30 August 2022: Original Paper
Clinical Validation of a Novel Scoring System Based on Donor and Recipient Risk Factors for Predicting Outcomes in Liver Transplantation
Lucas Souto Nacif1ABCDEF*, Daniel Reis Waisberg1CDE, Leonardo Yuri Zanini1BCDEF, Rafael Soares Pinheiro1ACD, Vinicius Rocha-Santos1ACD, Rubens Arantes Macedo1BD, Liliana DucattiDOI: 10.12659/AOT.936271
Ann Transplant 2022; 27:e936271
Abstract
BACKGROUND: Adequate donor and recipient matching in liver transplantation is crucial to improve patient survival. Our objective was to propose and validate a new model for predicting outcomes using donor and recipient scoring criteria.
MATERIAL AND METHODS: We retrospectively analyzed data of all patients (n=932) who underwent liver transplantation (n=1106) from January 2006 to December 2018. For score standardization, 30% (n=280) of patients were randomly selected for analysis and divided into 3 categories: ≤4 points, 5 to 8 points, and >8 points. Scoring system validation was performed on a dataset with 70% (n=652) of the patients.
RESULTS: Survival of the stratified group (30%) was significant (P<0.001). Scores of 4 to 8 points presented lower risk of death (1.74 [CI 0.97-3.13; P=0.062]), while >8 points presented higher risk (2.74 [CI 1.36-5.57; P=0.005]). In the validation score (70%), global survival was significant (P<0.0016); patients with scores of 4 to 8 points had lower risk of death (1.16 [CI 1.16-2.38; P=0.005]); and scores >8 points (2.22 [CI 1.40-3.50; P<0.001]), retransplant, fulminant hepatitis, previous large abdominal/biliary tree surgery, MELD score, and serum creatinine before liver transplantation >1.5 mg/dL (P<0.05) presented higher risk. Individual recipient factors with 4 to 8 points had a lower risk of death (2.29 [CI 1.82-2.87; P<0.0001]) than those with scores >8 points (4.02 [CI 2.22-7.26; P<0.0001]).
CONCLUSIONS: A novel prognostic-based scoring system using donor and recipient characteristics was proposed and clinically validated. Two-factor scoring indicated the superiority of the predictability outcome and improved prediction of higher mortality.
Keywords: Graft Survival, Liver Transplantation, Survival Analysis, Humans, Prognosis, Risk Factors, Tissue Donors
Background
Widely regarded as a model of justice and equality for organ allocation in liver transplantation (LT), the model of end-stage liver disease (MELD) score and its variations, such as the MELD-sodium (Na) and United Kingdom model for end-stage liver disease, use the principle of the sickest first. These allocation systems have been a useful tool in reducing mortality in LT waiting lists [1,2]. Moreover, it has been advocated to push the benefit of the transplant even further to very sick patients [3].
The increasing demand of patients on waiting lists, which is associated with the continuous expansion of indications, such as cholangiocarcinoma, colorectal liver metastasis, and acute alcoholic hepatitis, have been pushing LT services to the utilization of extended criteria liver grafts [4]. The combination of having the sickest patients and extended criteria liver graft has been associated with inferior outcomes [5,6].
Because the scores based on the “sickest first” policy are not the best to predict LT outcomes, utility-based systems, such as D-MELD, donor risk index, and others, have been investigated [2,6]. However, to date, no model has achieved the best donor and recipient match. Even though donors’ and recipients’ risk factors are well known, an ideal model relating them to transplant outcomes remains to be established, especially in Brazil, which has a high volume of liver transplants, with 2050 completed in 2020 [7]. Some regions, such as the state of São Paulo, the most populated area of the country, face an imbalance of donor availability and recipient demand, leading to organ scarcity. Brazil had 18.1 effective donors per million in 2020 [7]. In this context, it is crucial to better understand donor and recipient matching to improve graft utilization and posttransplant results. Aiming to investigate donor and recipient data and their correlation to LT results in our service, we performed this retrospective observational study to propose a new model for predicting outcomes.
Material and Methods
STUDY DESIGN:
We retrospectively analyzed clinical and laboratorial data of all consecutive patients who underwent LT in our center from January 2006 to December 2018. A total of 1101 LTs were performed, including 958 deceased donor LT (DDLT), 92 living donor LTs, 45 combined liver-kidney transplants, and 6 domino LTs. For DDLT, there were 932 patients who underwent 958 transplants. The number of transplants in DDLT was not the same as the number of patients because 21 patients underwent retransplant and another 5 patients needed a new transplant, thus totaling 958 LTs. The retransplant analysis considered just the first retransplant, therefore excluding eventual third LTs, since they were not common and in general occur a long time after the second LT. For building our model, we included all patients with DDLT, and randomly divided the patients into 2 stages: an initial group, 30% sample (n=280) and validation group, 70% of the patients (n=652).
STATISTICAL ANALYSIS:
The data analysis started with a descriptive exploration. Tables including a 95% confidence interval (95% CI) were used to summarize the results of qualitative variables. Quantitative variables were described using the mean, standard deviation (SD), median, and 25th (P25) and 75th (P75) percentiles.
Based on the clinical and laboratory characteristics of the patients, a scoring system was created. These scores were calculated using discrete quantitative variables. The final version was divided into 3 categories: ≤4 points, 5 to 8 points, and >8 points. In the first stage of the research, to standardize the score, a bank with 30% of the total number of patients was randomly selected for the analysis and adjustment of the score (initial group, 30% bank). In the second stage of the analysis, the score as well as the other variables were tested on a dataset with 70% of the patients (validation group, 70% bank
The risk factors related to recipient were analyzed separately, therefore excluding donor condition. The groups were separated by strata of scores as <4 points, 4 to 8 points, and >8 points. After, the scoring system using just the recipient factor was compared with the scoring using both donor and recipient scores.
The survival function considering the average estimate of time until patients reached death was performed using the Kaplan-Meier method. The hypothesis test for equality of means according to factors was performed using the non-parametric log-rank test (Mantel-Cox). Gross and adjusted hazard ratio (HR) estimates were performed using Cox regression.
All tests performed used a bidirectional α of 0.05 and a 95% CI. Analyses were done with IBM SPSS 25 (Statistical Package for the Social Sciences; IBM Corp, Armonk, NY, USA), Excel 2016 (Microsoft Office), and R package (R Core Team, Vienna, Austria).
PARAMETERS OF POINT SCORING: The factors of deceased donors analyzed for the scoring system were age ≥60 years (1 point), ≥65 years (2 points), ≥70 years (3 points), and <60 years old (0 point); pH <7.2 (1 point) and pH ≥7.2 (0 points); cardiorespiratory arrest >2 min (1 point) and without cardiorespiratory arrest (0 points); noradrenaline >1 mcg/kg/min (1 point) and without noradrenaline or <1 mcg/kg/min (0 points); moderate/severe liver steatosis (>30%, grade II/III; 1 point) and mild/absent (grade I or absence; 0 points); Intensive Care Unit (ICU) time >7 days (1 point) and ICU time <7 days (0 point) (Table 1).
The factors of recipient used for the scoring system were age ≥60 years (1 point), ≥65 years (2 points), and ≥70 years (3 points); liver retransplantation (2 points) and without retransplant (0 points); fulminant liver failure (2 points) and without fulminant liver failure (0 points); portal vein thrombosis (1 point) and without portal vein thrombosis (0 points); previous major abdominal surgery (1 point) and without previous surgery (0 point); MELD <24 (0 points), MELD ≥24 (1 point), MELD ≥29 (2 points), and MELD ≥35 (3 points); hemodialysis before LT or acute renal disease (creatinine >1.5 mg/dL) (2 points) and without hemodialysis or acute renal disease (0 points); severe cardiac risk (2 points) and mild/moderate cardiac risk (0 points); hospitalization for complications of cirrhosis (spontaneous bacterial peritonitis, upper digestive hemorrhage, cholangitis, hepatic encephalopathy, infection) (1 point) and without hospitalization (0 points) (Table 1).
PROGNOSTIC AND OTHER FACTORS:
Intraoperative factors evaluated were warm, cold, and total ischemia time, surgery time, temporary portal cava shunt performance, and intraoperative transfusion of fresh frozen plasma packs, red blood cells, and platelet concentrate packs.
In postoperative evaluation, we analyzed the serum creatinine levels in the first and third day after surgery, the total and ICU length of stay, and patient and graft survival after LT. Postoperative complications were classified according to the Clavien-Dindo score [8].
Results
INITIAL STAGE (30% OF THE SAMPLE):
In this group, 280 patients who underwent DDLT between January 2006 and December 2018 were evaluated. A total of 159 individuals were still alive until the end of this analysis, 77 had died, 23 were lost to follow-up, and 21 had undergone retransplantation. The global survival in this group is shown in Figure 1.
Each patient in this group received a score determined by the risk factors described above. The survival of the stratified group according the scoring system is shown in Figure 2 (P<0.001). Patients with scores of 4 to 8 points had a lower risk of death (1.74 [CI 0.97–3.13; P=0.062]), while those with scores >8 points had higher risk (2.74 [CI 1.36–5.57; P=0.005]).
The variables with P values <0.05 in the log-rank test were cardiopulmonary arrest >5 min, ≥7 days in ICU (donor), fulminant hepatitis, MELD (24–29), MELD (29–35), MELD (≥35), and serum creatinine before LT >1.5 mg/dL (Tables 2, 3).
VALIDATION STAGE (70% OF THE SAMPLE):
For validation of the score, we evaluated 652 patients who underwent DDLT in the same period as the first analysis group. A total 359 individuals were still alive until the end of this analysis, 177 had died, 46 were lost to follow-up, and 70 had undergone retransplantation. The global survival in this group is shown in Figure 3.
The risk factor scores applied in this validation group and the survival of the stratified groups according the scoring system are shown in Figure 4 (P<0.0016). Patients with scores of 4 to 8 points had a lower risk of death (1.16 [CI 1.16–2.38; P=0.005]), and those with scores >8 points had a higher risk (2.22 [CI 1.40–3.50; P<0.001]).
The variables with P values <0.05 in the log-rank test were retransplant, fulminant hepatitis, previous large abdominal/biliary tree surgery, MELD (24–29), MELD (29–35), MELD (≥35), and serum creatinine before LT >1.5 mg/dL (Table 4).
RECIPIENT DEPENDENT 1-FACTOR SCORING ANALYSIS:
For study of mortality with recipient factors isolated, we evaluated the total of 932 patients who underwent DDLT in the study period. A total of 254 patients died, 69 were lost to follow-up, and 91 underwent retransplantation. The risk factor scores applied in this group and survival were stratified using Kaplan-Meyer curves according the scoring system (Figure 5; P<0.0001). Individuals with scores of 4 to 8 points had a lower risk of death (2.29 [CI 1.82–2.87; P=0.0001]) than those with scores >8 points, who had a higher risk of death (4.02 [CI 2.22–7.26; P<0.0001]).
The variables with
COMPARING SCORE SYSTEM-RECIPIENT DEPENDENT 1-FACTOR SCORING VS DONOR-RECIPIENT 2-FACTOR SCORING:
The receiver operating characteristic (ROC) curve was drawn to compare the competence of these scores to predict mortality in liver transplantation. The area under the curve (AUC) using just the donor factors was 0.680 (P<0.001), while the AUC in donor-recipient 2-factor scoring was 0.70 (P<0.001; Figure 6). Both scoring systems individually showed a worst result with higher scores.
To demonstrate the distribution of our data, we comparatively evaluated the mortality involving each of the strata of scores of each type of scoring system (1-factor scoring vs 2-factor scoring; Table 4; Figure 7), which more effectively indicated the difference (superiority) of the predictability of outcomes of liver transplantation of the new scoring method (Table 5; Figure 7).
Discussion
It is paramount that LT offers good survival rates for patients after the procedure. On the other hand, it is also important to look after patients on the waiting list, allowing as many patients as possible to get the benefit of the transplant. This is the challenge that surgeons, hepatologists, and ICU staff face on a daily basis. Organ shortages, especially in some countries like Brazil, push transplant teams to use expanded criteria liver grafts. In our study, we developed a scoring system and validated it using clinical data in order to stratify patient groups to aid the organ allocation system.
Our hospital is one of the largest transplant centers in South America, currently performing more than 120 LT per year. This long-term study analyzed the data of 1101 patients (959 DDLT, 92 living donor LT, 45 combined liver-kidney transplants, and 6 domino LT). Optimized donor-recipient matching is still necessary to improve graft and patient survival. In this study, we identified prognostic factors for donor and recipient selection that may aid in avoiding futile LT.
Donor variables that have been previously identified as risk factors for early graft dysfunction include age (≥60 years), female sex (especially in male recipients), steatosis, race, elevated liver function tests, hypotension/increased vasopressor use, non-heart-beating donor (also known as donation after cardiac death donors), split liver grafts, elevated serum sodium levels, and prolonged cold ischemia time (>12 h) [9–12]. However, we have also identified others important donor variables as prognostic factors, such as cardiopulmonary arrest >5 min and donor ICU stay ≥7 days. On the other hand, we identified some recipient variables associated with worse prognosis, such as fulminant hepatitis, MELD (24–29), MELD (29–35), MELD (>35), and creatinine level before transplant >1.5 mg/dL. When using the combination of those risk factors, we developed a scoring system that significantly correlated to the clinical data. Patients with 4 to 8 points indeed presented a lower probability of death than those with more than 8 points. These findings may assist in conducting better patient selection for LT and in performing more adequate donor-recipient matching, which may ultimately not only improve posttransplant results but also decrease mortality on the waiting list.
This discussion of the literature makes a few points clear: no matter whether the allocation policy offers the graft directly to the patient or to the center, determining “who should get the liver graft” is increasingly being done with more sophisticated prognostic models based on patients’ specific data rather than defined measures [13–15]. In the present study, we proposed, developed, and validated a novel prognostic-based scoring system. Patients with scores of 4 to 8 points had a lower risk of death and those with score >8 points had a higher risk. Organ allocation systems with optimized donor and recipient selection may increase graft survival and reduce waiting list mortality.
The addition of donor factors in these scores resulted in a significant gain in the ROC curve. We observed that the donor-recipient 2-factor scoring in lower scores was able to determine better outcomes and to indicate a worst result with higher scores. The recipient-dependent 1-factor scoring demonstrated less accuracy of lower scores to indicate fewer deaths. The 2-factor scoring indicate the superiority of the predicting the outcome of liver transplantation. The model was able to predict worse results in liver transplantation. More donor variables must be studied and added to improve the capacity of this model.
As a single-center retrospective analysis, this study had some limitations. However, it proposes a novel prognostic-based scoring system for liver transplant recipients, which has been clinically validated. We hope that our findings can aid liver transplant centers worldwide to improve the matching of recipients and donors. This study may thus ultimately contribute to the increase in patient and graft survival.
Conclusions
In this study, we identified important risk factors in donor-recipient liver transplantation matching. A novel prognostic-based scoring system was proposed and clinically validated. The 2-factor scoring system was superior in predicting the outcome of liver transplantation. The additive analysis using donor factors (2-factor scoring) improved the capacity to predict higher mortality. Patients with scores of 4 to 8 points had a lower risk of death, and those with scores >8 points had a higher risk. Organ allocation systems with optimized donor and recipient selection may increase graft survival and reduce waiting list mortality.
Figures
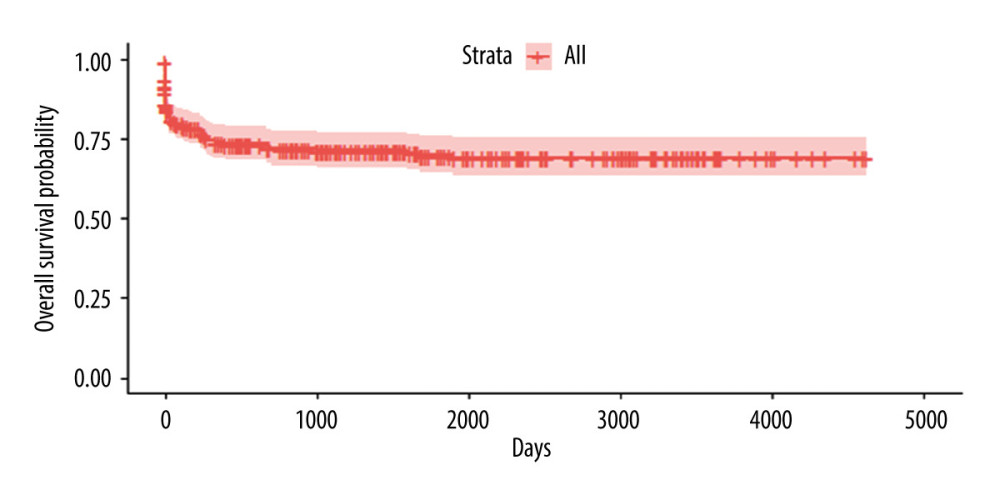 Figure 1. Kaplan-Meyer survival curve of patients who underwent DDLT – 30% sample, randomly selected.
Figure 1. Kaplan-Meyer survival curve of patients who underwent DDLT – 30% sample, randomly selected. 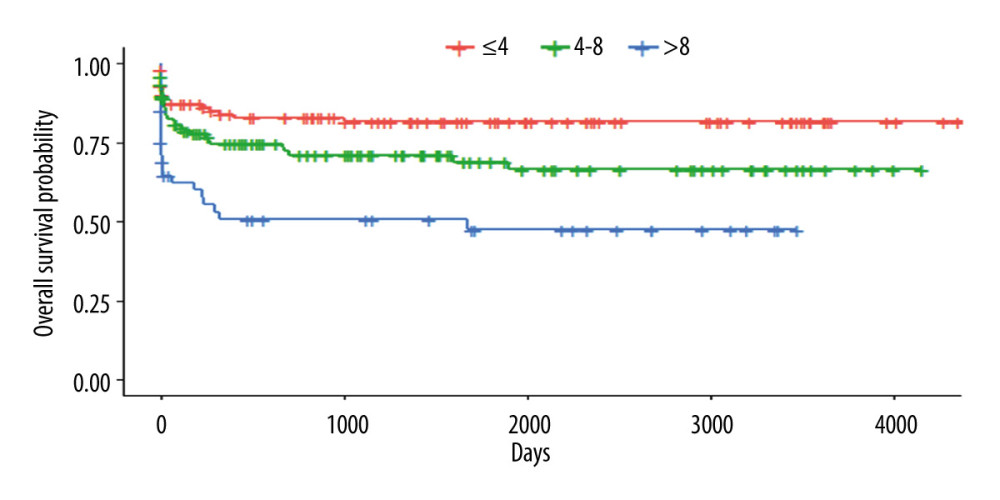 Figure 2. Kaplan-Meyer survival curves according to the scoring system – 30% sample, randomly selected (P<0.001).
Figure 2. Kaplan-Meyer survival curves according to the scoring system – 30% sample, randomly selected (P<0.001). 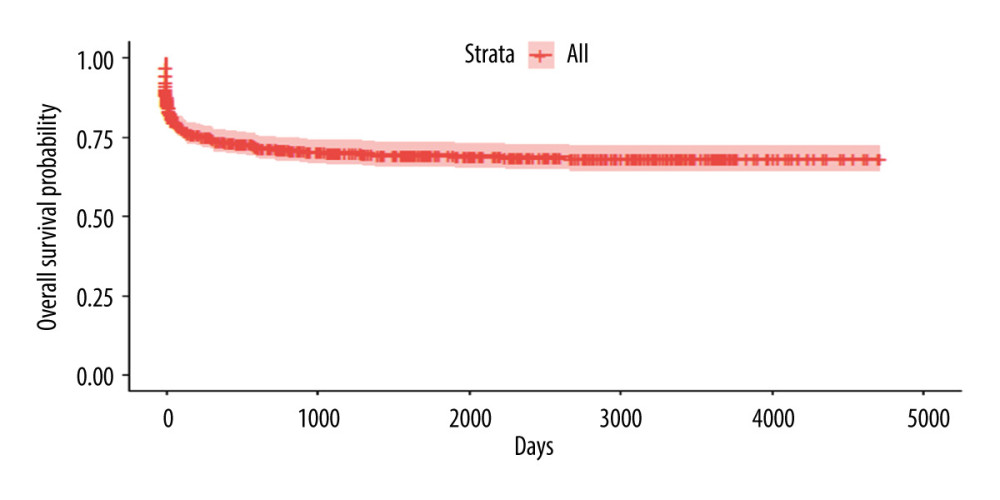 Figure 3. Kaplan-Meyer survival curve of patients who underwent DDLT – 70% sample, randomly selected.
Figure 3. Kaplan-Meyer survival curve of patients who underwent DDLT – 70% sample, randomly selected. 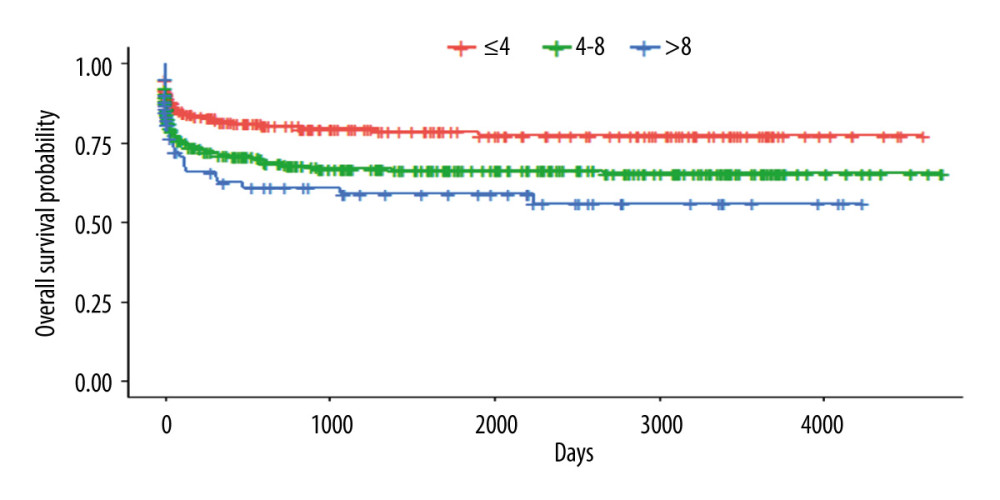 Figure 4. Kaplan-Meyer survival curves according to the scoring system – 70% sample, randomly selected (P<0.0016).
Figure 4. Kaplan-Meyer survival curves according to the scoring system – 70% sample, randomly selected (P<0.0016). 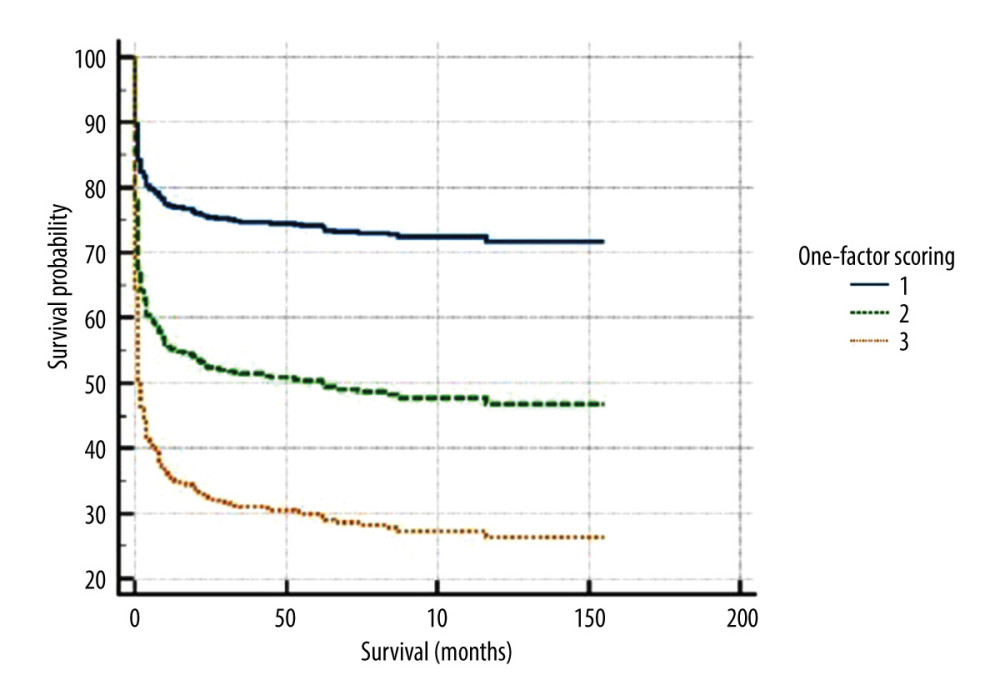 Figure 5. Kaplan-Meyer in recipient-dependent 1-factor scoring.
Figure 5. Kaplan-Meyer in recipient-dependent 1-factor scoring. 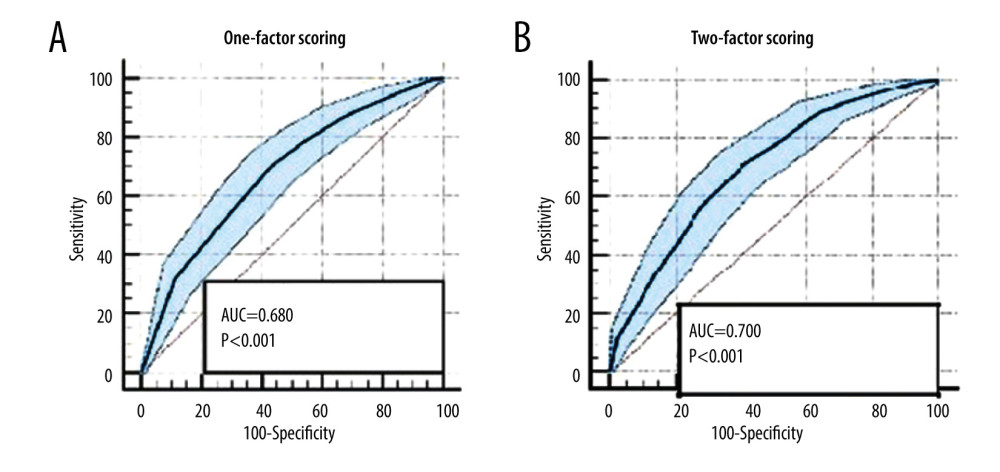 Figure 6. (A, B) Receiver operating characteristic curve of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring.
Figure 6. (A, B) Receiver operating characteristic curve of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring. 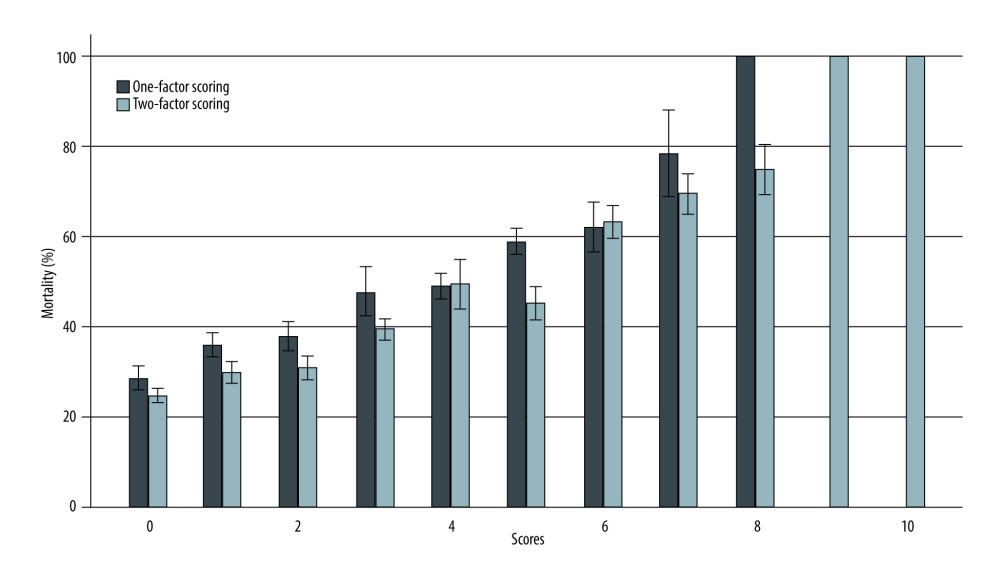 Figure 7. Mortality histogram of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring.
Figure 7. Mortality histogram of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring. Tables
Table 1. Score parameters based on donors’ and recipients’ risk factors.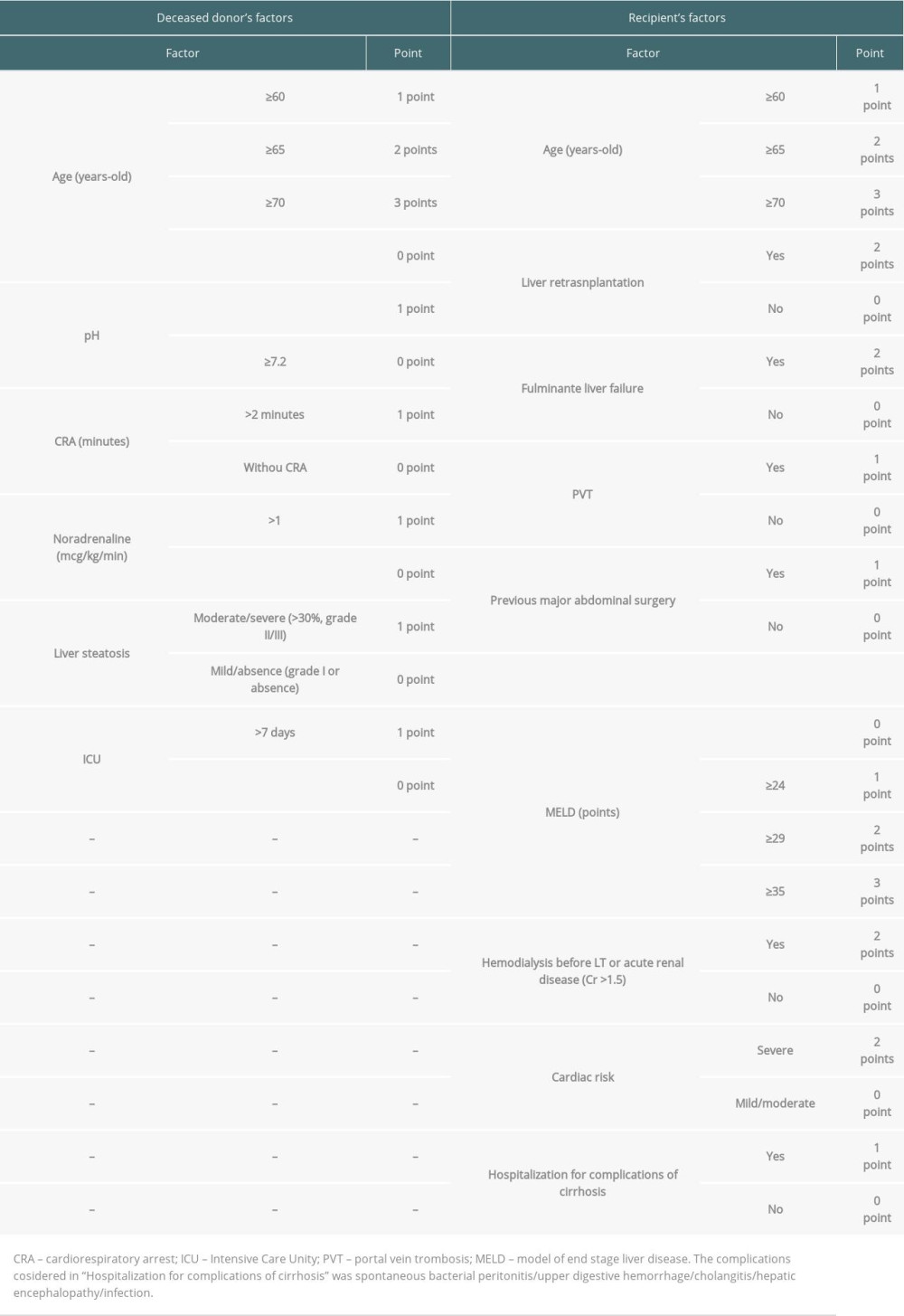 Table 2. Log-rank test of all scores variables (30% bank randomly selected).
Table 2. Log-rank test of all scores variables (30% bank randomly selected).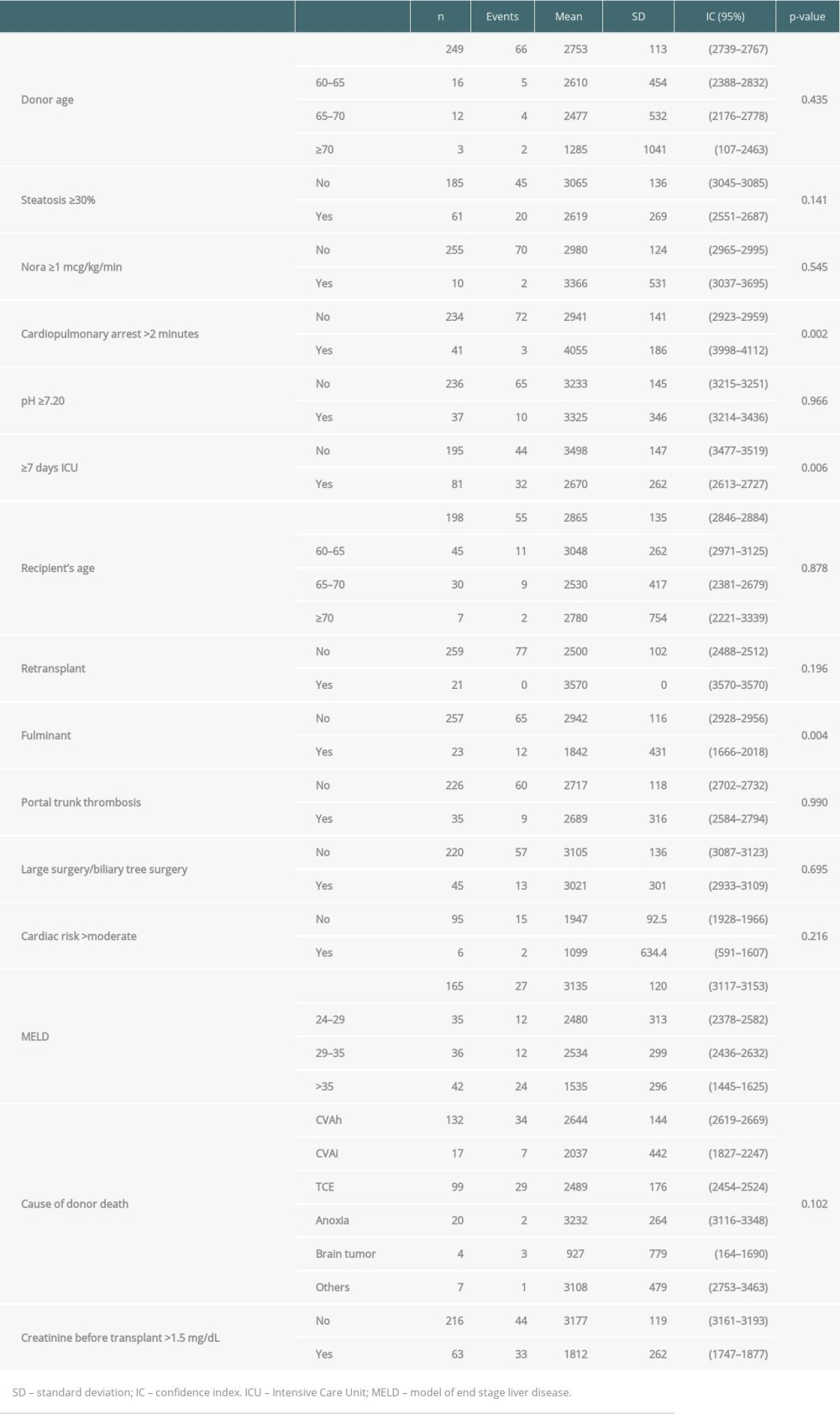 Table 3. COX regression analysis (30% bank randomly selected).
Table 3. COX regression analysis (30% bank randomly selected).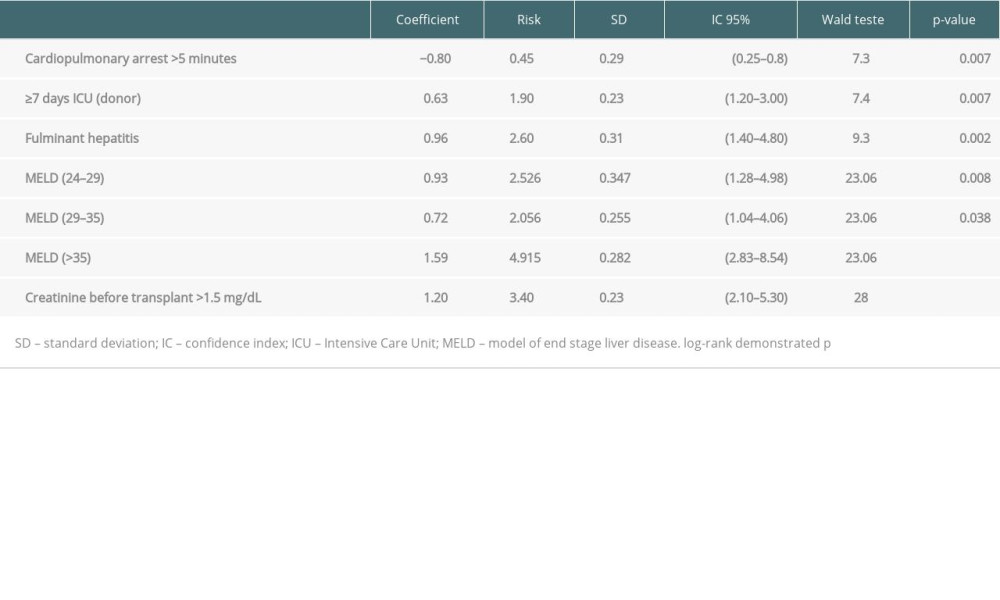 Table 4. COX regression analysis (validation group – 70% bank randomly selected).
Table 4. COX regression analysis (validation group – 70% bank randomly selected).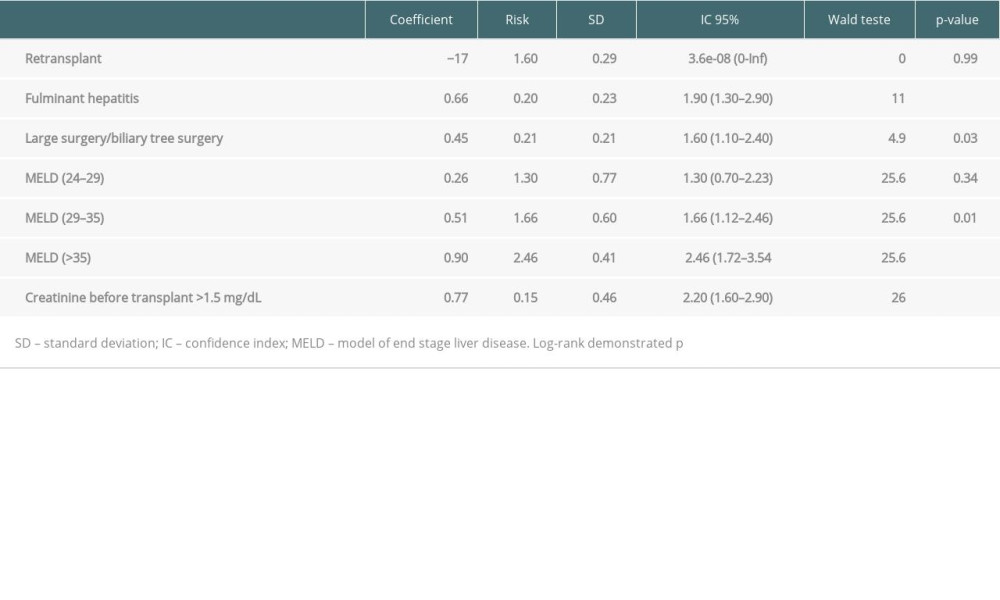 Table 5. Death rate according scores and comparison of groups.
Table 5. Death rate according scores and comparison of groups.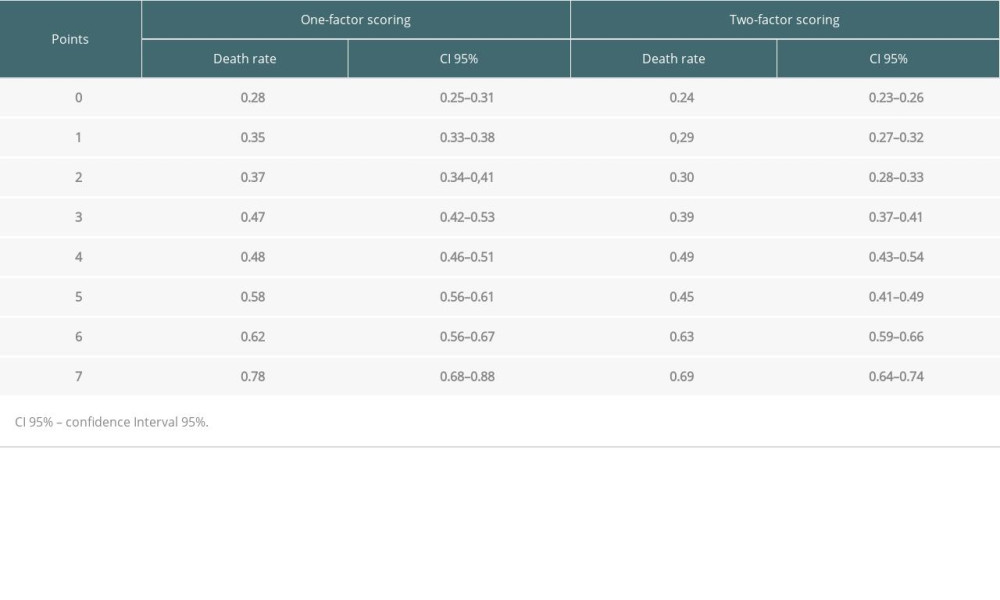
References
1. Freeman RB, Jamieson N, Schaubel DE, Who should get a liver graft?: J Hepatol, 2009; 50(4); 664-73
2. Cholongitas E, Burroughs AK, The evolution in the prioritization for liver transplantation: Ann Gastroenterol, 2012; 25(1); 6-13
3. Nadim MK, DiNorcia J, Ji L, Inequity in organ allocation for patients awaiting liver transplantation: Rationale for uncapping the model for end-stage liver disease: J Hepatol, 2017; 67(3); 517-25
4. Vodkin I, Kuo A, Extended criteria donors in liver transplantation: Clin Liver Dis, 2017; 21(2); 289-301
5. Nacif LS, Pinheiro RS, Rocha-Santos V, Better selection criteria with prognostic factors for liver transplantation: Transplant Proc, 2018; 50(3); 766-68
6. Haddad L, Cassenote AJF, Andraus W, Factors associated with mortality and graft failure in liver transplants: A hierarchical approach: PLoS One, 2015; 10(8); e0134874
7. Registro Brasileiro de Transplantes (RBT): Associação Brasileira de Transplantes de Órgãos (ABTO), 2020, Sao Paulo, RBT Jan a Dez/ABTO. Available from: https://site.abto.org.br/publicacao/xxvi-no-4-anual/
8. Dindo D, Demartines N, Clavien PA, Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey: Ann Surg, 2004; 240(2); 205-13
9. Zarrinpar A, Busuttil RW, Liver transplantation: Past, present and future: Nat Rev Gastroenterol Hepatol, 2013; 10(7); 434-40 Vol. 10
10. Renz JF, Kin C, Kinkhabwala M, Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation: Ann Surg, 2005; 242(4); 556-65
11. Tector AJ, Mangus RS, Chestovich P, Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival: Ann Surg, 2006; 244(3); 439-50
12. Feng S, Goodrich NP, Bragg-Gresham JL, Characteristics associated with liver graft failure: The concept of a donor risk index: Am J Transplant, 2006; 6(4); 783-90
13. Dutkowski P, Oberkofler CE, Slankamenac K, Are there better guidelines for allocation in liver transplantation?: A novel score targeting justice and utility in the model for end-stage liver disease era: Ann Surg, 2011; 254(5); 745-53
14. Halazun KJ, Quillin RC, Rosenblatt R, Expanding the margins: High volume utilization of marginal liver grafts among >2000 liver transplants at a single institution: Ann Surg, 2017; 266(3); 441-49
15. Asrani SK, Saracino G, O’Leary JG, Recipient characteristics and morbidity and mortality after liver transplantation: J Hepatol, 2018; 69(1); 43-50
Figures
 Figure 1. Kaplan-Meyer survival curve of patients who underwent DDLT – 30% sample, randomly selected.
Figure 1. Kaplan-Meyer survival curve of patients who underwent DDLT – 30% sample, randomly selected. Figure 2. Kaplan-Meyer survival curves according to the scoring system – 30% sample, randomly selected (P<0.001).
Figure 2. Kaplan-Meyer survival curves according to the scoring system – 30% sample, randomly selected (P<0.001). Figure 3. Kaplan-Meyer survival curve of patients who underwent DDLT – 70% sample, randomly selected.
Figure 3. Kaplan-Meyer survival curve of patients who underwent DDLT – 70% sample, randomly selected. Figure 4. Kaplan-Meyer survival curves according to the scoring system – 70% sample, randomly selected (P<0.0016).
Figure 4. Kaplan-Meyer survival curves according to the scoring system – 70% sample, randomly selected (P<0.0016). Figure 5. Kaplan-Meyer in recipient-dependent 1-factor scoring.
Figure 5. Kaplan-Meyer in recipient-dependent 1-factor scoring. Figure 6. (A, B) Receiver operating characteristic curve of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring.
Figure 6. (A, B) Receiver operating characteristic curve of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring. Figure 7. Mortality histogram of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring.
Figure 7. Mortality histogram of recipient-dependent 1-factor scoring vs donor-recipient 2-factor scoring. Tables
 Table 1. Score parameters based on donors’ and recipients’ risk factors.
Table 1. Score parameters based on donors’ and recipients’ risk factors. Table 2. Log-rank test of all scores variables (30% bank randomly selected).
Table 2. Log-rank test of all scores variables (30% bank randomly selected). Table 3. COX regression analysis (30% bank randomly selected).
Table 3. COX regression analysis (30% bank randomly selected). Table 4. COX regression analysis (validation group – 70% bank randomly selected).
Table 4. COX regression analysis (validation group – 70% bank randomly selected). Table 5. Death rate according scores and comparison of groups.
Table 5. Death rate according scores and comparison of groups. Table 1. Score parameters based on donors’ and recipients’ risk factors.
Table 1. Score parameters based on donors’ and recipients’ risk factors. Table 2. Log-rank test of all scores variables (30% bank randomly selected).
Table 2. Log-rank test of all scores variables (30% bank randomly selected). Table 3. COX regression analysis (30% bank randomly selected).
Table 3. COX regression analysis (30% bank randomly selected). Table 4. COX regression analysis (validation group – 70% bank randomly selected).
Table 4. COX regression analysis (validation group – 70% bank randomly selected). Table 5. Death rate according scores and comparison of groups.
Table 5. Death rate according scores and comparison of groups. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








