20 December 2022: Original Paper
Potential Utility of Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, and Neutrophil, Lymphocyte, and Platelet Ratios in Differential Diagnosis of Kidney Transplant Acute Rejection: A Retrospective, Propensity Score Matched Analysis
Aureliusz Kolonko1ABCDEF*, Tomasz Dwulit1BDE, Michał Skrzypek2CDE, Andrzej Więcek1EDOI: 10.12659/AOT.937239
Ann Transplant 2022; 27:e937239
Abstract
BACKGROUND: Acute kidney transplant rejection can negatively affect long-term graft function. The neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio have been proposed as non-invasive predictors of acute rejection in stable kidney transplant recipients. The aim of this study was to validate the predictive value of these ratios, as well as neutrophil, lymphocyte, and platelet ratios in the diagnosis of acute rejection during the early post-transplant period.
MATERIAL AND METHODS: After propensity score matching, we compared 71 kidney recipients with biopsy-proven acute rejection with 71 patients without rejection and also subjects with different histologic types of rejection. All 3 types of blood cell count-derived ratios were calculated 6 and 3 days prior to biopsy and on the day of biopsy.
RESULTS: There were 15 patients with T cell-mediated rejection, 33 with vascular rejection, and 23 with antibody-mediated rejection. The values of all examined ratios did not differ between subgroups with and without rejection. However, at all post-transplant study time-points, patients with antibody-mediated rejection had significantly higher values of all analyzed ratios than subjects with other types of rejection. In multivariate regression models, higher values of blood cell count-derived ratios were independently associated with the occurrence of antibody-mediated rejection.
CONCLUSIONS: In the early post-transplant period, the values of neutrophil-to-lymphocyte, platelet-to-lymphocyte, and neutrophil, lymphocyte, and platelet ratios were similar in patients with and without an acute rejection episode, but significantly higher values were found in subjects with antibody-mediated rejection as compared with other types of rejection and those without rejection. High values of analyzed ratios in patients with satisfactory early kidney graft function may be helpful in selecting subjects with increased risk of subclinical antibody-mediated rejection.
Keywords: Biopsy, Fine-Needle, Graft Rejection, Kidney Transplantation, Humans, Diagnosis, Differential, Neutrophils, propensity score, Lymphocytes, Kidney Diseases, Antibodies
Background
Kidney transplantation is the optimal method of treatment in patients with end-stage renal disease, however, acute rejection (AR) is one of main complications, which worsens the long-term graft function and patient survival [1,2]. Early kidney graft dysfunction can also be caused by delayed graft function, infection, nephrotoxicity, or surgical complications; however, kidney graft biopsy is usually performed in such patients during differential diagnosis. In contrast, in subjects with satisfactory early graft function, the ongoing subclinical rejection may be only diagnosed based on the early protocol biopsy [3]. The protocol biopsy program is not universally adopted, and in the transplant centers with such an approach, the timing of kidney graft protocol biopsies can vary widely, from the first post-transplant hospitalization to 12 months after transplantation [4].
Needle biopsy of the transplanted organ with subsequent histological evaluation remains the criterion standard for AR diagnosis. However, this procedure can be seriously complicated by hematoma, urinary bladder obstruction, need for blood transfusions or surgical procedure, graft loss, or even death [5,6], although major complications are rare if the biopsy is performed by an experienced operator under the guidance of an imaging method [7]. Moreover, various medical contraindications often present in the early post-transplant period may make this procedure challenging. Additional biopsy shortcomings like sampling errors and inter-observer variability furtherly limit the accuracy of this method. Therefore, different alternative non-invasive procedures were recently developed and tested, comprising imaging techniques (contrast media-enhanced ultrasound and magnetic resonance imaging), novel urine and serum biomarkers and microarray molecular analysis [8–11]. Additionally, a deep-learning computer-aided diagnostic system, based on the fusion of both imaging markers and clinical biomarkers, was reported to have high accuracy in early detection of AR in kidney transplant recipients (KTRs) [12].
In recent years, the utility of various blood cell count-derived ratios, such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), in the diagnosis of AR has been examined, based on the at least partly inflammatory nature of the acute rejection process [13,14]. Both reports described the significant differences in NLR values between stable KTRs groups of patients with and without AR, but the latter analysis unexpectedly founded NLR and PLR values that were several times higher in the rejection-free patients. Interestingly, neutrophil, lymphocyte, and platelet ratio (N/LP) were found to be associated with acute kidney injury after major abdominal surgery [15]. Thus, we performed a retrospective study to analyze the utility of inflammatory markers calculated from the individual types of cells counted in peripheral blood in the differential diagnosis of AR during the early period after kidney transplantation.
Material and Methods
STUDY GROUP:
We retrospectively analyzed all consecutive KTRs in our center from January 2013 to October 2020, in whom an AR episode was diagnosed based on the kidney graft biopsy performed during the first post-transplant hospital stay, which is the period from transplantation procedure to discharge from the hospital. Patients were identified in the center-operated prospective transplant register. The study was conducted in accordance with the Declaration of Helsinki. According to the opinion of the Bioethics Committee of the Medical University of Silesia (PCN/CBN/0022/KB/164/21), issued July 12, 2021, the present analysis based on anonymous patient data was permitted without obtaining individual informed consent.
Out of 898 KTRs, acute rejection during the first post-transplant hospital stay was diagnosed in 72 patients (8.0%). The control group consisted of patients without an early AR episode, transplanted during the same period of time. As there were substantial differences across the whole analyzed period concerning the percentage of highly-immunized patients or the structure of induction therapy algorithms, the control group without acute rejection was selected based on individual propensity scores, described in detail in the statistical section below.
IMMUNOSUPPRESSION REGIMEN:
Basically, triple immunosuppressive therapy, consisting of tacrolimus, mycophenolate mofetil, or sodium, and steroids, was used in all patients. The initial target tacrolimus levels were set at 5–15 ng/ml and did not change during the study period. At hospital discharge and thereafter, the target tacrolimus level was 6–12 ng/ml. The initial dose of mycophenolate was 500 mg BID or 750 mg BID, depending on patient’s baseline weight. During the operating procedure, 500 mg of methylprednisolone was given i.v., then 125 mg at the first post-operative day and then 20 mg of oral prednisone if the patient was on basiliximab induction. In subjects undergoing antithymocyte globulin induction, the dosing of oral prednisone after the ATG cessation was 20 mg/day, then was decreased to reach 10–15 mg/day at discharge.
KIDNEY GRAFT FUNCTION:
Immediate graft function (IGF) was defined as the serum creatinine concentration (SCr) at day 3 post-transplant below or equal to 3 mg/dl, slow graft function (SGF) as SCr above 3 mg/dl at day 3, and delayed graft function (DGF) requiring dialysis therapy during the first week after transplantation. Of note, we also classified subjects as developing DGF in cases of polyuria, high serum creatinine levels, and non-continuous kidney graft Doppler spectrum during the first post-operative days, despite the lack of need for dialysis therapy.
KIDNEY GRAFT BIOPSIES, ACUTE REJECTION CLASSIFICATION, AND TREATMENT:
Kidney graft biopsies were usually performed between the 8th and 10th post-transplant days. Between January 2013 and September 2015, biopsy was performed in patients with DGF that did not subside during the first week (n=11). Since October 2015, the protocol biopsies were introduced in our center in all patients, except those with clinical contraindications and those who did not give consent. During this period, 60 AR episodes were diagnosed. Throughout the whole study period, prior to the biopsy procedure, all subjects gave informed consent. Samples were stained with standard hematoxylin and eosin, periodic acid Schiff (PAS), methenamine silver, and trichrome. All biopsies were assessed according to the contemporary Banff recommendations. Patients with borderline changes were treated similarly to grade 1A T cell-mediated rejection (TCMR). All biopsy specimens were evaluated by the same experienced histopathologist. Based on the results of kidney histology and additional information (eg, presence of donor-specific antibodies, C4d staining) AR episodes were classified as tubulointerstitial (TCMR grade IA/B), vascular (VR, identical with TCMR grade IIA/B, III) or humoral, antibody-mediated rejection (ABMR). If there were only histologic features representative for T cell rejection without vascular lesions, patients received 3 methylprednisolone pulses at 500 mg/day. If intimal or transmural arteritis dominated in the histologic spectrum, but there was no clear evidence for antibody-mediated rejection (glomerulitis, PTC-itis), VR was diagnosed and patients were treated using ATG as the first-line treatment. Lastly, when ABMR was diagnosed, patients were initially treated with ATG, plasmaphereses and IVIG (if the presence of DSA was confirmed). Of note, since 2015 we introduced a novel protocol for an early AMBR treatment, based on plasmaphereses and bortezomib, with very promising mid-term results [16].
LABORATORY DATA:
The absolute white blood cell count, neutrophil count, lymphocyte count, and platelet count were extracted from complete blood counts taken: immediately before transplantation, 6 days before the biopsy (Bx-6), 3 days prior to biopsy (Bx-3), and at the day of biopsy (Bx). The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count; the PLR was calculated by dividing the absolute platelet count by calculated lymphocyte count (obtained by multiplying the absolute white blood cell count by the percentage of lymphocytes), and the N/LP ratio was calculated as:
STATISTICAL ANALYSIS:
Statistical analyses were performed using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA) and MedCalc version 20 (MedCalc Software, Ostend, Belgium). Data are presented as means with 95% confidence interval (95% CI), medians with Q25-Q75 quartile values, or frequencies. The subjects constituting the control group without acute rejection episode were selected out of 826 patients transplanted during the same time-span, based on 1: 1 matching with Caliper 0.2, using the “greedy” algorithm, based on individual propensity scores. The propensity scores were calculated, taking into account the following covariates: the recipient age, post-transplant follow-up period (defining the transplant era), number of transplantations, and the type of induction treatment used (none/basiliximab/antithymocyte globulin). All propensity score calculations were performed using SAS software (version 9.4, SAS Institute, Inc., Cary, North Carolina, USA). Finally, we used 2 matched cohorts of 71 patients with and 71 patients without acute rejection episode during the first post-transplant stay for all study analyses.
The initial comparison between these study subgroups was performed using the
Receiver operating characteristics (ROC) analyses were applied to determine the cut-off values for NLR. PLR and N/LP values, associated with the occurrence of AMBR. Univariate analyses were performed for the occurrence of ABMR, including age, HLA mismatch, cold ischemia time (CIT), extended criteria donor (ECD) status, and retransplantation, as well as NLR, PLR, and N/LP values calculated at Bx and Bx-3 time-points. Multiple regression analyses were performed for the occurrence of ABMR as the dependent variable, including CIT, retransplantation, and each out of 6 evaluated blood count-based ratios as potential independent variables. The stepwise selection method was used. The significance level to allow a variable to enter into the model and to stay in the model was set at 0.2 and 0.3, respectively. In all statistical tests,
Results
STUDY SUBGROUPS:
After the propensity score matching, 2 cohorts were compared: patients with biopsy-proven AR during the first post-transplant hospital stay (median duration of 24 (14–35) days) and patients without AR (median length of stay 15 (13–19) days; P<0.001). The clinical characteristics of study subgroups are given in Table 1. Patients in both subgroups did not differ in sex, BMI, dialysis vintage, the occurrence of pre-transplant diabetes, cold ischemia time, or HLA mismatch. Potential kidney transplant candidates had been screened for the presence of panel-reactive antibodies (PRA) and anti-HLA antibodies (class I, class II, and anti-MICA). Out of all those parameters of pre-transplant immunologic status, the only difference was a significantly higher percentage of anti-HLA class I antibodies in the AR subgroup (71.8 vs 42.3% in the control group; P<0.01). Additionally, there were no significant differences regarding donor data and early kidney graft function, as well as initial immunosuppression measures within the first 2 post-transplant weeks. In the whole study cohort, the causes of chronic kidney disease were as follows: glomerulonephritis (39.4%), diabetic nephropathy (9.9%), pyelonephritis (8.5%), autosomal dominant polycystic kidney disease (11.3%), hypertensive nephropathy (10.6%), and other and unknown (20.3%). There were no significant differences in the structure of the pathogenesis of chronic kidney disease between the 2 study subgroups (P=0.55).
KIDNEY BIOPSY RESULTS:
The first kidney graft protocol biopsy, which was the basis of AR diagnosis, was performed at a median of 9 (8–9) days after transplantation. In the whole AR subgroup, there were 15 TCMR, 33 vascular, and 23 ABMR cases. In a rejection-free group, most patients (66.6% of the whole cohort) were biopsied at the same post-transplant time (between the 8th and 10th days), whereas in those transplanted before 2015, biopsy was not routinely performed except in subjects with DGF longer than 1 week.
The distribution of different types of AR significantly differed depending on the type of induction therapy (ATG vs IL2-RB vs none: χ2=32.9;
BLOOD COUNT-BASED RATIOS IN PATIENTS WITH AND WITHOUT AR EPISODE:
Table 2 presents median values of NLR, PLR, and N/LP calculated at all study time-points (preoperatively, Bx-6, Bx-3, and Bx). There were no significant differences between subjects with and without AR. Of note, the concomitant C-reactive protein (CRP) values were also similar (data not shown). There was also no association between NLR, PLR, and N/LP values and concomitant CRP values in both study subgroups.
Prior to transplantation, median values of all 3 analyzed markers in the subgroups of patients with different type of induction therapy (ATG vs IL2-RB vs none) were similar. After transplantation, at all 3 study time-points, the highest values of NLR were noted in the ATG subgroup [Bx-6: 41.4 (27.4–59.0) vs 10.0 (5.5–13.8) in IL2-RB and 7.2 (4.5–20.2) in subgroup without induction; all
BLOOD COUNT-BASED RATIOS IN PATIENTS WITH DIFFERENT TYPES OF REJECTION:
When considering the pre-transplantation values of all 3 ratios, they were comparable in the 3 analyzed subgroups (TCMR, VR, ABMR), except for significantly greater NLR in the TCMR subgroup vs the ABMR subgroup (4.1 (3.1–4.9) vs 2.7 (1.8–3.8); P<0.05). However, at all post-transplant study time-points, including the day of kidney graft biopsy, patients with ABMR had significantly higher NLR than subjects with TCMR or VR [Bx-6: 24.4 (14.5–41.5) vs 10.6 (4.3–11.5) vs 11.1 (5.7–25.1), respectively; both P<0.01; Bx-3: 17.1 (3.7–32.0) vs 3.4 (2.3–7.7); P<0.01 vs 4.0 (2.8–5.7); P<0.001, respectively; Bx: 14.6 (3.8–28.4) vs 3.8 (3.2–7.2); P<0.05 vs 3.8 (2.4–8.4); p<0.01, respectively] (Figure 1).
Similarly, there were significantly higher PLR values in the ABMR subgroup than in the TCMR and VR subgroups [Bx-6: 440 (281–967) vs 198 (153–242); P<0.01 vs 236 (150–341); P<0.05), respectively; Bx-3: 356 (131–1158) vs 152 (121–179) vs 149 (108–196), respectively, both P<0.05; Bx: 311 (164–562) vs 164 (115–203) vs 170 (106–247), respectively, both P<0.05] (Figure 2).
Also, N/LP values were significantly higher in the ABMR subgroup than in the TCMR or VR subgroups [Bx-6: 17.8 (6.9–29.6) vs 5.1 (2.2–8.2) vs 5.8 (2.5–18.2), respectively; both P<0.01; Bx-3: 11.1 (2.7–23.6) vs 2.0 (1.2–4.1); P<0.01 vs 1.9 (1.2–3.6); P<0.001, respectively; Bx: 9.0 (2.8–21.8) vs 2.0 (1.1–2.9); P<0.05 vs 1.8 (0.9–3.6); P<0.001, respectively] (Figure 3).
Notably, NLR, PLR, and N/LP values did not differ between the TCMR and VR subgroups, but they were significantly greater in the ABMR subgroup as compared with patients without AR at Bx-3 and Bx time-points (data not shown), except for the PLR difference at the Bx-3 time-point (
In the period from pre-transplant measurement to the 3rd post-transplant day (Bx-6 time-point), the median values of NLR increased by a significantly greater multiplier in ABMR [*9.9 (6.1–20.4)] than in VR [*3.5 (1.8–5.9);
ROC ANALYSES:
The ROC analysis for the Bx time-point revealed that NLR, PLR, and N/LP values above 8.7, 284, and 2.78, respectively, increased the risk for ABMR, with 65% (95% CI: 43–84) sensitivity and 81% (67–91%) specificity for NLR, 61% (39–80%) sensitivity and 88% (75–95%) specificity for PLR, and 78% (56–93%) sensitivity and 71% (56–83%) specificity for N/LP (Figure 4A–4C). Similar analyses for the Bx-3 time-point showed the following cut-off values: 8.26, 212, and 6.37 for NLR, PLR, and N/LP, respectively, with 70% (95% CI: 47–87%) sensitivity and 85% (72–94%) specificity for NLR, 70% (47–87%) sensitivity and 81% (67–91%) specificity for PLR, and 70% (47–87%) sensitivity and 85% (72–94%) specificity for N/LP (Figure 5A–5C).
Notably, there were significant correlations between NLR, PLR, and N/LP values at both analyzed time-points [Bx: NLR and PLR (r=0.813); NLR and N/LP (r=0.950); PLR and N/LP (r=0.695); Bx-3: NLR and PLR (r=0.766); NLR and N/LP (r=0.960); PLR and N/LP (r=0.652), all
UNIVARIATE AND MULTIVARIATE ANALYSES:
In those analyses, we compared ABMR cases with all no-ABMR cases (TCMR, VR, and rejection-free patients). The results of univariate analyses are shown in Table 3. In multivariate regression models, including CIT, retransplantation, and different blood count-based ratios, all 3 ratios (NLR, PLR, and N/LP) measured at both Bx and Bx-3 time-point were found to be independently associated with the occurrence of ABMR in protocol biopsy (Table 4). Moreover, CIT was also independently associated with ABMR occurrence in models including NLR, PLR, or N/LP values calculated at the day of the biopsy, but not at the Bx-3 time-point.
Discussion
In the present study we examined the utility of different inflammatory markers calculated based on the blood cell count in the differential diagnosis of an early post-transplant acute rejection episode in kidney transplant recipients. None of the analyzed markers could distinguish between patients with or without AR in the early post-transplant period. However, we found significantly higher values of NLR, PLR, and N/LP ratios in patients with antibody-mediated rejection as compared to other histologic types.
It is worth to noting that 2 earlier analogic analyses in stable kidney transplant patients yielded conflicting results. Ergin et al [13], in a small study of 22 subjects with AR, noted that NLR > 2.5 was associated with rejection episode, whereas Naranjo et al [14] observed substantially lower NLR and PLR values in AR cases. Importantly, only cellular rejection episodes were analyzed in both studies and kidney graft biopsies were performed “for-cause” in the stable transplant recipients months and years after transplantation. There were neither multivariate analysis nor any clinical characteristics of subjects with or without AR episode in the latter study, while 3 independent analyses performed by Ergin et al, due to the limited sample size, included only uric acid level, steroid dose, and NLR as potential independent variables. Additionally, the type of donor and median time after transplantation were not analyzed in the multivariate analysis despite between-group differences.
In contrast with these previous studies, in our study all analyzed biopsies were performed within the first 2 weeks post-transplant. There were no significant differences in NLR, PLR, or N/LP between patients with and without early post-transplantation AR. Of note, after the procedure, the values of all analyzed parameters were significantly higher on the 3rd day and subsequently subsided. Importantly, in the group of patients with confirmed ABMR episode, this initial increase was significantly more pronounced than in VR and TCMR subjects and the values of all 3 inflammatory ratios decreased only partially until the day of kidney graft biopsy. Consequently, the significant differences in NLR, PLR, and N/LP values between patients with ABMR and other types of rejection or no-rejection were still seen 3 days before and on the day of biopsy.
The inflammatory markers analyzed in our study were previously reported to be associated with mortality and morbidity in acute coronary syndrome [17], surgical outcomes in cancer patients [18], and having a prognostic value in gangrenous appendicitis [19]. In patients with chronic kidney disease (CKD), NLR and PLR values were shown to be higher than in healthy controls [20] and were positively correlated with other inflammatory markers [21]. Importantly, NLR was found to be predictive for cardiovascular events and all-cause mortality in CKD subjects [22,23], whereas the evidence concerning its utility as a marker for CKD progression remains inconclusive [24,25]. In hemodialysis patients, NLR may be a good marker of stenosis and restenosis of native and prosthetic arteriovenous fistulas [26]. In KTRs, elevated preoperative NLR was associated with DGF after transplantation [20]. Later after transplant, NLR gradually decreased until it became stable at 3 months post-transplant, but a substantial increase was noted in patients who then developed malignant disease [28].
To date, none of these analyzed ratios have been studied in association with early post-transplant AR. However, Fahim et al investigated the amount and composition of immune cells within glomeruli and peritubular capillaries (PTC) in cellular and humoral allograft rejection occurring relatively shortly after kidney transplantation [29]. They found that not the absolute number of different immune cells within capillaries, but rather their composition can distinguish humoral from cellular rejection, with inflammatory cells accumulating in glomeruli and PTC, especially in C4d-positive biopsies [29]. Of note, expression of pentraxin-3, an acute-phase reactant produced by a variety of cells at sites of local inflammation, was shown to be higher in rejection biopsies [30]. Moreover, plasma and urinary levels of endocan, a proteoglycan exclusively secreted by vascular endothelium, were significantly higher in ABMR than no-rejection or TCMR rejection subjects [31]. Also, inflammatory lesions in areas of atrophy-fibrosis (i-IFTA), which are associated with increased risk of graft failure, were more often found in indication biopsies with concomitant ABMR, but few with TCMR [32].
All the above-mentioned findings may partly explain our recent observation concerning the greater and more pronounced increase of blood cell count-derived inflammatory ratios in patient with ABMR than in other types of rejection, as well as than in rejection-free patients. As ABMR episodes, especially those that are C4d-negative, are frequently underestimated using conventional criteria [33], and subclinical ABMR had the poorest long-term kidney graft survival as compared with TCMR patients [34], we may speculate that based on the abnormal evolution of analyzed inflammatory markers, the suspicion of subclinical ABMR could be raised earlier after transplantation, resulting in earlier final biopsy-proven diagnosis, more efficient treatment, and better long-term kidney transplantation outcomes. This is especially relevant in patients with good early post-transplant kidney graft function, without any clinical suspicion of AR. Most ABMR episodes during this study emerged despite the previous ATG induction, which was prescribed due to the high pre-transplant measures of immunization. Although the specificity and sensitivity of the analyzed markers in predicting ABMR are lower than for newly developed donor-derived cell-free DNA [35], the possibility to calculate those ratios with no additional costs is still attractive.
Limitations of the present study are its retrospective design and small sample size. Also, the routine protocol biopsy program was not being implemented in our center during the whole study period, which could have caused bias, because during the approximately 1/5 of study period, only for-cause biopsies were performed and analyzed. However, this is the first study to concurrently investigate the efficacy of NLR, PLR, and N/LP as a potential markers of rejection in the very early period after kidney transplantation, including different types of AR. Additionally, in the effort to improve the quality of our research, we used propensity score matching to create the control group of patients without an AR episode, including factors that potentially influence the blood cell count, and we also analyzed inflammatory ratios: patient age, transplant era, retransplantation, and the type of induction treatment.
Conclusions
There was no difference in NLR, PLR, and N/LP values between patients with and without AR in the early post-transplant period. As subjects with ABMR presented significantly higher values of analyzed inflammatory markers, the routine assessment of those markers might be of value for identifying patients without clinical suspicion of rejection, who may benefit from early kidney graft biopsy. However, these retrospective data should be confirmed in a prospective study.
Figures
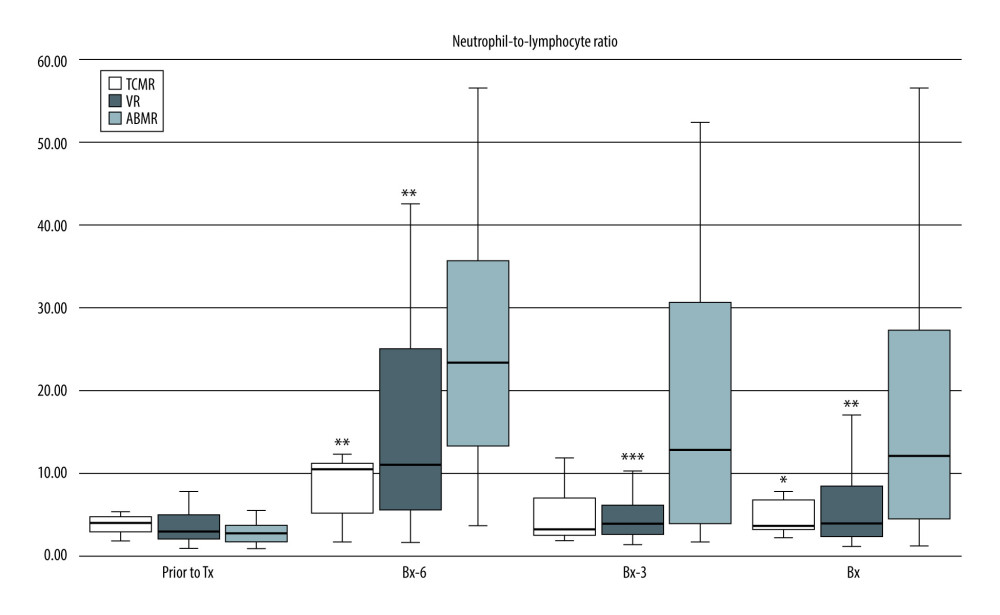 Figure 1. Pre-transplant and early post-transplant median values of NLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco Inc., Palo Alto, CA, USA).
Figure 1. Pre-transplant and early post-transplant median values of NLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco Inc., Palo Alto, CA, USA). 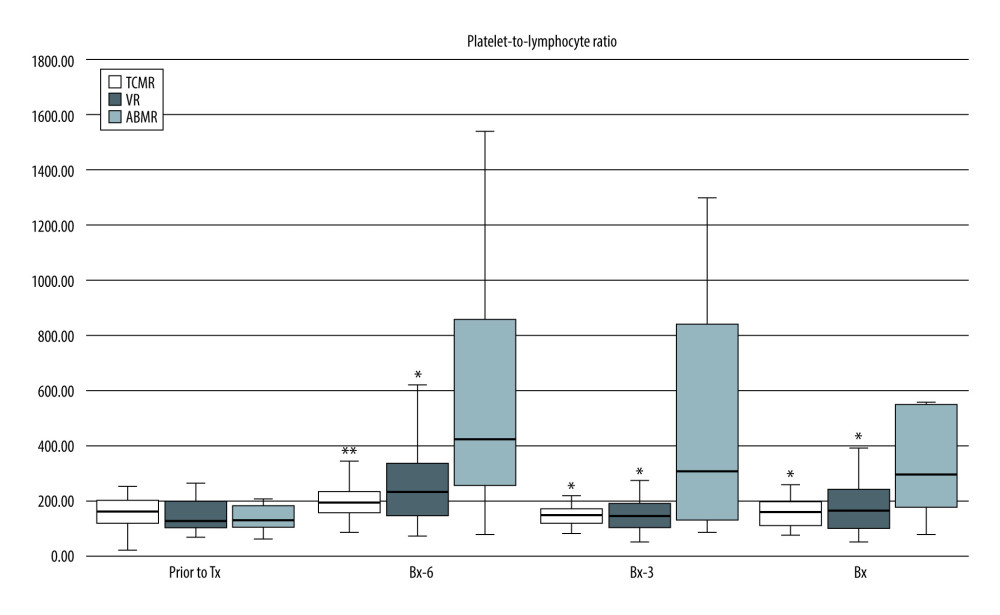 Figure 2. Pre-transplant and early post-transplant median values of PLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 2. Pre-transplant and early post-transplant median values of PLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). 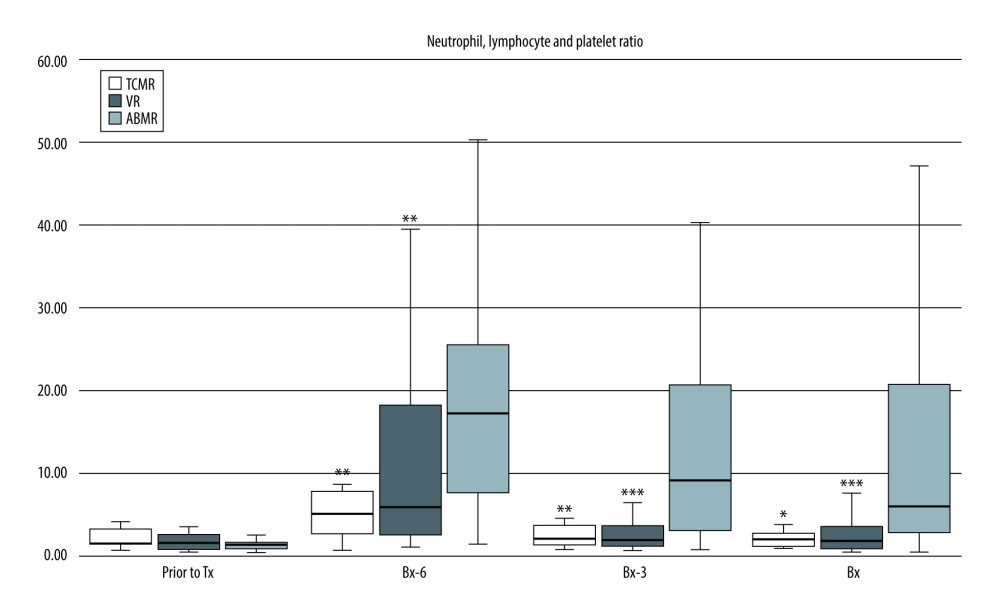 Figure 3. Pre-transplant and early post-transplant median values of N/LP in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 3. Pre-transplant and early post-transplant median values of N/LP in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). 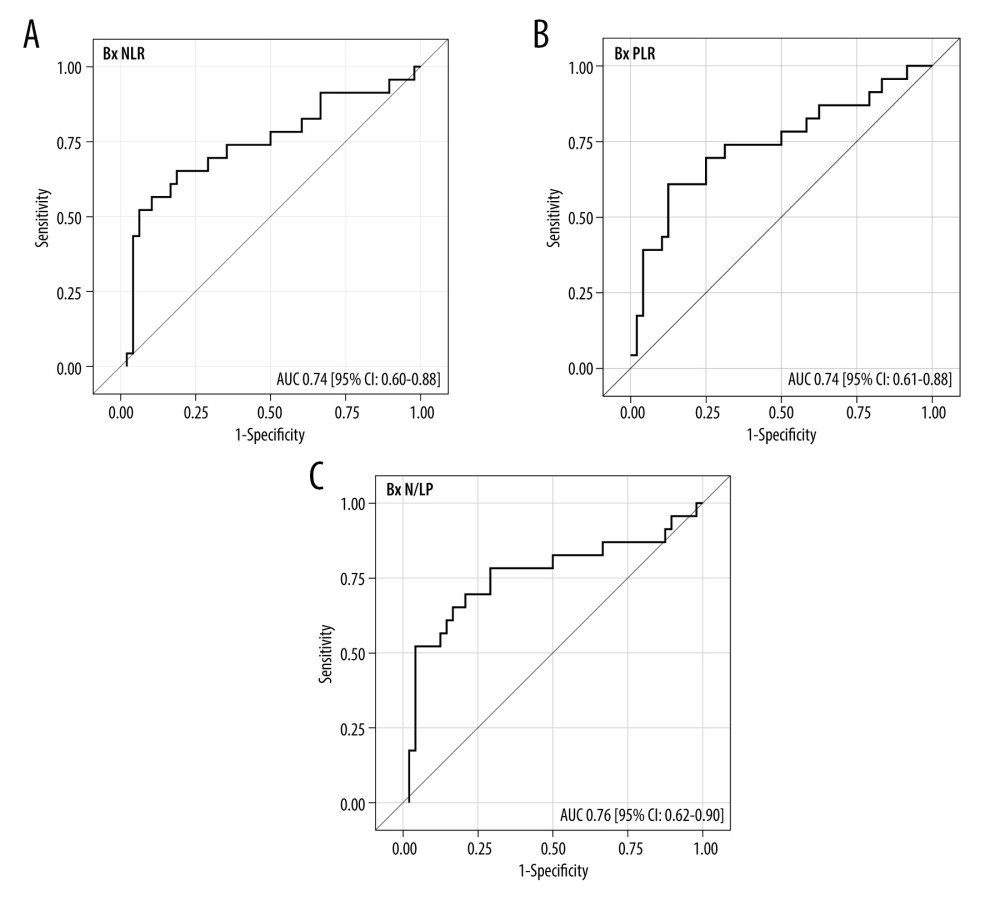 Figure 4. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the time of kidney biopsy (Bx), which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 4. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the time of kidney biopsy (Bx), which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). 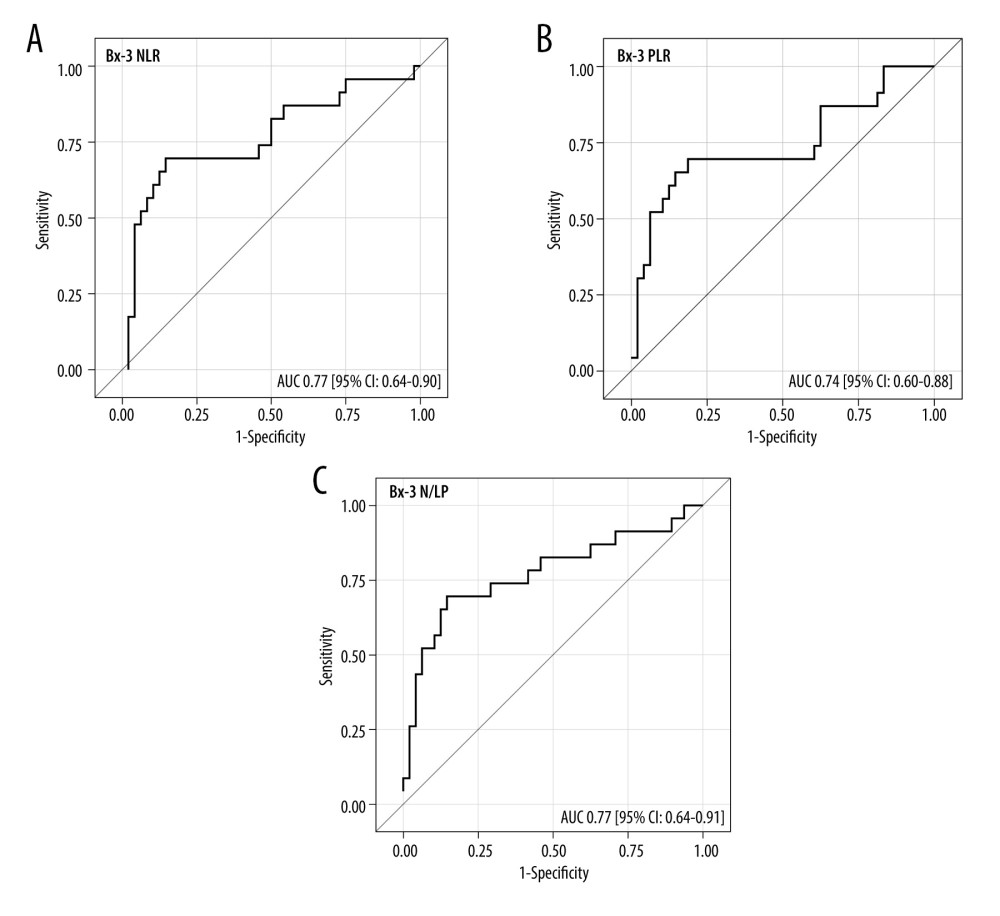 Figure 5. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the Bx-3 time-point, which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 5. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the Bx-3 time-point, which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). Tables
Table 1. Clinical characteristics of study subgroups with and without early acute rejection episode in the early post-transplant period.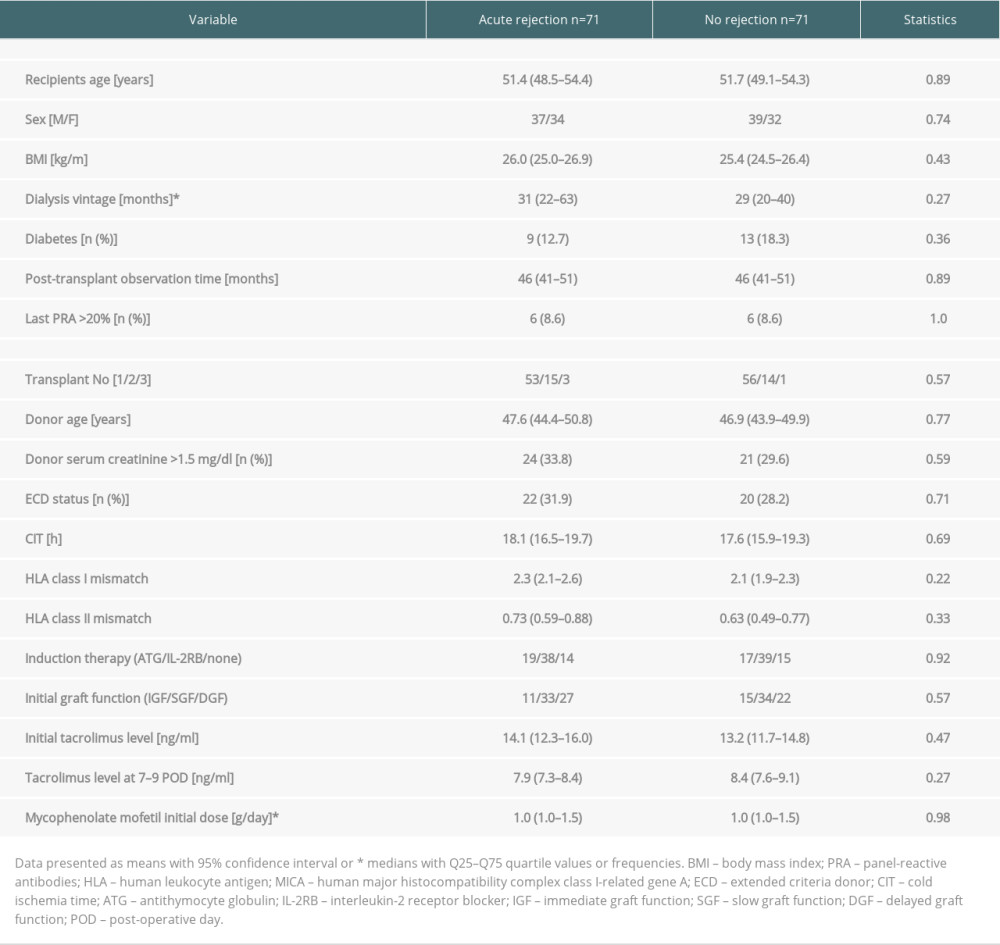 Table 2. Median values of NLR, PLR, and N/LP ratios calculated at all study time-points in subjects with and without acute rejection episode.
Table 2. Median values of NLR, PLR, and N/LP ratios calculated at all study time-points in subjects with and without acute rejection episode.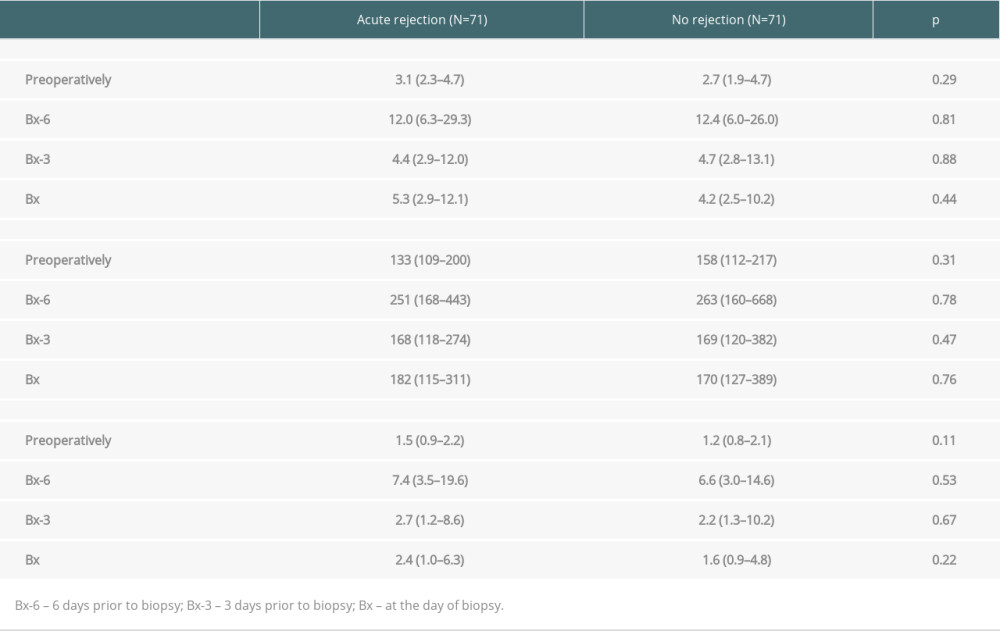 Table 3. The results of univariate analysis for the occurrence of ABMR episode.
Table 3. The results of univariate analysis for the occurrence of ABMR episode.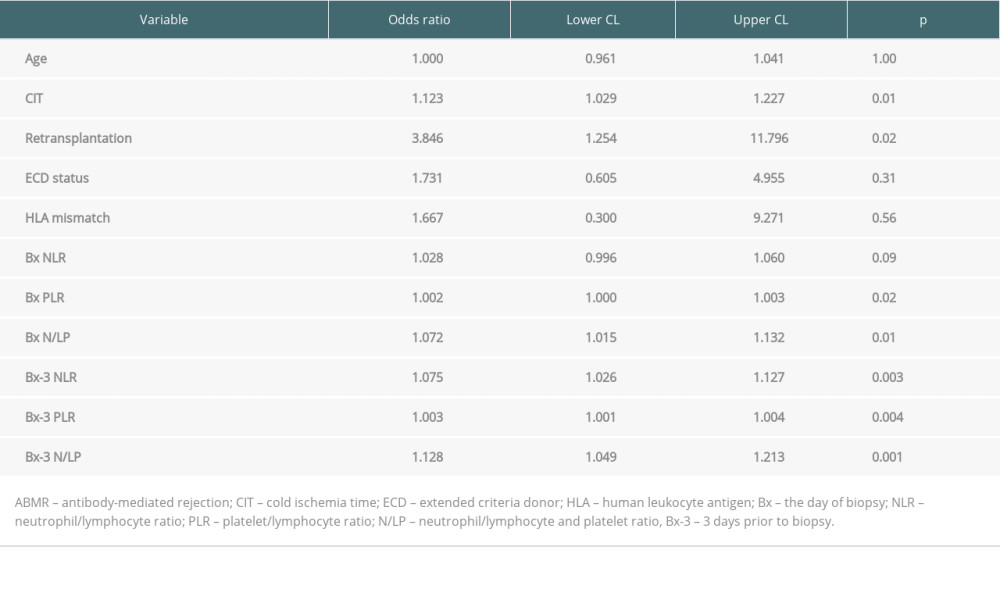 Table 4. Results of multiple regression analysis for the occurrence of ABMR episode.
Table 4. Results of multiple regression analysis for the occurrence of ABMR episode.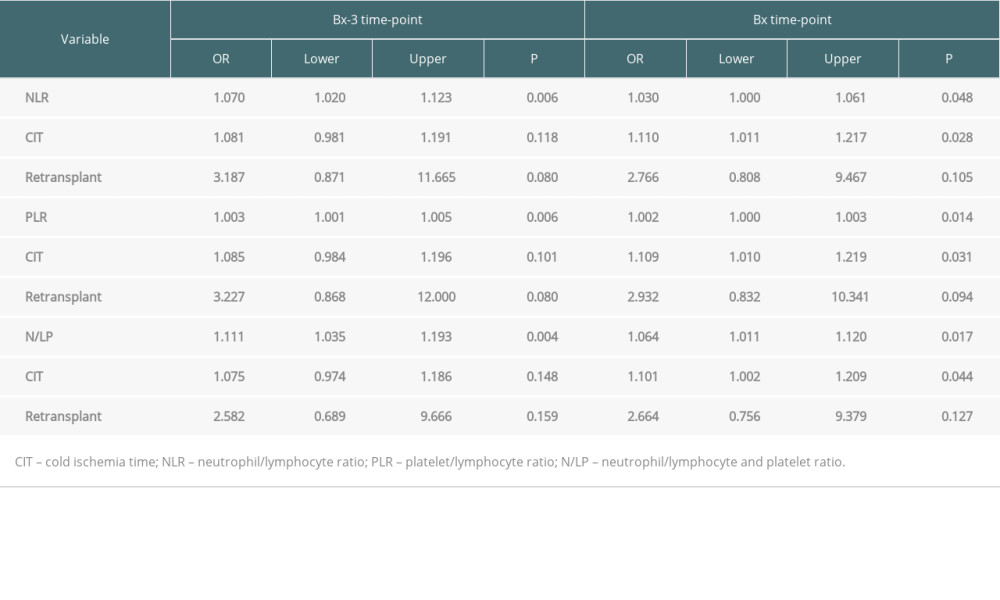
References
1. Wolfe RA, Ashby VB, Milford EL, Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant: N Engl J Med, 1999; 341; 1725-30
2. Clayton PA, McDonald SP, Russ GR, Chadban SJ, Long-term outcomes after acute rejection in kidney transplant recipients: An ANZDATA analysis: J Am Soc Nephrol, 2019; 30; 1697-707
3. Filippone EJ, Farber JL, The problem of subclinical antibody-mediated rejection in kidney transplantation: Transplantation, 2021; 105; 1176-87
4. Chapman JR, Do protocol transplant biopsies improve kidney transplant outcomes?: Curr Opin Nephrol Hypertens, 2012; 21; 580-86
5. Whittier W, Korbet SM, Timing of complications in percutaneous renal biopsy: J Am Soc Nephrol, 2004; 15; 142-47
6. Prasad N, Kumar S, Manjunath R, Real-time ultrasound-guided percutaneous renal biopsy with needle guide by nephrologist decreases post-biopsy complications: Clin Kidney J, 2015; 8; 151-56
7. Laute M, Vanholder R, Voet D, Safety and sample adequacy of renal transplant surveillance biopsies: Acta Clin Belg, 2013; 68; 161-65
8. Jehn U, Schuette-Nuetgen K, Kentrup D, Renal allograft rejection: Noninvasive ultrasound- and MRI-based diagnostics: Contrast Media Mol Imaging, 2019; 2019; 3568067
9. Suthanthiran M, Schwartz JE, Ding R, Urinary-cell mRNA profile and acute cellular rejection in kidney allografts: N Engl J Med, 2013; 369; 20-31
10. Halloran PF, Reeve JP, Pereira AB, Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: New insights from the Genome-Canada studies of kidney transplant biopsies: Kidney Int, 2014; 85; 258-64
11. Cao Y, Alexander SI, Chapman JR, Integrative analysis of prognostic biomarkers for acute rejection in kidney transplant recipients: Transplantation, 2021; 105; 1225-37
12. Abdeltawab H, Shehata M, Shalaby A, A novel CNN-based CAD system for early assessment of transplanted kidney dysfunction: Sci Rep, 2019; 9; 5948
13. Ergin G, Deger SM, Kopru B, High neutrophil-to-lymphocyte ratio predicts acute allograft rejection in kidney transplantation: A retrospective study: Turk J Med Sci, 2019; 49; 525-30
14. Naranjo M, Agrawal A, Goyal A, Rangaswami J, Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predicts acute cellular rejection in the kidney allograft: Ann Transplant, 2018; 23; 467-74
15. Gameiro J, Finseca JA, Dias JM, Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery: BMC Nephrol, 2018; 19; 320
16. Kolonko A, Słabiak-Błaż N, Karkoszka H, The preliminary results of bortezomib used as a primary treatment for an early acute antibody-mediated rejection after kidney transplantation – a single-center case series: J Clin Med, 2020; 9; 529
17. Dong CH, Wang ZM, Chen SY, Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis: Clin Biochem, 2018; 52; 131-36
18. Hung HY, Chen JS, Yeh CY, Effect of preoperative neutrophil-lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy: Int J Colorectal Dis, 2011; 26; 1059-65
19. Ishizuka M, Shimizu T, Kubota K, Neutrophil-to-lymphocyte ratio has a close association with gangrenous appendicitis in patients undergoing appendectomy: Int Surg, 2012; 97; 299-304
20. Cankaya E, Bilen Y, Keles M, Neutrophil-lymphocyte ratio is significantly decreased in preemptive renal transplant patients: Transplant Proc, 2015; 47; 1364-68
21. Turkmen K, Erdur FM, Ozcicek F, Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients: Hemodial Int, 2013; 17; 391-96
22. Ao G, Wang Y, Qi X, Wang F, Wen H, Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: A meta-analysis: Clin Exp Nephrol, 2021; 25; 157-65
23. Zhao W-M, Tao S-M, Liu G-L, Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis: Ren Fail, 2020; 42; 1059-66
24. Yuan Q, Wang J, Peng Z, Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE): J Transl Md, 2019; 17; 86
25. Altunoren O, Akkus G, Sezal DT, Does neutrophil to lymphocyte ratio really predict chronic kidney disease progression?: Int Urol Nephrol, 2019; 51; 129-37
26. Valga F, Monzon T, Henriquez F, Anton-Perez G, Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as biological markers of interest in kidney disease: Nefrologia, 2019; 39; 243-49
27. Halazun KJ, Marangoni G, Hakeem A, Elevated preoperative recipient neutrophil-lymphocyte ratio is associated with delayed graft function following kidney transplantation: Transplant Proc, 2013; 45; 3254-57
28. Ohtaka M, Kawahara T, Takamoto D, Neutrophil-to-lymphocyte ratio in renal transplant patients: Exp Clin Transplant, 2018; 5; 546-49
29. Fahim T, Bohmig GA, Exner M, The cellular lesion of humoral rejection: Predominant recruitment of monocytes to peritubular and glomerular capillaries: Am J Transplant, 2007; 7; 385-93
30. Imai N, Nishi S, Yoskita K, Pentraxin-3 expression in acute renal allograft rejection: Clin Transplant, 2012; 26(Suppl 24); 25-31
31. Lee YH, Kim S-Y, Moon H, Endocan as a marker of microvascular inflammation in kidney transplant recipients: Sci Rep, 2019; 9; 1854
32. Halloran PF, Matas A, Kasiske BL, Molecular phenotype of kidney transplant indication biopsies with inflammation in scarred areas: Am J Transplant, 2019; 19; 1356-70
33. Halloran PF, Reeve JP, Pereira AB, Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: New insights from the Genome Canada studies of kidney transplant biopsies: Kidney Int, 2014; 85; 259-64
34. Loupy A, Vernerey D, Tinel C, Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts: J Am Soc Nephrol, 2015; 26; 1721-31
35. Cheng D, Liu F, Xie K, Donor-derived cell-free DNA: An independent biomarker in kidney transplant patients with antibody-mediated rejection: Transplant Immunol, 2021; 69; 101404
Figures
 Figure 1. Pre-transplant and early post-transplant median values of NLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco Inc., Palo Alto, CA, USA).
Figure 1. Pre-transplant and early post-transplant median values of NLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco Inc., Palo Alto, CA, USA). Figure 2. Pre-transplant and early post-transplant median values of PLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 2. Pre-transplant and early post-transplant median values of PLR in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). Figure 3. Pre-transplant and early post-transplant median values of N/LP in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 3. Pre-transplant and early post-transplant median values of N/LP in kidney recipients with different types of acute rejection. P values as compared with the corresponding ABMR group; * P<0.05, ** P<0.01, *** P<0.001. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). Figure 4. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the time of kidney biopsy (Bx), which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 4. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the time of kidney biopsy (Bx), which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). Figure 5. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the Bx-3 time-point, which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA).
Figure 5. (A–C) The ROC analysis for the values of NLR, PLR, and N/LP ratios calculated at the Bx-3 time-point, which increased the risk for antibody-mediated rejection. Figure created using STATISTICA 13.3 (Tibco, Inc., Palo Alto, CA, USA). Tables
 Table 1. Clinical characteristics of study subgroups with and without early acute rejection episode in the early post-transplant period.
Table 1. Clinical characteristics of study subgroups with and without early acute rejection episode in the early post-transplant period. Table 2. Median values of NLR, PLR, and N/LP ratios calculated at all study time-points in subjects with and without acute rejection episode.
Table 2. Median values of NLR, PLR, and N/LP ratios calculated at all study time-points in subjects with and without acute rejection episode. Table 3. The results of univariate analysis for the occurrence of ABMR episode.
Table 3. The results of univariate analysis for the occurrence of ABMR episode. Table 4. Results of multiple regression analysis for the occurrence of ABMR episode.
Table 4. Results of multiple regression analysis for the occurrence of ABMR episode. Table 1. Clinical characteristics of study subgroups with and without early acute rejection episode in the early post-transplant period.
Table 1. Clinical characteristics of study subgroups with and without early acute rejection episode in the early post-transplant period. Table 2. Median values of NLR, PLR, and N/LP ratios calculated at all study time-points in subjects with and without acute rejection episode.
Table 2. Median values of NLR, PLR, and N/LP ratios calculated at all study time-points in subjects with and without acute rejection episode. Table 3. The results of univariate analysis for the occurrence of ABMR episode.
Table 3. The results of univariate analysis for the occurrence of ABMR episode. Table 4. Results of multiple regression analysis for the occurrence of ABMR episode.
Table 4. Results of multiple regression analysis for the occurrence of ABMR episode. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








