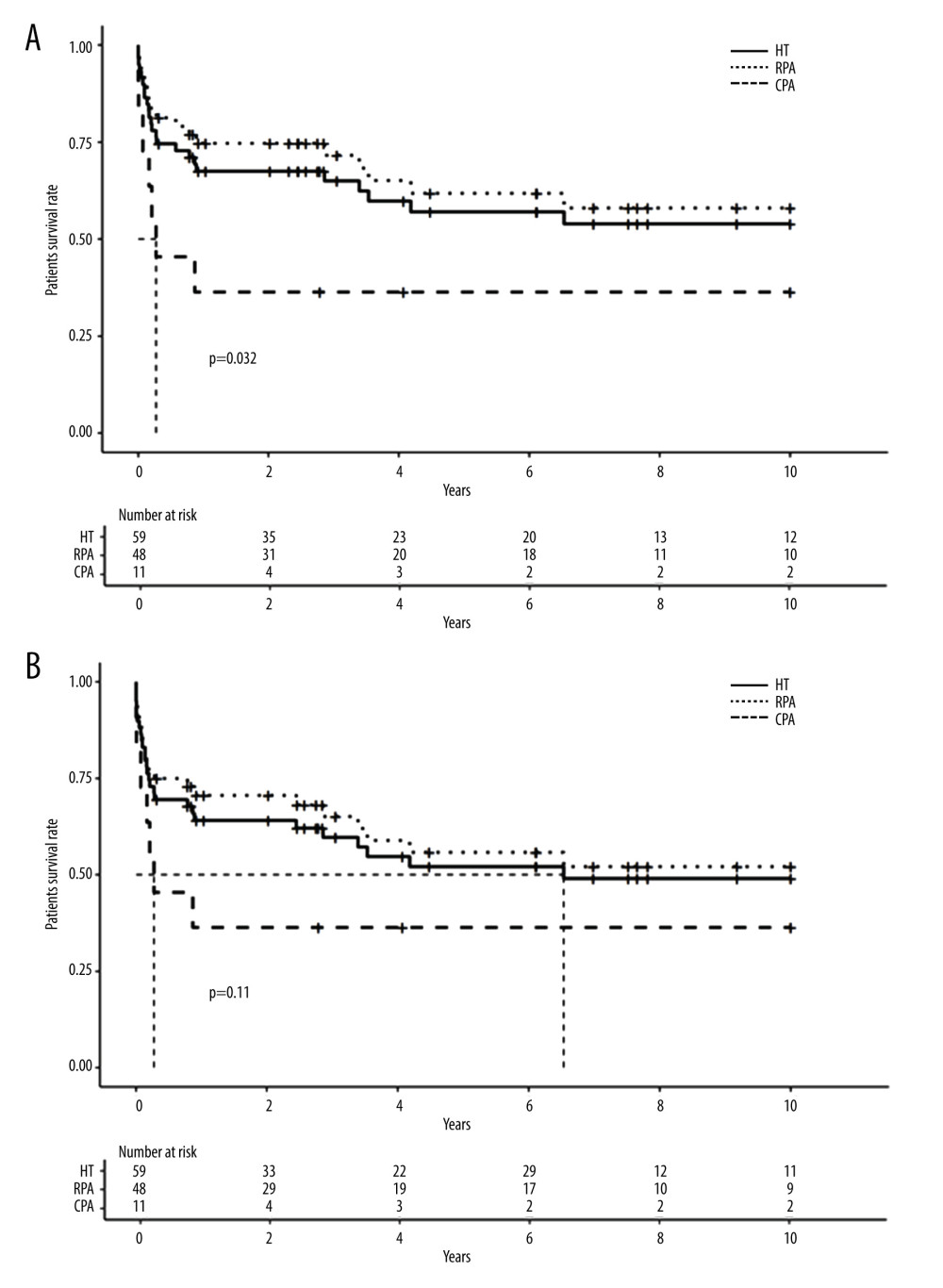 Figure 2. Long-term survival of patients and grafts after hemitransposition (HT) using cavoportal anastomosis (CPA) or renoportal anastomosis (RPA). (A). Long-term patient survival; (B) Long-term graft survival.
Figure 2. Long-term survival of patients and grafts after hemitransposition (HT) using cavoportal anastomosis (CPA) or renoportal anastomosis (RPA). (A). Long-term patient survival; (B) Long-term graft survival. 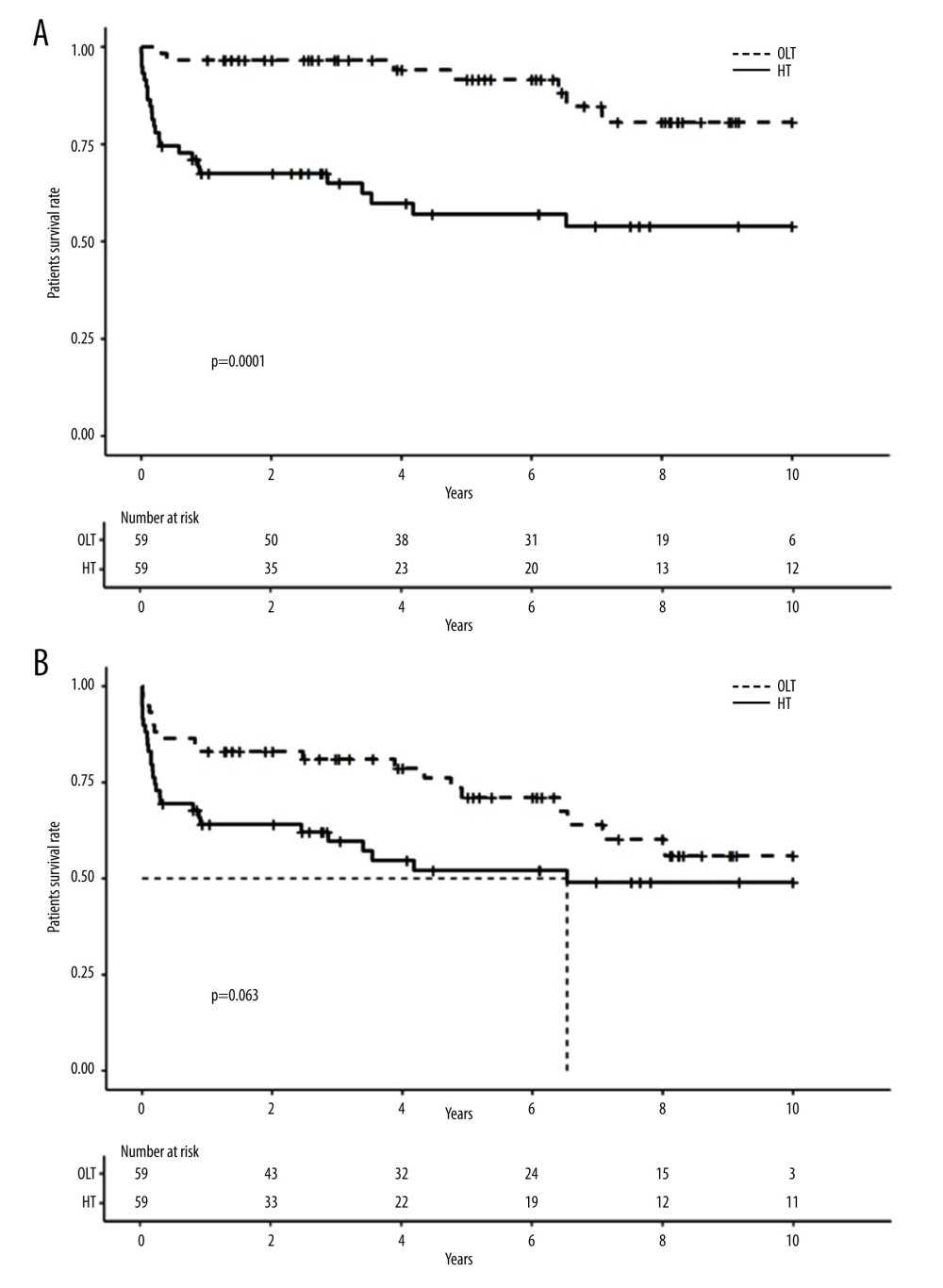 Figure 3. Long-term survival of patients and grafts after HT or orthotopic liver transplantation (OLT). (A). Long-term patient survival; (B) Long-term graft survival.
Figure 3. Long-term survival of patients and grafts after HT or orthotopic liver transplantation (OLT). (A). Long-term patient survival; (B) Long-term graft survival. Tables
Table 1. Hemitransposition case reports and short series.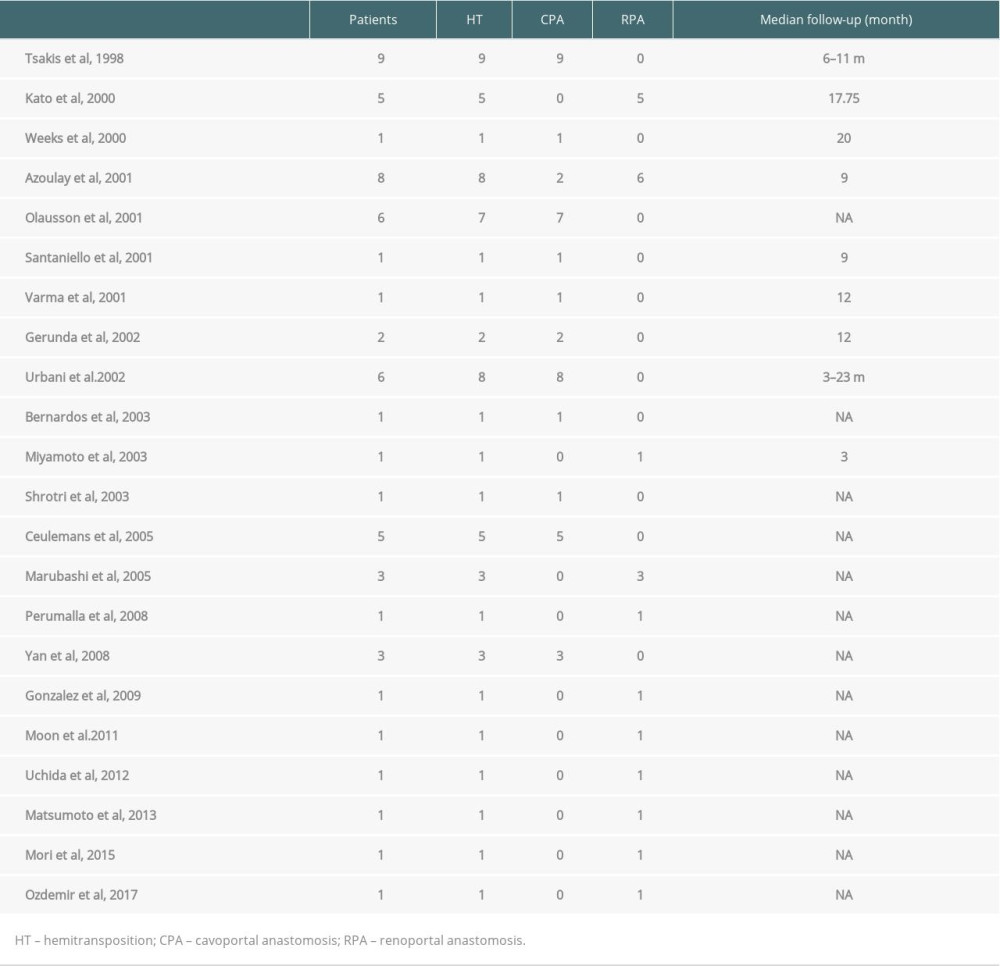 Table 2. Variables used for univariate analysis.
Table 2. Variables used for univariate analysis. Table 3. Patient, graft and perioperative characteristics.
Table 3. Patient, graft and perioperative characteristics.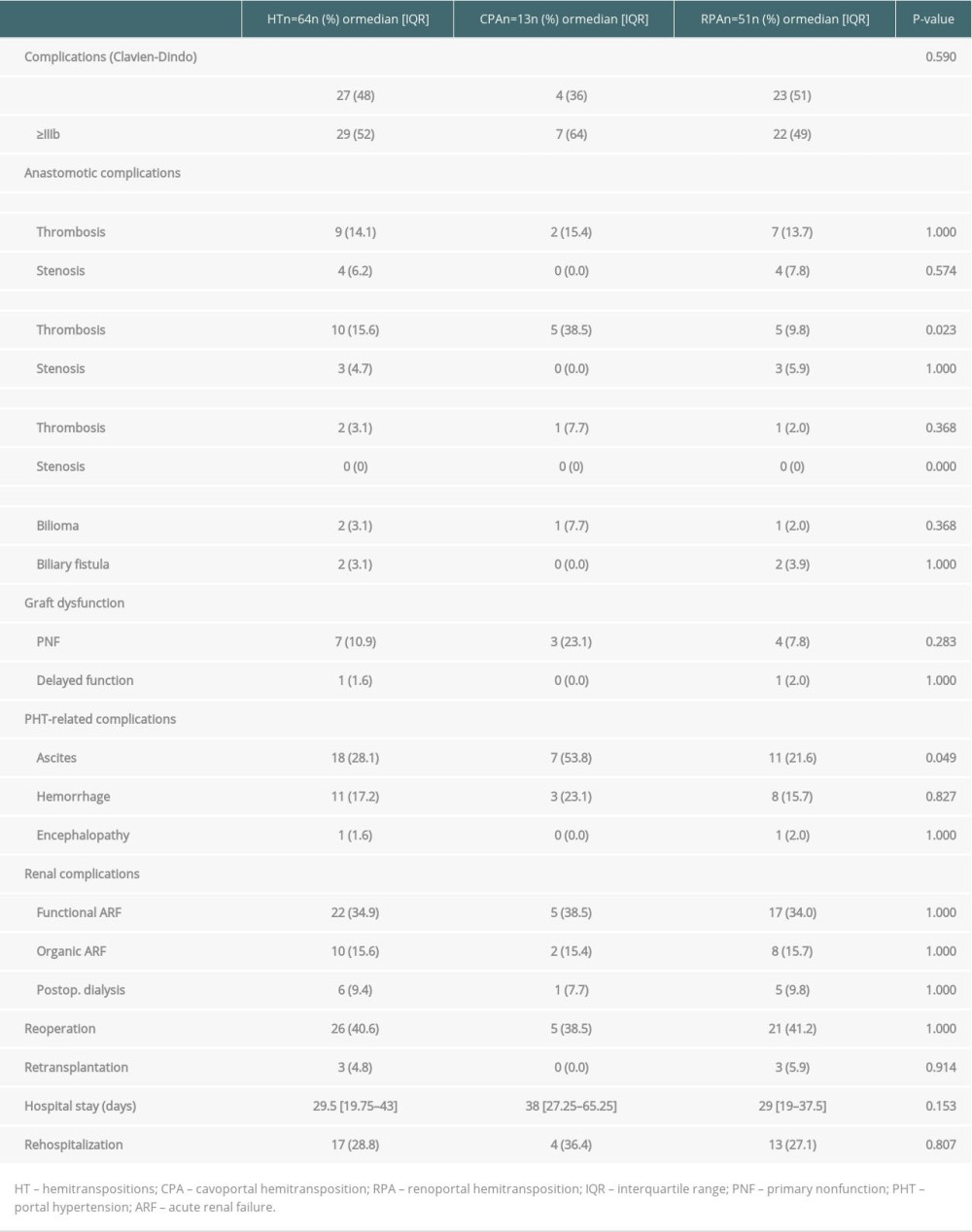 Table 4. Perioperative complications in HT.
Table 4. Perioperative complications in HT.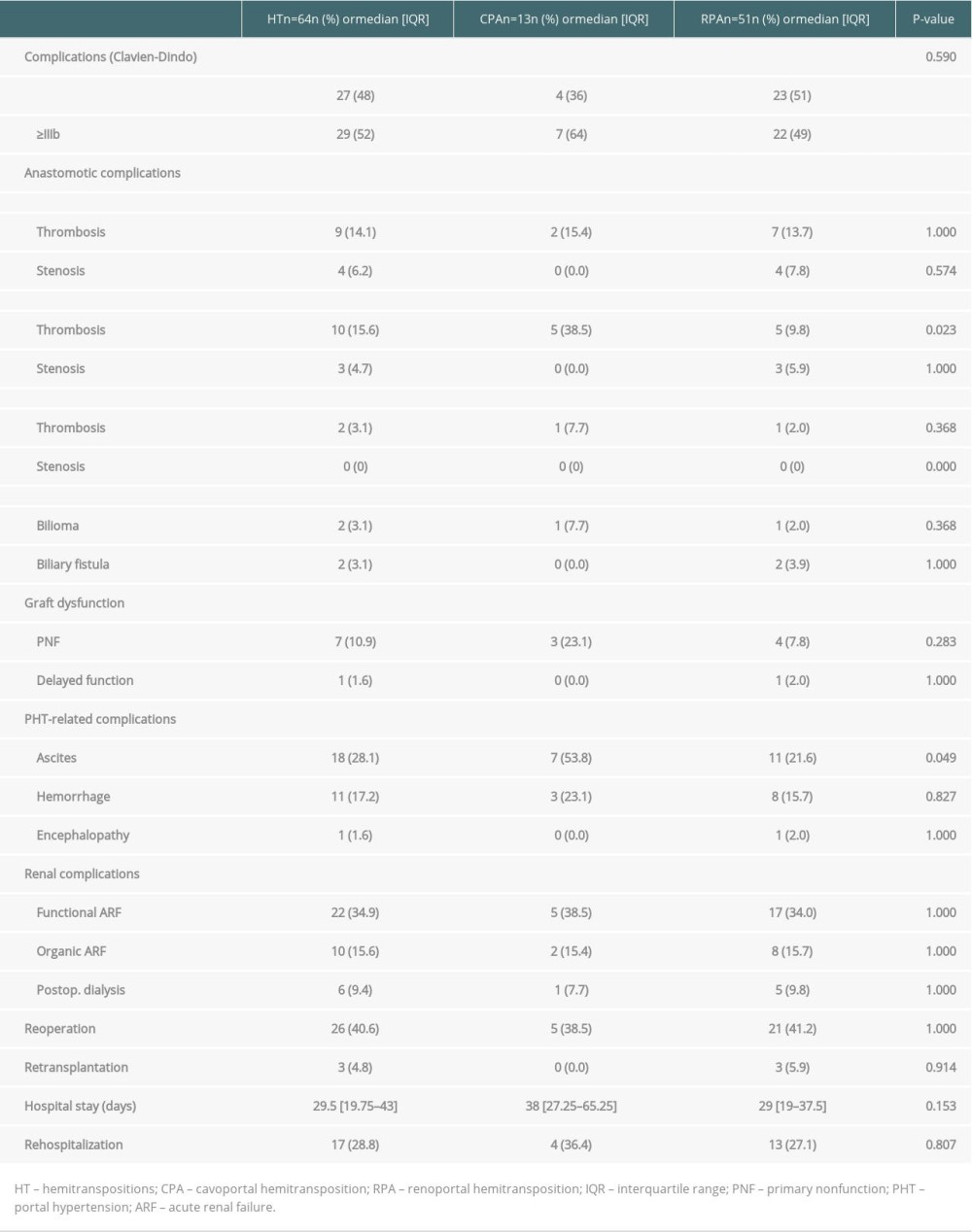 Table 5. Patient, graft and preoperative characteristics after HT or OLT.
Table 5. Patient, graft and preoperative characteristics after HT or OLT.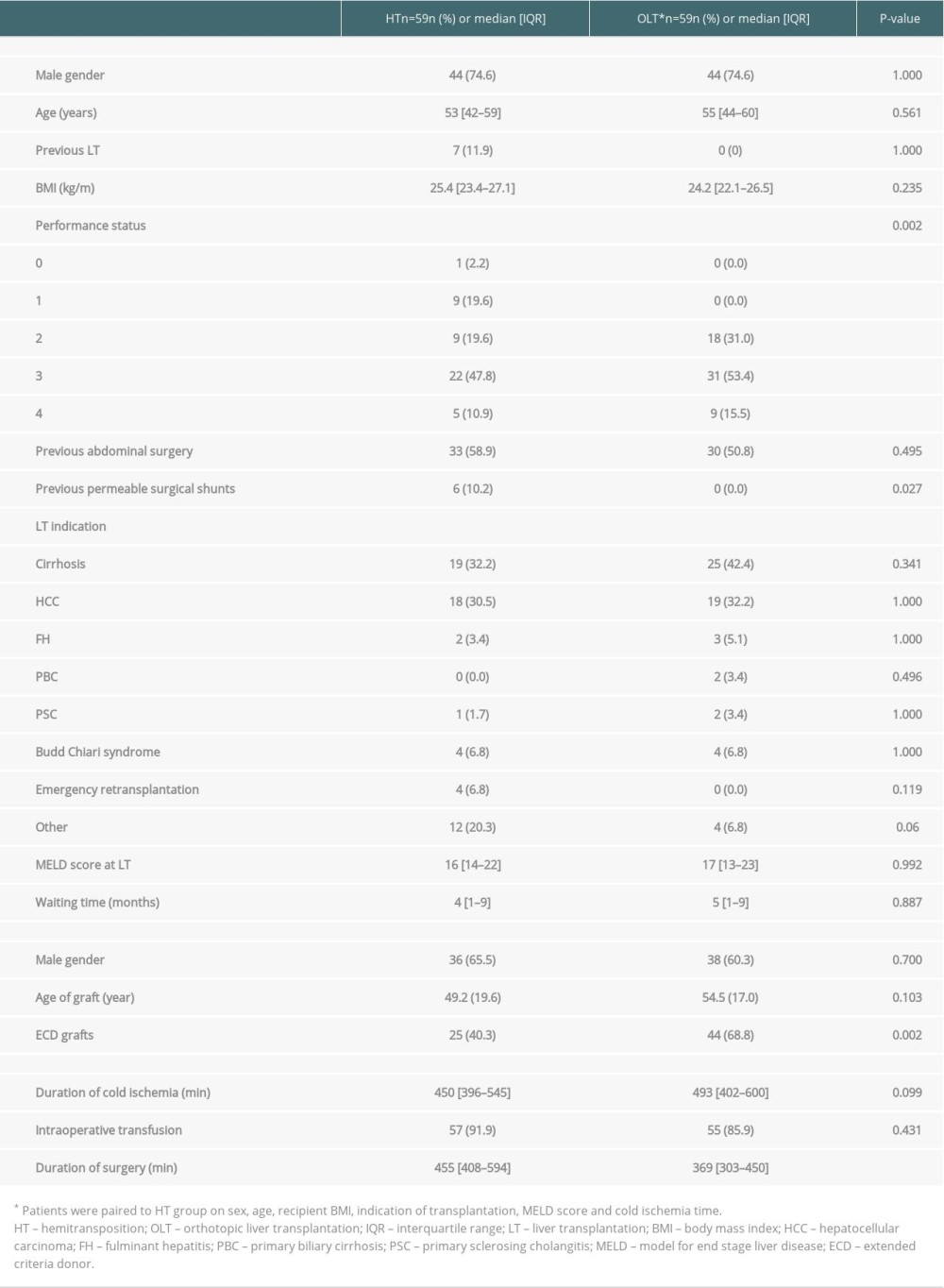 Table 6. Comparison of perioperative complications between the HT and OLT groups.
Table 6. Comparison of perioperative complications between the HT and OLT groups.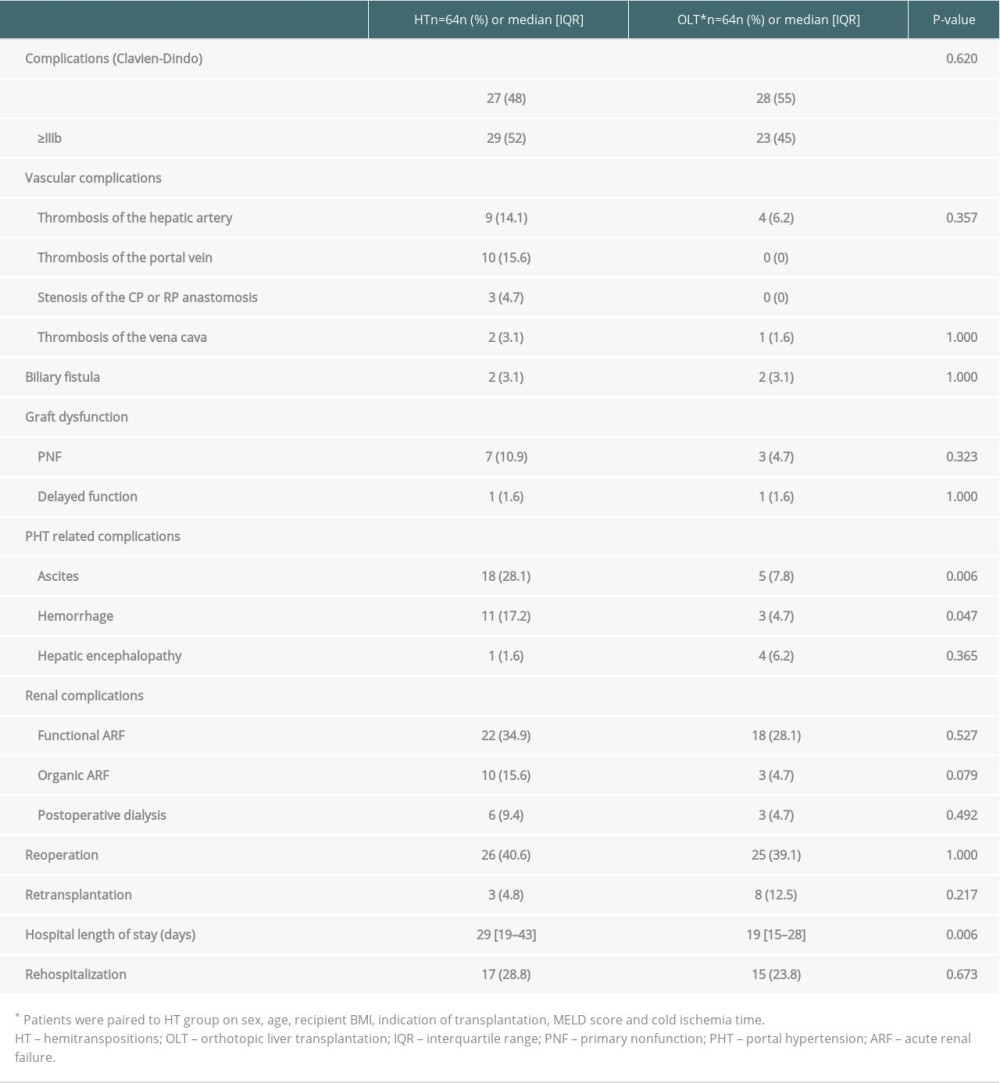 Table 7. Univariable and multivariable analysis of prognosis factors for patient survival after HT.
Table 7. Univariable and multivariable analysis of prognosis factors for patient survival after HT.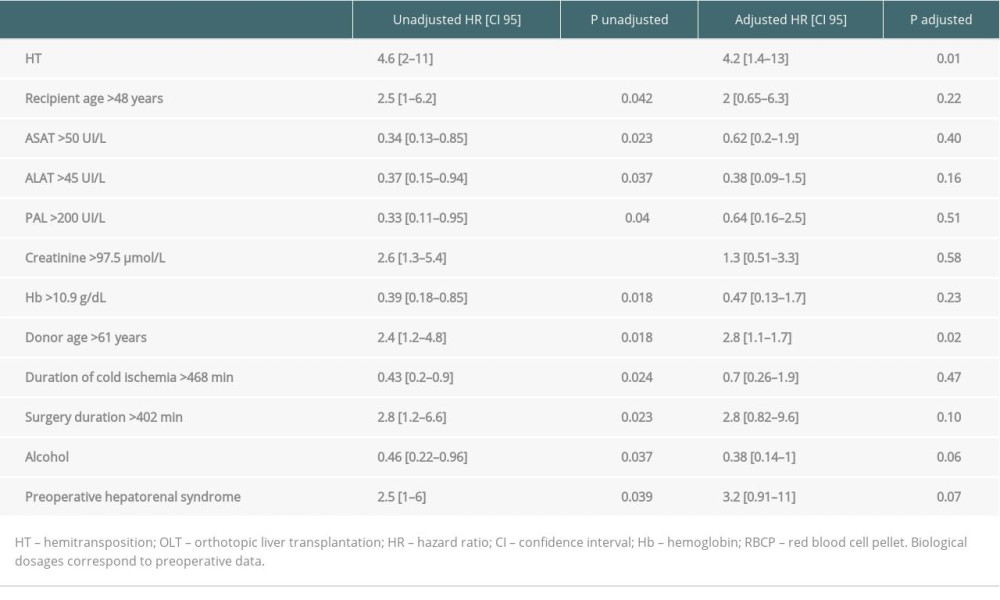
References
1. : Agence de la biomédecine https://rams.agence-biomedecine.fr/greffe-hepatique
2. Yerdel MA, Gunson B, Mirza D, Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome: Transplantation, 2000; 69(9); 1873-81
3. Bhangui P, Lim C, Salloum C, Caval inflow to the graft for liver transplantation in patients with diffuse portal vein thrombosis: A 12-year experience: Ann Surg, 2011; 254(6); 1008-16
4. Tao Y-F, Teng F, Wang Z-X, Liver transplant recipients with portal vein thrombosis: A single center retrospective study: Hepatobiliary Pancreat Dis Int, 2009; 8(1); 34-39
5. Sheil AG, Stephen MS, Chui AK, A liver transplantation technique in a patient with a thrombosed portal vein and a functioning renal-lieno shunt: Clin Transplant, 1997; 11(1); 71-73
6. Tzakis AG, Kirkegaard P, Pinna AD, Liver transplantation with cavoportal hemitransposition in the presence of diffuse portal vein thrombosis: Transplantation, 1998; 65(5); 619-24
7. Pinna AD, Nery J, Kato T, Liver transplant with portocaval hemitransposition: Experience at the University of Miami: Transplant Proc, 2001; 33(1–2); 1329-30
8. Selvaggi G, Weppler D, Nishida S, Ten-year experience in porto-caval hemitransposition for liver transplantation in the presence of portal vein thrombosis: Am J Transplant, 2007; 7(2); 454-60
9. Kato T, Levi DM, DeFaria W, Liver transplantation with renoportal anastomosis after distal splenorenal shunt: Arch Surg, 2000; 135(12); 1401-4
10. Weeks SM, Alexander JR, Sandhu J, Mechanic and pharmacologic treatment of a saddle embolus to the portal vein after liver transplantation and portacaval hemitransposition: Am J Roentgenol, 2000; 175(2); 537-39
11. Azoulay D, Adam R, Castaing DLiver transplantation with cavoportal or renoportal anastomosis: A solution in cases of diffuse portal thrombosis: Gastroenterol Clin Biol, 2002; 26(4); 325-30 [in French]
12. Olausson M, Norrby J, Mjörnstedt L, Liver transplantation using cavoportal hemitransposition – a life-saving procedure in the presence of extensive portal vein thrombosis: Transplant Proc, 2001; 33(1–2); 1327-28
13. Santaniello W, Ceriello A, Defez M, Liver transplant with cavoportal hemitransposition for portal and mesenteric thrombosis: Case report: Transplant Proc, 2001; 33(1–2); 1488-89
14. Varma CR, Mistry BM, Glockner JF, Cavoportal hemitransposition in liver transplantation: Transplantation, 2001; 72(5); 960-63
15. Gerunda GE, Merenda R, Neri D, Cavoportal hemitransposition: A successful way to overcome the problem of total portosplenomesenteric thrombosis in liver transplantation: Liver Transp, 2002; 8(1); 72-75
16. Urbani L, Cioni R, Catalano G, Cavoportal hemitransposition: Patient selection criteria and outcome: Transplant Proc, 2002; 34(8); 3331-33
17. Bernardos A, Serrano J, Gomez MA, Portal vein thrombosis: An emergency solution for blood flow in liver transplantation: Transpl Int, 2003; 16(8); 500-1
18. Miyamoto A, Kato T, Dono K, Living-related liver transplantation with renoportal anastomosis for a patient with large spontaneous splenorenal collateral: Transplantation, 2003; 75(9); 1596-68
19. Shrotri M, Sudhindran S, Gibbs P, Case report of cavoportal hemitransposition for diffuse portal vein thrombosis in liver transplantation: Transplant Proc, 2003; 35(1); 397-98
20. Ceulemans B, Aerts R, Monbaliu D, Liver transplantation using cavoportal transposition: An effective treatment in patients with complete splanchnic venous thrombosis: Transplant Proc, 2005; 37(2); 1112-14
21. Marubashi S, Dono K, Nagano H, Living-donor liver transplantation with renoportal anastomosis for patients with large spontaneous splenorenal shunts: Transplantation, 2005; 80(12); 1671-75
22. Perumalla R, Jamieson NV, Praseedom RK, Left renal vein as an option for portal inflow in liver transplant recipients with portal vein thrombosis: Transpl Int, 2008; 21(7); 701-3
23. Yan M-L, Zeng Y, Li B, Postoperative complications after liver transplantation with cavoportal hemitransposition: Hepatobiliary Pancreat Dis Int, 2008; 7(3); 322-24
24. González-Pinto IM, Miyar A, García-Bernardo C, Renoportal anastomosis as a rescue technique in postoperative portal thrombosis in liver transplantation: Transplant Proc, 2009; 41(3); 1057-59
25. Moon D-B, Lee S-G, Ahn C-S, Side-to-end renoportal anastomosis using an externally stented polytetrafluoroethylene vascular graft for a patient with a phlebosclerotic portal vein and a large spontaneous splenorenal shunt: J Am Coll Surg, 2011; 212(3); e7-11
26. Uchida H, Sakamoto S, Shigeta T, Living donor liver transplantation with renoportal anastomosis for a patient with congenital absence of the portal vein: Case Rep Surg, 2012; 2012; 670289
27. Matsumoto Y, Ikegami T, Morita K, Renoportal anastomosis in right lobe living donor liver transplantation: Report of a case: Surg Today, 2013; 43(11); 1316-20
28. Mori A, Iida T, Iwasaki J, Portal vein reconstruction in adult living donor liver transplantation for patients with portal vein thrombosis in single center experience: J Hepatobiliary Pancreat Sci, 2015; 22(6); 467-74
29. Ozdemir F, Kutluturk K, Barut B, Renoportal anastomosis in living donor liver transplantation with prior proximal splenorenal shunt: World J Transplant, 2017; 7(1); 94-97
30. Azoulay D, Quintini C, Rayar M, Renoportal anastomosis during liver transplantation in patients with portal vein thrombosis: First long-term results from a multicenter study: Ann Surg, 2021 [Online ahead of print]
31. Dindo D, Demartines N, Clavien P-A, Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey: Ann Surg, 2004; 240(2); 205-13
32. Madec J, Bouzillé G, Riou C, eHOP Clinical Data Warehouse: From a prototype to the creation of an Inter-Regional Clinical Data Centers Network: Stud Health Technol Inform, 2019; 264; 1536-37
33. van Buuren S, Groothuis-Oudshoorn K, MICE: Multivariate imputation by chained equations in R: Journal of Statistical Software, 2011; 45(3); 1-67
34. Cheng Y, Chen Y, Jiang Y, Zhang X, Ten-year survival after portal vein arterialization in liver transplantation: Ann Palliat Med, 2019; 8(5); 790-92
35. Abu-Elmagd KM, Costa G, Bond GJ, Five hundred intestinal and multivisceral transplantations at a single center: Major advances with new challenges: Ann Surg, 2009; 250(4); 567-81
36. Grant D, Abu-Elmagd K, Mazariegos G, Intestinal transplant registry report: Global activity and trends: Am J Transplant, 2015; 15(1); 210-19
37. Vianna RM, Mangus RS, Kubal C, Multivisceral transplantation for diffuse portomesenteric thrombosis: Ann Surg, 2012; 255(6); 1144-50
Figures
 Figure 1. Hemitransposition anastomosis. (A) Renoportal anastomosis; (B). Cavoportal anastomosis. * Splenoral spontaneous or surgical shunt. ** Staplle line on the IVC downstream to the cavoportal anastomosis.
Figure 1. Hemitransposition anastomosis. (A) Renoportal anastomosis; (B). Cavoportal anastomosis. * Splenoral spontaneous or surgical shunt. ** Staplle line on the IVC downstream to the cavoportal anastomosis. Figure 2. Long-term survival of patients and grafts after hemitransposition (HT) using cavoportal anastomosis (CPA) or renoportal anastomosis (RPA). (A). Long-term patient survival; (B) Long-term graft survival.
Figure 2. Long-term survival of patients and grafts after hemitransposition (HT) using cavoportal anastomosis (CPA) or renoportal anastomosis (RPA). (A). Long-term patient survival; (B) Long-term graft survival. Figure 3. Long-term survival of patients and grafts after HT or orthotopic liver transplantation (OLT). (A). Long-term patient survival; (B) Long-term graft survival.
Figure 3. Long-term survival of patients and grafts after HT or orthotopic liver transplantation (OLT). (A). Long-term patient survival; (B) Long-term graft survival. Tables
 Table 1. Hemitransposition case reports and short series.
Table 1. Hemitransposition case reports and short series. Table 2. Variables used for univariate analysis.
Table 2. Variables used for univariate analysis. Table 3. Patient, graft and perioperative characteristics.
Table 3. Patient, graft and perioperative characteristics. Table 4. Perioperative complications in HT.
Table 4. Perioperative complications in HT. Table 5. Patient, graft and preoperative characteristics after HT or OLT.
Table 5. Patient, graft and preoperative characteristics after HT or OLT. Table 6. Comparison of perioperative complications between the HT and OLT groups.
Table 6. Comparison of perioperative complications between the HT and OLT groups. Table 7. Univariable and multivariable analysis of prognosis factors for patient survival after HT.
Table 7. Univariable and multivariable analysis of prognosis factors for patient survival after HT. Table 1. Hemitransposition case reports and short series.
Table 1. Hemitransposition case reports and short series. Table 2. Variables used for univariate analysis.
Table 2. Variables used for univariate analysis. Table 3. Patient, graft and perioperative characteristics.
Table 3. Patient, graft and perioperative characteristics. Table 4. Perioperative complications in HT.
Table 4. Perioperative complications in HT. Table 5. Patient, graft and preoperative characteristics after HT or OLT.
Table 5. Patient, graft and preoperative characteristics after HT or OLT. Table 6. Comparison of perioperative complications between the HT and OLT groups.
Table 6. Comparison of perioperative complications between the HT and OLT groups. Table 7. Univariable and multivariable analysis of prognosis factors for patient survival after HT.
Table 7. Univariable and multivariable analysis of prognosis factors for patient survival after HT. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








