25 October 2022: Original Paper
Risk Factors for Polyomavirus, Cytomegalovirus, and Viruria Co-Infection for Follow-Up of Renal Transplant Patients
Ho Trung Hieu1ABCDEFG*, Bui Tien Sy2ACEFDOI: 10.12659/AOT.937771
Ann Transplant 2022; 27:e937771
Abstract
BACKGROUND: The interaction of viral infection may be associated with increased morbidity after renal transplantation. This study aimed to identify the incidence and risk factors of viruria infections in renal transplant recipients.
MATERIAL AND METHODS: In this longitudinal study, 502 episodes recorded in 81 kidney transplant patients from 1/2019 to 12/2021 in a hospital in Vietnam were included. BK, JC polyomaviruses, CMV, EBV, and HSV were detected. Multivariable Cox regression analysis was performed to evaluate risk factors for the viruria infection.
RESULTS: Fifty-six patients (69.1%) had viruria co-infection. The incidence of JC, CMV, and BK infection was the most common viruria, with 67.9%, 61.7%, and 56.8%, respectively. Cox regression revealed that the risk factors for JC were single infection, dose of MMF (HR 1.002), corticoid (HR 1.02), hypertension (HR 1.65), and hematuria (HR 2.03); risk factors for CMV infection were male sex (HR 1.92) and eGFR (HR 0.98); risk factors for BK single infection were hypertension (HR 1.67), proteinuria (HR 3.80), higher tacrolimus trough level (HR 1.17), and dose of MMF (HR 1.002). Hypertension (HR 1.68), fasting plasma glucose (HR 1.13), proteinuria (HR 6.01), tacrolimus trough level (HR 1.12), and dose of MMF (HR 1.004) were independent risk factors for the viruria co-infection.
CONCLUSIONS: Kidney function was associated with the incidence of viruria. Higher tacrolimus trough level and dose of MMF were associated with higher risk of BK, JC, and co-infection.
Keywords: BK Virus, Cytomegalovirus, JC Virus, Kidney Transplantation, Urinary Tract Infections, Vietnam, Humans, Male, Female, Polyomavirus, Polyomavirus Infections, Tacrolimus, Follow-Up Studies, coinfection, Longitudinal Studies, Blood Glucose, Kidney Diseases, Risk Factors, Cytomegalovirus Infections, Proteinuria, Hypertension
Background
Urinary tract infections are a major cause of graft dysfunction or failure in renal transplant patients [1]. The mortality and graft survival prevalence in post-transplant patients have remarkably improved with the development of immunosuppressive agents [2]. However, the renal recipients faced a greater risk for infections owing to sufficient immunosuppression. Immunosuppression therapy is a notable factor leading to an increased incidence of polyomavirus-associated diseases [3]. A low dose of anti-thymocyte globulin (ATG) (1 mg/kg/day for 4 days) lowers survival free of CMV than basiliximab at a dose of 20 mg on day −0 and day −4 post-transplant.
Viruria usually results in an asymptomatic infection that hides in the urinary tract [4]. JCV and BKV are 2 of 5 human polyomaviruses associated with clinical disease [3,5]. BKV in urine is one of the early markers of risk for BK viremia and nephropathy [6]. BKV is a polyomavirus associated with nephropathy in early post-renal transplant (RTx) [7]. The impact of JCV on nephropathy was shown in a few cases [8]. However, there is evidence showing that JCV nephropathy occurs at both early and late after renal transplantation [3,4]. The interaction of viral infection may be associated with increased post-RTx morbidity [9]. BKV and CMV co-infection have significantly lower eGFR after RTx than a single BK or CMV infection [9,10].
The treatment for post-transplantation viral infections is not straightforward due to difficulties in identifying potential pathogens [2]. Therefore, identifying active viral infections is vital in therapy decisions and monitoring transplant recipients [11]. There is limited information on the incidence of viral infections in Vietnamese kidney recipients. Therefore, the present study was conducted to assess the incidence of common viral infections in the urine of RTx recipients and to identify the risk factors for viruria infection after renal transplantation.
Material and Methods
SUBJECTS:
From 1/2019 to 12/2021, we enrolled 101 patients with first-time renal transplants and underwent treatment therapy after transplantation in the Department of Nephrology and Transplantation, 108 Military Central Hospital, Vietnam. Eighty-nine patients who had at least 6 months of follow-up were included. In 3 years, 501 urine samples were collected, and at least 1 virus was detected in 369 samples of 81 patients (Figure 1).
This study complied with the principles of the Declaration of Helsinki. The study proposal, related appendix, participant information sheet, and written informed consent were reviewed and approved by the Institutional Review Board in Biomedical Research of 108 MCH (approval ID: 2021-1539/HDDD).
STUDY DESIGN:
We conducted a longitudinal study to follow up renal transplant patients with repeated monitoring of health status and relevant infections every 3 months. Patients’ age, sex, etiology of renal failure, and donor-related factors were also collected.
DETECTING INFECTION PATHOGENS:
Physicians diagnosed all clinically pertinent infections. Clinically relevant infections included probable and proven viral infections and viral syndromes. To detect opportunistic pathogens in patients’ urine specimens, we performed real-time PCR using TaqMan probes (Bio-Rad, USA) for various types of viral infection (CMV, BK, JC, EBV, and HSV).
DATA ANALYSIS:
Statistical analysis was conducted using SPSS software version 21. Categorical variables were presented as frequency (n) and percentage (%). Continuous variables (age, months in renal replacement therapy, time of follow-up, eGFR, dose, and blood level of drugs) were presented as means and standard deviations. Multivariable Cox regression analysis was performed to evaluate risk factors for viruria infection.
Results
CHARACTERISTICS OF RENAL TRANSPLANT PATIENTS:
Clinical characteristics and transplant-related data are illustrated in Table 1. Among 81 renal transplant patients included in the study (mean age 40.9±12.0 years, 76.5% male), 25 (30.9%) patients had only 1 type of viruria infection (viruria single group, VS), and 56 (69.1%) had at least 2 viruses (viruria co-infection group, VC). The leading cause of end-stage renal disease was glomerulonephritis (71.6%); 88% of patients had used renal replacement therapy. The mean follow-up duration after RTx was 28.8 months. Deceased donor and time of dialysis were significantly different between groups. The mean eGFR at baseline and at the end-point showed a significant improvement in kidney graft (paired t test, P<0.05), but there was no association with creatinine values.
Antibiotic prophylaxis and immunosuppressive therapy, which included calcineurin inhibitor (tacrolimus), antimetabolite (mycophenolate mofetil [MMF]) and prednisone, were administered for 73 (90.1%) RTx patients. Myfortics were replaced by MMF in 6 (7.4%) patients; 1 (1.2%) patient used cyclosporin instead of tacrolimus. The number of patients and dose of mycophenolate mofetil, and the amount and blood level of tacrolimus were significantly greater in the single viruria groups. There was no significant difference in the type of induction therapy, either ATG or basiliximab, and the prednisolone dose.
CHARACTERISTICS OF VIRURIA INFECTION IN RENAL TRANSPLANTATION:
Overall, CMV and polyomaviruses (BK and JC) infection were the most common viruria in the current study. The mean time until viruria in the co-infection group was not significantly different form that of the single-infection group (Table 2). Human Epstein-Barr virus and Herpes Simplex Virus were rare.
Figures 2 and 3 showed the frequency of viruria infection by the number of episodes. It is clear that the number of BKV infection episodes was highest, but the incidence of BK co-infection was lower than CMV and JCV. In contrast, the frequency of CMV detected was lower, but there were 89 episodes (80%) of CMV co-infection (Figure 3), and the number of patients who had CMV was highest, at 55 (67.9%) (Table 2). Table 2 shows that among 50 patients with BK infection, three-quarters of them had recurrent BK infection. There were 32/46 (69.6%) recurrent JC infections, and 35/55 (63.6%) patients had CMV, but no significant difference was recorded.
RISK FACTORS FOR VIRAL INFECTION IN RENAL TRANSPLANTATION:
Potential risk factors were age of recipients at renal transplantation time, sex, renal function (eGFR, hematuria, and proteinuria), donor-related factors (sex, age, deceased donor), immunosuppressive therapy, hypertension, blood glucose, and time until detection of the first viruria. In the last step, the fitting Cox multiple regression only showed significant variables.
Age (HR 0.97, P<0.01) and the first time BK was detected (HR 0.91, P<0.001) were associated with lower risk of BK infection. Renal recipients who had hypertension (HR 1.67, P<0.01), proteinuria (HR 3.80, P<0.001), higher tacrolimus trough levels (HR 1.17, P<0.001), and dose of MMF (HR 1.002, P<0.001) had a greater risk of a single BK infection (Table 3).
Cox regression analysis revealed that the dose of MMF (HR 1.002, P<0.001) and corticoid (HR 1.02, P <0.01), hypertension (HR 1.65, P<0.05), and hematuria (HR 2.03, P<0.001) were associated with a higher risk of single JC infection. Improved eGFR (HR 0.99, P<0.05) and late detection of JC (HR 0.88, P<0.001) were associated with lower risk of JC infection (Table 4). In patients with single CMV infection, eGFR (HR 0.98, P<0.01) and time until the first CMV (HR 0.91, P<0.001) were protective factors (Table 5). Males had a higher risk of CMV infection (HR 1.92, P<0.01).
Cox regression analysis showed age (HR 0.95, P<0.001), hypertension (HR 1.68, P<0.05), fasting plasma glucose (HR 1.13, P<0.05), eGFR (HR 0.98, P<0.05), proteinuria (HR 6.01, P<0.001), tacrolimus trough level (HR 1.12, P<0.01), and dose of MMF (HR 1.004, P<0.001) were independent risk factors for the occurrence of co-infection (Table 6).
Discussion
The study is the first to investigate these 5 common viruses that cause asymptomatic urinary tract infection in RTx patients who were screened in Vietnam. The results showed that CMV and polyomaviruses were the most common viruria infections.
The viruria infection rate of cytomegalovirus (CMV) in our study was 67.9%, which is higher than in other studies [12–16]. The difference may be due to the price of antiviral prophylaxis drugs, and we used acyclovir as a pre-emptive treatment. Valacyclovir had greater efficacy than acyclovir in preventing CMV infection [17]. Although there was evidence that a low dose of ATG was associated with lower CMV-free survival than basiliximab [18], there was no significant difference in the infection rate between the 2 groups in our study. This could be because in our study only 8 patients received ATG as induction therapy, while 64 patients received basiliximab.
Our study’s rate of polyomavirus infection in urine was 61.73 for BK infection and 56.79 for JC infection. These results were higher than in the studies by Eidgahi et al [16] and Babazadeh et al [19]. The different outcomes may be due to the use of cyclosporin in other studies and use of tacrolimus in our research. Regarding the maintenance of immunosuppression therapy, we followed the guideline of tacrolimus blood level 7–10 ng/mL and a 1.5–2 g/day dose of MMF [18]. A higher tacrolimus trough level was reported to be an independent risk factor for BK infection in Chinese and Tunisian renal transplant recipients [20]. Our study also found that tacrolimus and MMF were associated with BK, JC, and co-infection in the urine. In immunocompetent patients, polyomavirus infection was asymptomatic; however, sufficient triple-drug immunosuppressive in RTx patients can reactivate polyomavirus in the urine [21]. On average, it would take a few months for BK viruria to cause BK viremia in 10–15% of kidney recipients [7,22]. BK viruria was an important marker indicating when to start therapeutic intervention to reduce the risk of developing BK viremia and nephropathy [6].
We also showed the connection between viruria infection and renal function, including eGFR and proteinuria status. Excess proteins in the urine caused a greater risk of kidney damage; therefore, the damaged glomerular basement membrane created a suitable environment for the development of infection [23]. The current evidence identified risk factors for each single viruria or co-infection. Potential risk factors of viral infection included the age of recipients at renal transplantation time, sex, renal function, donor age, deceased donor, immunosuppressive therapy, and co-morbidities [10,14–16,19]. Our study showed a negative association between age and risk of viruria infection. In contrast, studies by Eidgahi et al and Babazadeh et al found the age of RTx patients was associated with increasing risk of BKV infection [16] and CMV infection [15,19]. A study from Argentina also reported that older patients, deceased donors, and length of hospital stay after transplantation were risk factors for CMV infection [15]. It could be explained that although immunosuppressive agents may predispose elderly patients to the risk of over-immunosuppression [24], older people theoretically had a slower immune response than younger people [25]. In addition, our patients were younger than in these other studies. As a result, the incidence of infection was lower as age increased in our study.
The current study showed that more than 40% of viruria co-infection episodes were related to CMV and BK, which contrasted with Jehn et al, who showed that although CMV and BK accounted for most RTx patients, the CMV and BK co-infection were rare [10]. Our study illustrated that in RTx patients who were routinely screened for viruria, those with late detection had lower risk of repeated BKV, JCV, and CMV viruria infection than those with early detection. We found that the degree of immunosuppression at 1–6 months after transplant was the leading cause of opportunistic infections or pathogens reactivation in recipients with latent infection [7,13]. After 6 months post-transplant, the patient’s dose of immunosuppressive agents was adjusted and reduced, making it a protective factor against viruria infection. These findings highlighted the need for post-transplant routine screening of viruses in blood and urine for all kidney transplant recipients until the end of the first post-transplant year [7,19,26].
Conclusions
In conclusion, renal function, including eGFR, proteinuria, and hematuria, were associated with the incidence of viruria. Late detection of viruria significantly reduced the risk of repeated single viruria infection. Higher tacrolimus trough level and dose of MMF increased the risk of single BK, JC, and co-infection. In addition, age, hypertension, and fasting plasma glucose level were independent risk factors for the occurrence of co-infection. The study suggests the need for routine screening of viruses in blood and urine for all kidney transplant recipients in the first post-transplant year.
Figures
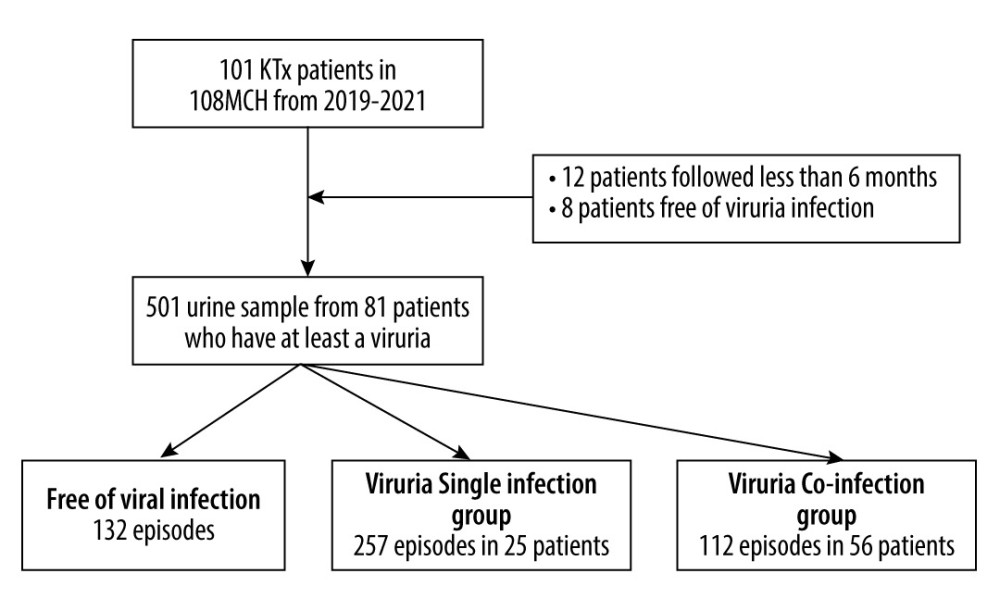 Figure 1. Flowchart of patient inclusionThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072).
Figure 1. Flowchart of patient inclusionThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072). 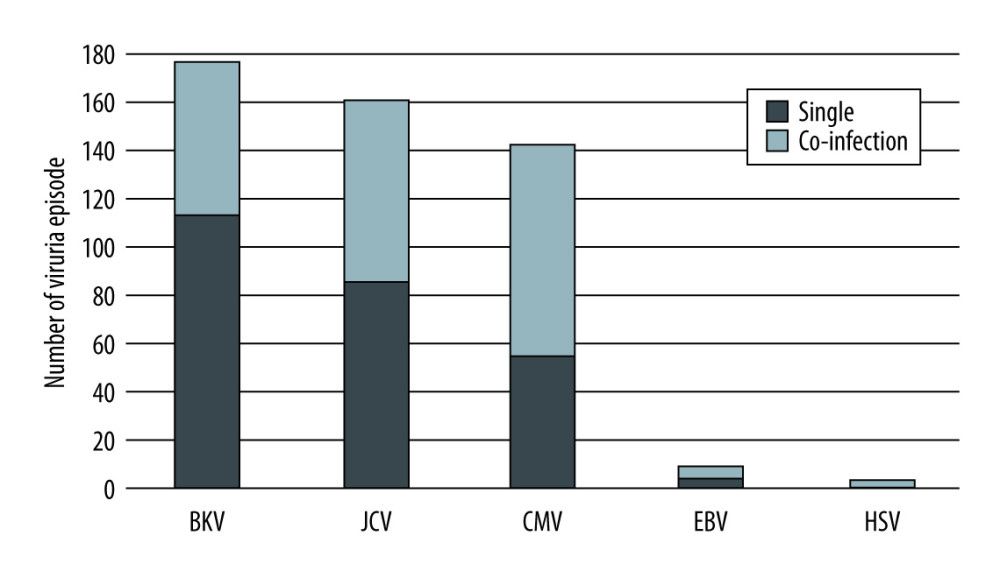 Figure 2. Frequency of viruria episodes among renal transplantation recipients in the 2 groupsThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072).
Figure 2. Frequency of viruria episodes among renal transplantation recipients in the 2 groupsThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072). 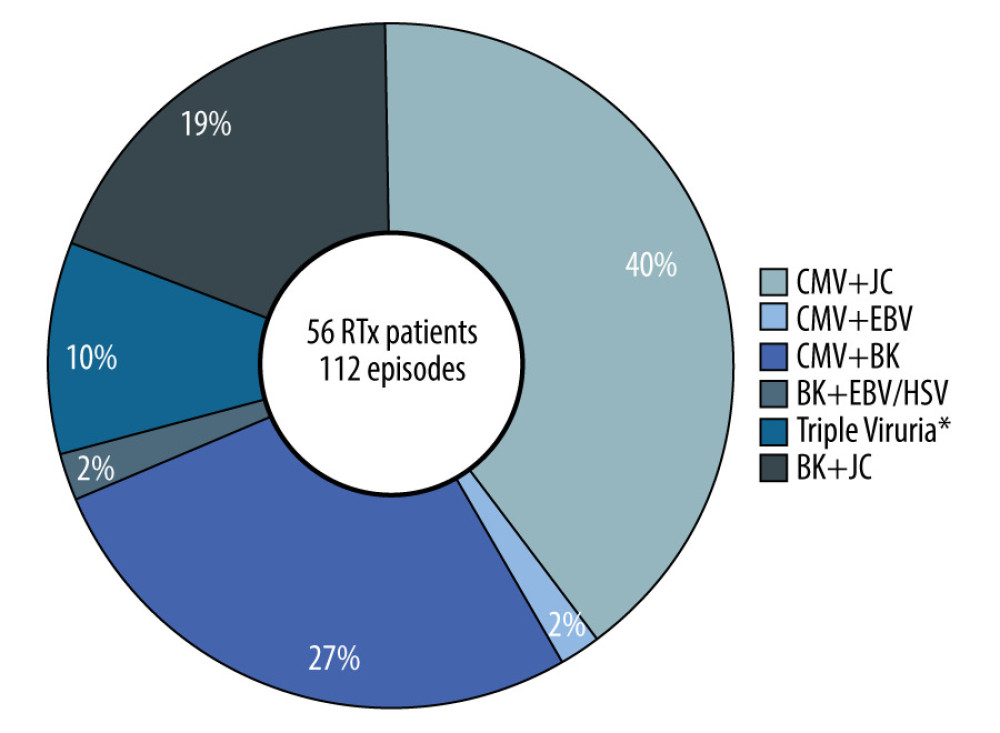 Figure 3. Association of frequency of episodes with the type of viruria co-infection among renal transplantation recipients* Triple viruria were CMV and BK co-infection with either JCV or EBV. The figure is created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072).
Figure 3. Association of frequency of episodes with the type of viruria co-infection among renal transplantation recipients* Triple viruria were CMV and BK co-infection with either JCV or EBV. The figure is created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072). Tables
Table 1. Characteristics of renal transplant recipients.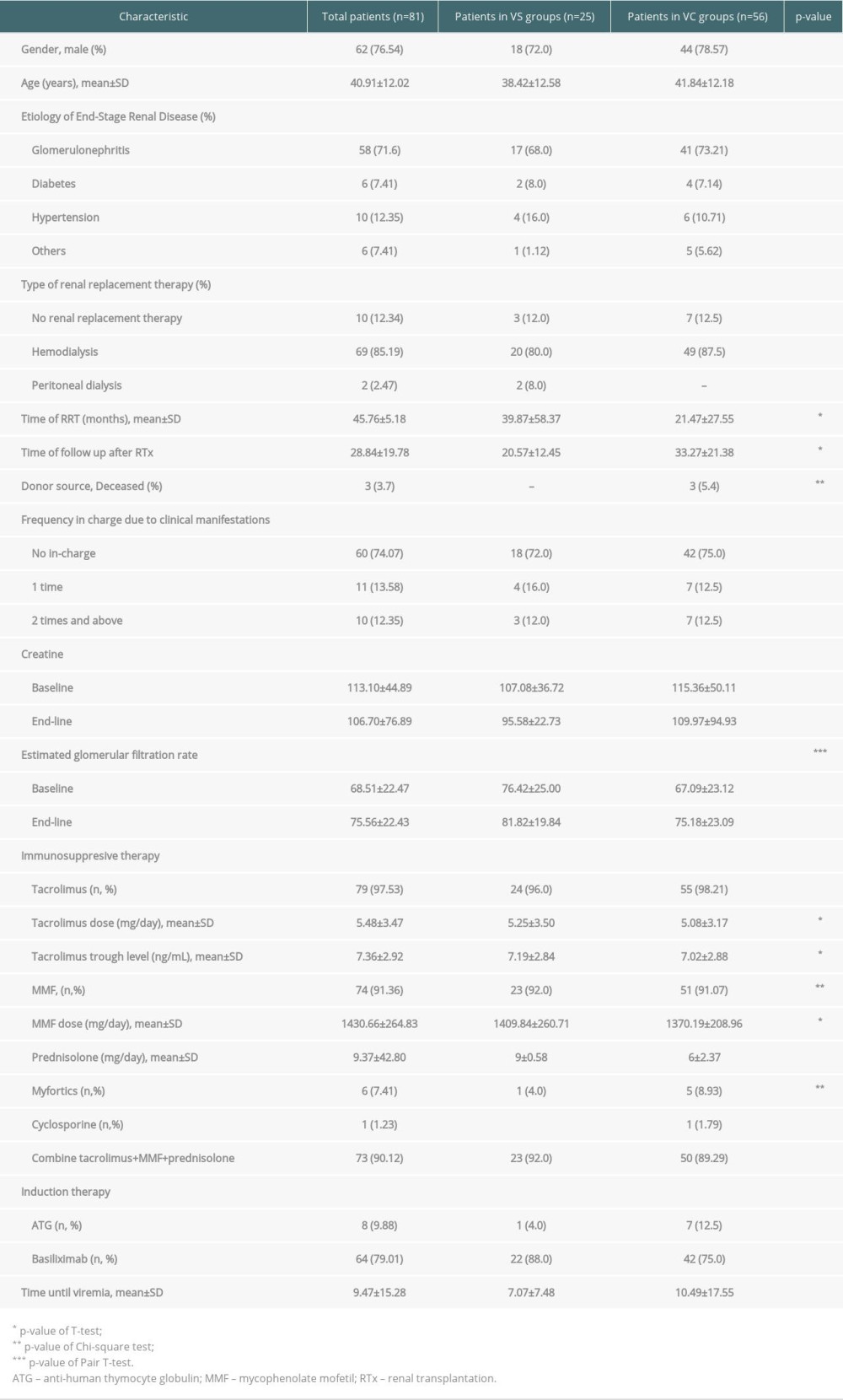 Table 2. Characteristics of viruria infection in renal transplant recipients (n, %).
Table 2. Characteristics of viruria infection in renal transplant recipients (n, %).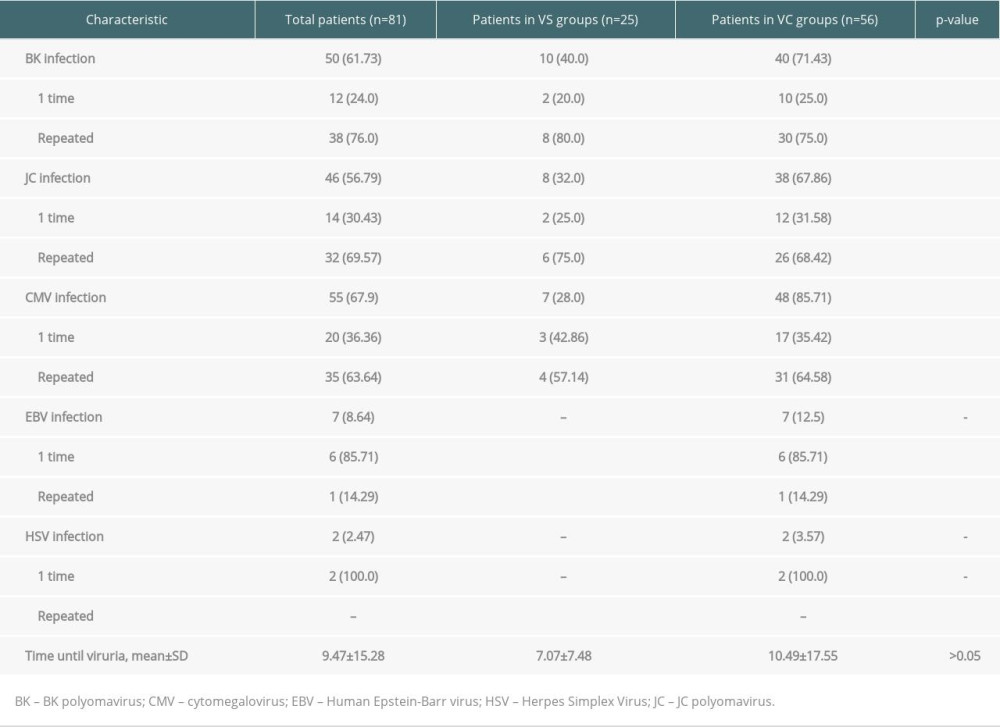 Table 3. Cox-regression for the development of BK polyomavirus only.
Table 3. Cox-regression for the development of BK polyomavirus only.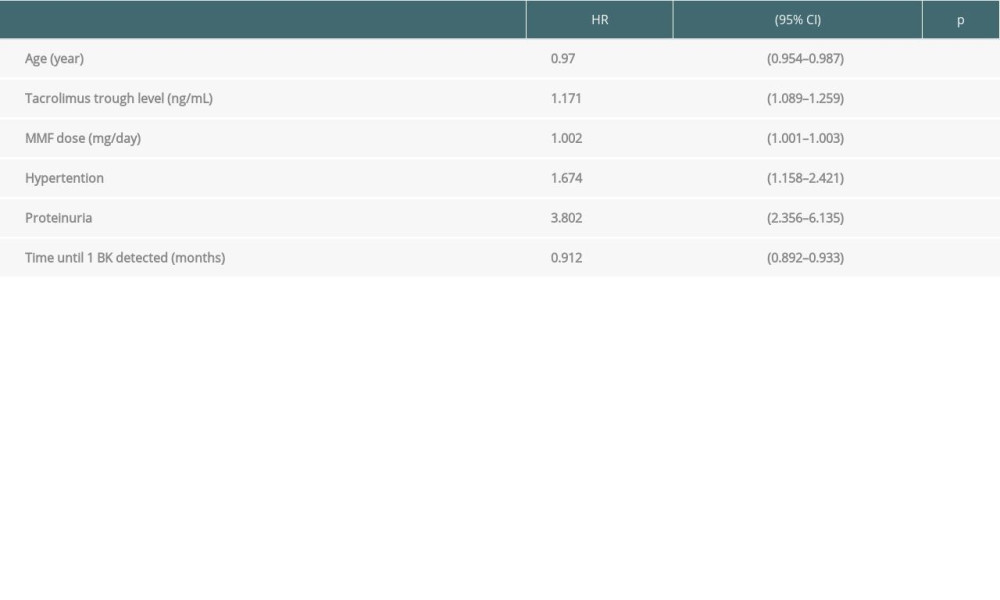 Table 4. Cox-regression for the development of JC polyomavirus only.
Table 4. Cox-regression for the development of JC polyomavirus only.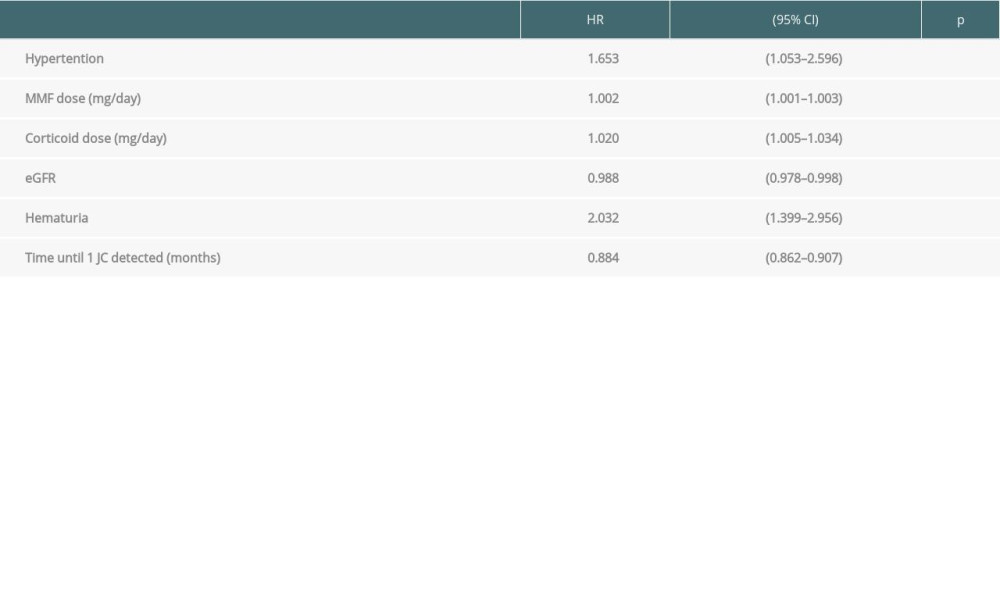 Table 5. Cox-regression for the development of CMV only.
Table 5. Cox-regression for the development of CMV only.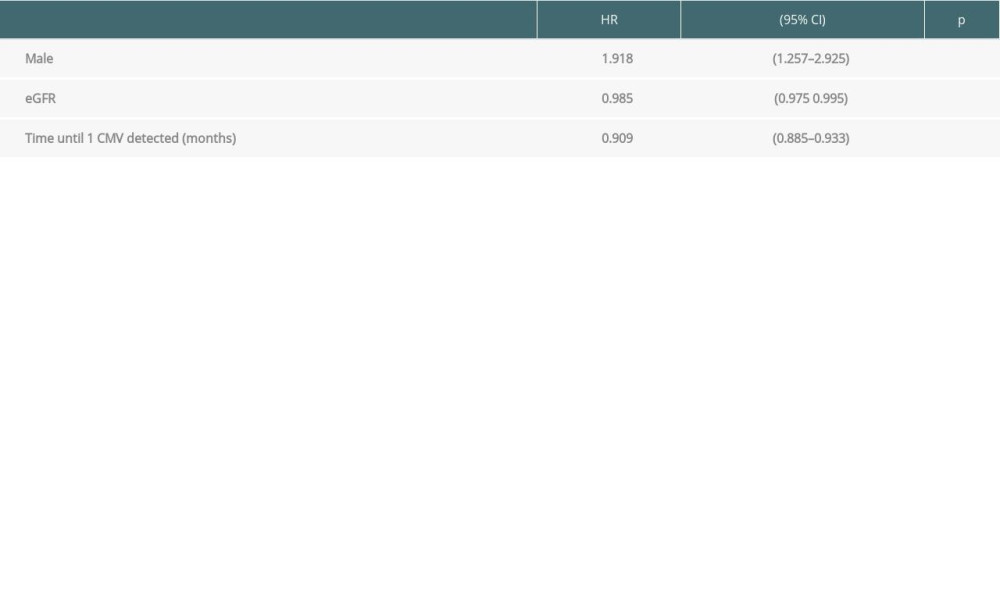 Table 6. Cox-regression for the development of viruria co-infection.
Table 6. Cox-regression for the development of viruria co-infection.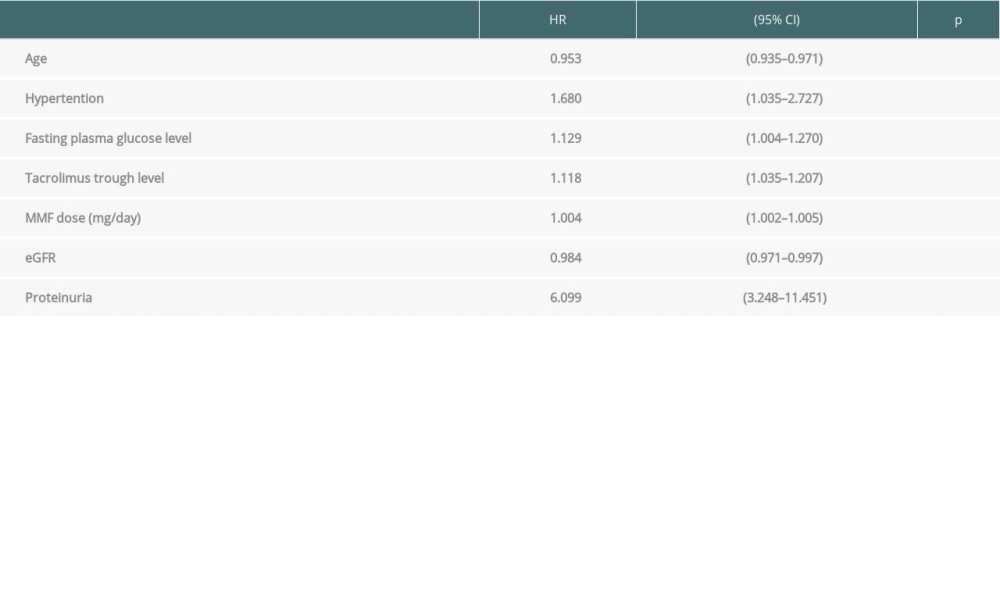
References
1. Nambiar P, Silibovsky R, Belden KA, Infection in kidney transplantation: Contemporary kidney transplantation, organ and tissue transplantation, 2018; 307-27, Springer International Publishing AG
2. Nakanishi K, Kaito H, Ogi M, Three severe cases of viral infections with post-kidney transplantation successfully confirmed by polymerase chain reaction and flow cytometry: Case Rep Nephrol Dial, 2018; 8; 198-206
3. Safaei F, Mohebbi A, Hassanpour M, Viruria of human BK virus and John Cunningham virus among renal transplant recipients and healthy control in Southeast of Caspian Sea: Intervirology, 2021; 64; 111-18
4. Hildreth JEK, Alcendor DJ, JC polyomavirus and transplantation: Implications for virus reactivation after immunosuppression in transplant patients and the occurrence of PML disease: Transplantology, 2021; 2; 37-48
5. Cook L, Polyomaviruses: Microbiology spectrum, 2016; 4
6. Brochot E, Descamps V, Handala L, BK polyomavirus in the urine for follow-up of kidney transplant recipients: Clin Microbiol Infect, 2019; 25; 112e1-e5
7. Khosravi M, Dadras M, Monfared A, Investigating risk factors for the development of BK virus infection in kidney transplant recipients in Guilan Province during 2007–2015: Urol J, 2020; 17; 620-25
8. Delbue S, Ferraresso M, Ghio L, A review on JC virus infection in kidney transplant recipients: Clin Dev Immunol, 2013; 2013; 926391
9. Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, BKV, CMV, and EBV Interactions and their effect on graft function one year post-renal transplantation: Results from a large multi-centre study: EBioMedicine, 2018; 34; 113-21
10. Jehn U, Schütte-Nütgen K, Bautz J, Clinical features of BK-polyomavirus and cytomegalovirus co-infection after kidney transplantation: Sci Rep, 2020; 10; 22406
11. Hasannia T, Moosavi Movahed SM, Vakili R, Active CMV and EBV infections in renal transplant recipients with unexplained fever and elevated serum creatinine: Ren Fail, 2016; 38; 1418-24
12. Van Delden C, Stampf S, Hirsch HH, Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study: Clin Infect Dis, 2020; 71; e159-e69
13. Agrawal A, Ison MG, Danziger-Isakov L, Long-term infectious complications of kidney transplantation: Clin J Am Soc Nephrol, 2022; 17(2); 286-95
14. Kim JS, Jeong KH, Lee DWKorean Organ Transplantation Registry Study Group, Epidemiology, risk factors, and clinical impact of early post-transplant infection in older kidney transplant recipients: The Korean organ transplantation registry study: BMC Geriatr, 2020; 20(1); 519
15. Saad EJ, Fernández P, Cardozo Azua AE, Infections in the first year after renal transplant: Medicina, 2020; 80; 611-21
16. Eidgahi ES, Lotfi Z, Tayefi M, Incidence and risk factors of common viral infections among renal transplant recipients during the first year post-transplant in North-eastern Iran: Saudi J Kidney Dis Transpl, 2019; 30; 597-605
17. Zhang Y, Zhou T, Huang M, Prevention of cytomegalovirus infection after solid organ transplantation: A Bayesian network analysis: Ann Clin Microbiol Antimicrob, 2020; 19(1); 34
18. Andrade-Sierra J, Heredia-Pimentel A, Rojas-Campos E, Cytomegalovirus in renal transplant recipients from living donors with and without valganciclovir prophylaxis and with immunosuppression based on anti-thymocyte globulin or basiliximab: Int J Infect Dis, 2021; 107; 18-24
19. Babazadeh A, Javanian M, Oliaei F, Incidence and risk factors for cytomegalovirus in kidney transplant patients in Babol, northern Iran: Caspian J Intern Med, 2017; 8; 23-29
20. Li P, Cheng D, Wen J, Risk factors for BK virus infection in living-donor renal transplant recipients: A single-center study from China: Ren Fail, 2018; 40; 442-46
21. Unal E, Topcu A, Demirpolat MT, Özkan ÖF, Viral infections after kidney transplantation: An updated review: International Journal of Virology and AIDS, 2018; 5; 40-43
22. Hirsch HH, Babel N, Comoli P, European perspective on human polyomavirus infection, replication and disease in solid organ transplantation: Clin Microbiol Infect, 2014; 20(Suppl 7); 74-88
23. Haider MZ, Aslam A, Proteinuria: StatPearls, 2022, Treasure Island (FL), StatPearls Publishing Copyright© 2022, StatPearls Publishing LLC
24. Krenzien F, El Hajj S, Tullis SG, Gabardi S, Immunosenescence and immunosuppressive drugs in the elderly: Handbook of immunosenescence: Basic understanding and clinical implications, 2017; 2147-67, Switzerland, Springer International Publishing
25. Krenzien F, ElKhal A, Quante M, A rationale for age-adapted immunosuppression in organ transplantation: Transplantation, 2015; 99; 2258-68
26. Chadban SJ, Ahn C, Axelrod DA, KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation: Transplantation, 2020; 104; S11-103
Figures
 Figure 1. Flowchart of patient inclusionThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072).
Figure 1. Flowchart of patient inclusionThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072). Figure 2. Frequency of viruria episodes among renal transplantation recipients in the 2 groupsThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072).
Figure 2. Frequency of viruria episodes among renal transplantation recipients in the 2 groupsThe figure was created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072). Figure 3. Association of frequency of episodes with the type of viruria co-infection among renal transplantation recipients* Triple viruria were CMV and BK co-infection with either JCV or EBV. The figure is created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072).
Figure 3. Association of frequency of episodes with the type of viruria co-infection among renal transplantation recipients* Triple viruria were CMV and BK co-infection with either JCV or EBV. The figure is created using Microsoft® Word for Microsoft Office 2019 MSO (Version 2208 Build 16.0.15601.20072). Tables
 Table 1. Characteristics of renal transplant recipients.
Table 1. Characteristics of renal transplant recipients. Table 2. Characteristics of viruria infection in renal transplant recipients (n, %).
Table 2. Characteristics of viruria infection in renal transplant recipients (n, %). Table 3. Cox-regression for the development of BK polyomavirus only.
Table 3. Cox-regression for the development of BK polyomavirus only. Table 4. Cox-regression for the development of JC polyomavirus only.
Table 4. Cox-regression for the development of JC polyomavirus only. Table 5. Cox-regression for the development of CMV only.
Table 5. Cox-regression for the development of CMV only. Table 6. Cox-regression for the development of viruria co-infection.
Table 6. Cox-regression for the development of viruria co-infection. Table 1. Characteristics of renal transplant recipients.
Table 1. Characteristics of renal transplant recipients. Table 2. Characteristics of viruria infection in renal transplant recipients (n, %).
Table 2. Characteristics of viruria infection in renal transplant recipients (n, %). Table 3. Cox-regression for the development of BK polyomavirus only.
Table 3. Cox-regression for the development of BK polyomavirus only. Table 4. Cox-regression for the development of JC polyomavirus only.
Table 4. Cox-regression for the development of JC polyomavirus only. Table 5. Cox-regression for the development of CMV only.
Table 5. Cox-regression for the development of CMV only. Table 6. Cox-regression for the development of viruria co-infection.
Table 6. Cox-regression for the development of viruria co-infection. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








