16 December 2022: Original Paper
Acute Pancreatitis in Patients After Liver Transplantation
Alzbeta Hujova1BCDEF, Peter Macinga1AEF, Jana Jarosova1E, Jiri Fronek2A, Pavel Taimr1A, Julius Spicak1A, Tomas Hucl1ACDEF*DOI: 10.12659/AOT.938114
Ann Transplant 2022; 27:e938114
Abstract
BACKGROUND: Acute pancreatitis (AP) is a relatively rare but serious complication that can occur after organ transplantation.
MATERIAL AND METHODS: The aim of this study was to evaluate the incidence, potential risk factors, and course of AP in patients following liver transplantation at a single large-volume transplant center.
RESULTS: Out of a total of 1850 transplanted patients, 49 (2.8%) were diagnosed with AP. Of this group, 37 (75.5%) had a mild form of AP and 12 (24.5%) had a severe form of AP. The mortality rate was 10% overall and 42% in the group of patients with severe AP. An early form of AP (<30 days from transplantation) occurred in 13 patients (26.5%), most of whom presented with severe AP (10 patients, 76.9%); 4 patients died (40%). A late form of AP was diagnosed in 36 patients (73.5%), most of whom had mild AP (34 patients, 94.4%); 1 of 2 patients with severe AP died. The most common AP etiologies were post-ERCP (38.8%), idiopathic (34.7%), and postoperative (18.4%). Chronic HBV infection was a risk factor for development of AP (P=0.01).
CONCLUSIONS: AP in liver transplant recipients was more frequent and more severe than in the general population. This unfavorable course was associated with the occurrence of AP in the early post-transplant period. Liver transplantation due to complications of HBV infection was a risk factor for the development of AP.
Keywords: Hepatitis B, Liver Transplantation, Pancreatitis, Humans, Acute Disease, Risk Factors, Incidence
Background
Acute pancreatitis (AP) is a disease of the pancreas, characterized by intraglandular zymogen activation, leading to cell damage and induction of an inflammatory response. The most common causes are cholecystolithiasis and alcohol consumption. Overall mortality reaches up to 5% [1].
Although geographically heterogeneous, the incidence of AP is increasing in developed countries, with annual cases ranging from 5 to 80 per 100 000 inhabitants [2,3].
Clinically, AP can manifest as either mild or severe. Severe pancreatitis is characterized by necrosis, distant failure, or the presence of other complications. It occurs in about 20% of patients, and is associated with a high mortality rate, ranging between 15% and 30%.
AP is a well-documented complication of organ transplantation, which occurs in up to 35% of transplant patients [4]. The incidence of AP following kidney transplantation is 1.2–7% and is associated with a high mortality rate [5,6]. AP has been reported in 1% of stem cell transplantations [7], 5.5% of intestinal transplantations [8], 2–18% of heart transplantations [9], and in up to 35% of pancreas transplantations [10]. A higher risk has been attributed to patients with lower age [7,11], hypercalcemia [5], alcohol abuse [11], gallstones [5,6,11,12], congenital abnormalities of the pancreas [5,7], viral infections (CMV, VZV, HBV) [5], and history of AP prior to transplantation [11] or after endoscopic retrograde cholangiopancreatography (ERCP) [13]. The use of several immunosuppressive drugs, including corticosteroids, tacrolimus, cyclosporine, azathioprine, and mycophenolate mofetil, is associated with the development of acute pancreatitis [6,7,12,13].
The reported incidence of acute pancreatitis in liver transplant recipients is 1.5–8% [14–16]. A higher risk is associated with hepatitis B infection as an indication for transplantation [17], retransplantation, extent of pancreatic dissection, type of biliary reconstruction, duration of venous bypass (>90 min), quantity of intraoperative i.v. calcium chloride administration, longer surgical time in association with hypotension, or use of an aortohepatic interposition graft or ERCP [15,18,19].
Mortality due to AP in transplant recipients is typically greater than in the general population, reaching up to 63.6% [14–16,19]. Higher mortality was reported in a group of patients with early pancreatitis (within 30 days of transplantation) and in individuals with a history of pancreatitis during the pre-transplant period. The presence of pancreatitis has also been strongly associated with a higher risk of graft failure [16].
The aim of our study was to determine the incidence, risk factors, and clinical course of AP in patients after liver transplantation.
Material and Methods
We performed a retrospective analysis of the medical records, laboratory results, and imaging findings of all patients meeting the diagnostic criteria of AP who underwent liver transplantation (or in combination with another organ transplantation) at the Institute for Clinical and Experimental Medicine (IKEM) between September 1996 and November 2020.
Diagnostic criteria were defined as the presence of at least 2 of the following 3 findings: (1) characteristic abdominal pain, (2) elevation of serum amylase beyond 3 times the upper limit of normal, (3) imaging findings typical for AP based on ultrasound or CT. For the purpose of our study, we categorized patients into 2 groups: those with mild pancreatitis (mild AP according to the revised Atlanta classification) and those with severe pancreatitis (moderate and severe forms of the revised Atlanta classification) [20]. Early pancreatitis was defined as AP occurring within 30 days after transplantation, with late pancreatitis defined as AP occurring 30 days or more after transplantation.
During the post-transplant period, all patients received standard treatment with a triple combination of calcineurin inhibitors (cyclosporine or later tacrolimus), mycophenolate, and corticosteroids (with a gradual reduction over time). Acute rejection treatment comprised administration of high-dose corticosteroids or, in selected cases, intravenous antithymocyte immunoglobulin. The diagnosis of chronic HBV infection as an indication for transplantation was based on the latest EASL clinical guidelines [21].
AP treatment was either conservative or interventional, based on need in accordance with standard recommendations. Briefly, conservative treatment consisted of intravenous fluids, analgesics, enteral or parenteral nutrition (based on need) and antibiotics. Interventional treatment involved radiology, endoscopy or surgery.
Data are presented as means and ranges. A 2-sample
Results
In total, 1850 patients underwent liver transplantation at our center since the start of the transplantation program in 1996. The group consisted of 1078 men and 772 women, with a mean age of 49.6 years (range, 0–76). The mean follow-up time was 90.5 months.
Acute pancreatitis occurred in 49 (2.6%) of all transplanted patients, consisting of 30 men and 19 women, with a mean age of 53.0 years (range, 12–71). There were no differences in sex representation or mean age between the groups with and without pancreatitis. Furthermore, there was no difference in the incidence of pancreatitis in the first vs second half of the study period: 13/606 patients (2.2%) vs 36/1244 patients (2.9%) (
Mild AP was diagnosed in 37 patients (75.5%), with severe AP occurring in 12 patients (24.5%). There were no deaths in the group of patients with mild AP. Five patients (41.7%) from the severe AP group died (Table 1).
Thirteen patients (26.5%) had an early form of AP (<30 days after transplantation). AP was diagnosed on average within 10 days after the procedure. Most of these patients had severe AP (10 patients, 76.9%), 4 of whom died (40%). Late AP occurred in 36 patients (73.5%). In this group, AP was diagnosed on average 40 months after the procedure. Most of these patients had mild AP (34 patients, 94.4%), while 2 patients had severe AP (5.6%), 1 of whom died. These results are summarized in Table 1 and Figures 1–3.
The most frequent cause of AP after liver transplantation was endoscopic retrograde cholangiopancreaticography (ERCP) (19 patients, 38.8%). In 17 patients (34.7%) the cause of AP was unclear, and in 9 patients (18.4%) AP was classified as postoperative (within 10 days after transplantation). Three patients (6.1%) had acute biliary pancreatitis (Table 2). All cases of post-ERCP pancreatitis (PEP) were mild, 13 cases (68.4%) followed the first ERCP, and in all of these patients there were other risk factors present for the development of PEP. In 6 patients (31.6%) the indication for ERCP was extraction of the internal biliary stent with or without cholestasis, and another frequent indication was stenosis of the biliary anastomosis or, in 1 case, biliary leak. During the study period, 346 liver transplant patients had a total of 1055 ERCPs, and the overall incidence of PEP in our patient group was 1.8%.
The most frequent indications for liver transplantation in patients without AP were alcoholic liver cirrhosis (21.2%), hepatocellular cancer (15.9%), primary sclerosing cholangitis (10.5%), and hepatitis C cirrhosis (8.2%). In patients with AP, the most common indications were alcoholic liver cirrhosis (14.3%), hepatitis C cirrhosis (14.3%), hepatocellular cancer (12.2%), and hepatitis B cirrhosis (12.2%). HBV infection was a statistically significantly more frequent indication for liver transplantation in patients with AP compared to patients without AP (7 patients, 14.3% vs 45 patients, 2.5%,
In most cases, AP occurred after the first liver transplant (45 patients, 91.8%), in 3 patients after the second transplant (6.1%), and in 1 patient after the third transplant (2.0%). The proportion of patients without AP who underwent 1 (92.3%), 2 (6.9%), or 3 (0.8%) transplants was not significantly different from those with AP.
Five patients with AP (10.2%) underwent hepaticojejunostomy, which was comparable to the proportion of patients without AP (168 patients, 9.7%) who underwent the same anastomosis procedure.
Complications of AP occurred in 12 patients (24.5% of all patients), manifesting as either local or systemic. Necrosis occurred in 9 patients, acute or chronic fluid collection in 6 patients, multiorgan failure in 6 patients, and colonic perforation and hemoperitoneum in 1 patient.
In total, 5 patients died (10.2%), all of whom had severe AP (mortality 42%). Four of these patients had early pancreatitis and 1 had late pancreatitis.
The first patient was a 64-year-old man with polycystic disease diagnosed with AP on the 8th postoperative day after liver and renal transplant. He developed infected necrosis, sepsis, and multiorgan failure. Despite repeated mini-invasive endoscopic necrosectomies, the patient died 20 days after diagnosis of AP.
The second patient was a 58-year-old man transplanted for liver cirrhosis due to non-alcoholic steatohepatitis. AP was diagnosed on the 29th postoperative day. The patient died the next day due to uncontrolled multiorgan failure.
The third patient was a 44-year-old woman transplanted for fulminant liver failure of unknown cause. Necrotising AP was diagnosed on the 8th postoperative day. Although the patient underwent repeated endoscopic necrosectomies for infected necrosis, sepsis progressed to multiorgan failure, culminating in death 47 days after diagnosis of AP.
The fourth patient was a 23-year-old woman who developed AP 6 days after transplantation for primary sclerosing cholangitis. Diffuse necrosis and edema of the pancreas resulted in compression and ultimate failure of the liver graft. Surgical revisions were unsuccessful, with the patient dying of multiorgan failure 6 days after diagnosis.
The fifth patient was a 50-year-old man transplanted for HBV liver cirrhosis who developed a late form of AP of unknown cause. Symptoms started approximately 15 years (5368 days) after transplantation. He developed infected necrosis deemed unsuitable for invasive intervention. The patient died 35 days after AP diagnosis. These results are summarized in Table 4.
Discussion
Acute pancreatitis can have a poor prognosis and can complicate the course of organ transplantation. It has been repeatedly documented in patients following kidney, bone marrow, intestine, heart, pancreas, and liver transplantations [4,13]. AP incidence and mortality in this group are often higher than in the general population. However, most of the available data derive from a limited number of retrospective studies dating from the 1990s, and many had small sample sizes [14,15,17,18,22]. In our study, we evaluated all patients transplanted and followed up at a single transplant center over a period of 24 years. We found a considerable incidence of acute pancreatitis in our population of liver transplant recipients, as well as a high mortality rate (10% overall and 42% in patients with severe AP).
According to reports, the incidence of post-liver transplant acute pancreatitis ranges between 1.5 and 8% [14–16]. In the largest study performed thus far, Krokos et al analyzed 1832 patients with 2161 transplantations, with pancreatitis occurring in 55 patients (3%) [15]. Producing very similar results, our study included 1850 patients representing 2005 transplants, with AP diagnosed in 49 patients (2.6%). In both studies, the incidence was within the lower part of the reported range.
The incidence of AP is higher in liver transplant patients than in the general population [23–25]. This difference can be attributed at least in part to the characteristics of patients after liver transplantation – these patients underwent a major intra-abdominal intervention in the hepatopancreatobiliary region of the abdominal cavity. Long-term immunosuppressive therapy in combination with other drugs is common, as are multiple post-transplant complications [13].
Postoperative pancreatitis typically occurs after surgery of the stomach, biliary tract, intra-abdominal vessels, or heart, where direct intraoperative trauma to the pancreas and/or ischaemia are suggested to cause pancreatitis. These factors can also be encountered during liver transplantation [13]. In the Krokos study, the risk of AP was higher among patients who underwent retransplantation. Only 52.7% of AP occurred in first-transplant patients, despite this group representing 72% of all transplants (
Infection is a well-known but rare etiology of AP. Agents typically include mumps, measles, or herpes viruses, bacteria such as legionella, leptospira, or mycoplasma, and some fungal or parasitic agents [26].
The hepatitis B virus exhibits highest affinity for hepatocytes. Although extrahepatic manifestations of the infection are known, they rarely involve acute pancreatitis. In a study involving 124 patients with acute viral hepatitis, 7 patients (5.7%) experienced mild acute pancreatitis. The most common cause was hepatitis E (4 patients), with hepatitis B confirmed in only 1 case [27]. In a recent systematic review, Panic et al reported 73 published cases of AP in patients with viral hepatitis, 6 of whom (8.2%) had HBV [28]. Interestingly, the only reported case of chronic pancreatitis associated with viral hepatitis was due to HBV. Since both HBsAg and HCV antigens were detected in pancreatic tissue, the authors hypothesized that chronic infection with HBV or HCV viruses might also affect the pancreas [28]. In 1988, Alexander et al compared HBsAg+ and HBsAg− liver transplantation patients. AP occurred in 4/27 HBsAg+ patients but not in HBsAg− patients. Interestingly, acute hepatitis B of the graft was present in all patients with AP [17]. In the largest study to date of AP occurrence in the post-transplant population, 31% of 55 patients with AP were transplanted for HBV infection, which represented only 6% of all indications for transplantation [15]. In our study, we confirmed this association. HBV infection as an indication for transplantation was 6 times more common among patients with AP than among those without AP. A mild form of AP with an uneventful course of disease was present in most cases (5 patients, 71.4%). All of these patients presented with a late form of AP. Two patients had severe AP. The first patient developed severe AP 20 days after a transplantation complicated by sepsis. No intervention was indicated and the patient was discharged 40 days later. The second patient developed AP 14 years after transplantation. AP was complicated by infection and the patient died. Although the exact mechanism behind the association of AP and HBV infection is unclear, 2 hypotheses have been advanced. The first assumes direct toxicity of the virus and its components in acinar cells. Cavallari et al describe the case of a 32-year-old man who underwent liver transplantation following fulminant liver failure associated with HBV infection. On the 60th postoperative day, the patient developed necrotising pancreatitis resulting in death. Histological examination revealed the presence of HBsAg and HBV DNA in acinar cells of the pancreas in contrast to a corresponding absence in other organs, namely the spleen, kidney, lung, and brain [22].
The second hypothesis posits that HBV-infected acinar cells can trigger an immune response similar to that triggered by HBV-infected hepatocytes. Another contention is that this chronic process can result in subclinical acinar cell damage, increasing vulnerability to other, well-defined insults.
Biliary complications represent one of the most common post-transplant complications. Stenosis and leakage of biliary anastomosis are indications for endoscopic retrograde cholangiopancreatography (ERCP), a procedure associated with acute pancreatitis risk. The level of risk in the general population ranges between 3% and 8%, depending also on the level of technical complexity, which is often higher in liver transplant patients [29]. In a study evaluating 234 cases of ERCP performed in a post-liver transplant population, a form of uncomplicated AP developed in 9 patients (3.9%) [30]. In another study, evaluating 730 ERCP procedures involving 301 patients, the AP incidence was 3%. ERCP performed on a native papilla was associated with pancreatitis (OR=4.04, 95% CI 1.40–11.65), while the use of prednisone was found to have a protective effect [31].
In 2 previous studies, ERCP was the cause of pancreatitis after liver transplantation in about 11% of patients [32,33]; however, the number of patients included was small. In contrast, the results of our study confirmed ERCP as the most common cause of AP, accounting for more than one-third of cases (39%). There are various explanations for this difference. First, our group of patients was significantly larger. In one of the smaller studies, ERCP as an etiology represented 11% of AP cases, accounting for only one-ninth of all patients [33]. Clearly, studies involving such low numbers have a risk of bias. Second, the incidence of AP correlates with the proportion of patients requiring ERCP. Third, prophylactic measures such as administration of non-steroidal anti-inflammatory drugs or pancreatic stents have only entered into standard use in recent years [29]. Most of our patients developed AP at a time when prophylactic measures were not yet being used. The overall incidence of PEP in our study group was 1.8%. According to some studies, the incidence may be lower than in the general population due to the protective role of immunosuppression [31].
The role of immunosuppression in the development of AP is not entirely clear. Several studies have reported an increased risk of AP owing to the use of ciclosporin, corticosteroids, everolimus, sirolimus, or tacrolimus [34–37]. The role of azathioprine in the development of AP is better understood. For example, up to 7% of patients with inflammatory bowel disease on azathioprine develop AP [38]. In the post-transplant population, its role is not as evident. Frick et al found no association between the use of azathioprine and AP in patients after kidney transplantation [39].
In our study, we were unable to identify a typical cause of AP in our group of 26 patients (53.1%). Nine of these patients, who developed pancreatitis within 10 days of transplantation, were classified as postoperative AP. Ligation of the gastroduodenal artery in patients with suboptimal hepatic artery inflow and extensive lymphadenopathy in patients with hepatocellular carcinoma may be associated with pancreatic injury, but neither of these proceedures was done in any of our 9 patients. Thus, only some level of unspecific pancreatic injury associated with major abdominal surgery can be considered. The remaining 17 patients were classified as true idiopathic. All of these patients received combined immunosuppressive treatment involving cyclosporine/tacrolimus, corticosteroids, and mycophenolate. Except in 2 cases, all patients had late pancreatitis. Of the 3 patients with a severe form, 2 died. One patient on azathioprine developed AP approximately 3 years after transplantation, while another patient had been given NSAIDs prior to the development of AP.
In our AP group, 11 patients were transplanted for alcoholic liver cirrhosis. Alcohol use was the cause of AP only in 1 patient (2% of AP and 11% of patients transplanted for alcoholic cirrhosis). Results are similar for liver graft failure where the proportion of cases caused by alcohol is low, as is the recurrence rate of alcohol abuse after transplantation (approximately 10–20%). In a group of patients studied by Verran et al, alcohol was the cause of a one-third of pancreatitis cases after transplantation. However, other studies have found alcohol to be a rare cause [33,37].
The clinical course of AP in patients after liver transplantation can be modified both by past major intra-abdominal surgery and/or immunosuppressive therapy. Currently, there is no recommended treatment for AP in this specific group of patients. The only probable exception is the use of prophylactic antibiotics. In our study population, we administered antibiotics to all patients diagnosed with AP knowing that immunosuppressive therapy would potentially put them at higher risk of infection. Infected necrosis developed in 9 out of 12 patients with severe AP (75%). No intervention was required in the 7 surviving patients. All 5 deceased patients developed early infected necrosis: 2 were treated conservatively, another 2 underwent endoscopic necrectomy, while the remaining patient was indicated for early surgical revision. The mortality rate of early necrectomy was 100%, a not entirely surprising result. As reported in most studies, the mortality of open necrectomy in the general population exceeds 50% when performed early [40,41].
Our study shows that the proportional representation of different types of pancreatitis was similar across post-transplant and general populations, despite a poorer prognosis in the post-transplant population [1,20]. Although mild pancreatitis dominated, the infrequent severe form had a high mortality rate (42%). We further confirmed earlier observations of different prognoses for early vs late forms of AP [32]. The early form, defined as pancreatitis occurring within 30 days, has been associated with a high mortality rate of up to 63%. Late pancreatitis has a more favorable prognosis, with mortality rates between 0% and 11% [32,33]. In our group of patients, overall mortality was 10% and mortality from severe pancreatitis was 42%. Overall mortality rates of early pancreatitis and late pancreatitis were 31% and 3%, respectively. Compared to the study by Krokos, published more than 25 years ago, overall mortality in our study was lower (63.6% vs 10.2%) [15].
Our study has several shortcomings. The retrospective nature of the work limits the quality of the data evaluated, making verification impossible in some cases. Furthermore, the classification of etiology in patients with postoperative and idiopathic pancreatitis was arbitrary and therefore does not categorically exclude unrecognised etiological factors. Knowledge of perioperative hemodynamic parameters such as the presence of prolonged hypotension, the extent of peripancreatic tissue dissection, or the use of an aortohepatic graft would be appropriate for a more precise evaluation of postoperative pancreatitis. We had no up-to-date information on the level of viremia in HBV-infected patients who had developed AP, even though active replications were unlikely given the immunization, normal liver function tests, and normal liver function.
Conclusions
Acute pancreatitis occurs relatively rarely after liver transplantation, but more frequently than in the general population. The main risk factors include major intra-abdominal surgery, HBV infection as an indication for transplantation, administration of immunosuppressive drugs, and post-transplant biliary complications requiring ERCP. The early form is associated with a severe course and high mortality. Although based on retrospective analysis and requiring definitive confirmation by larger prospective studies, the results of our study, especially given the large number of patients evaluated, warrant the attention of physicians specializing in the care of patients after liver transplantation.
Figures
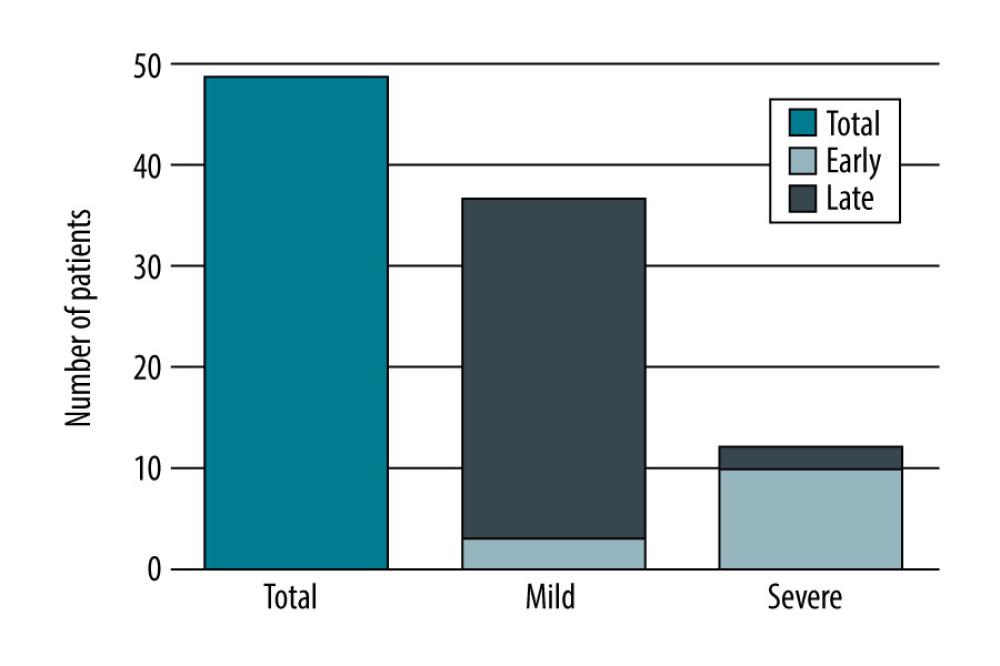 Figure 1. Number of patients with AP after liver transplantation based on its severity, created in Numbers (version 4.1.1, 4338).
Figure 1. Number of patients with AP after liver transplantation based on its severity, created in Numbers (version 4.1.1, 4338). 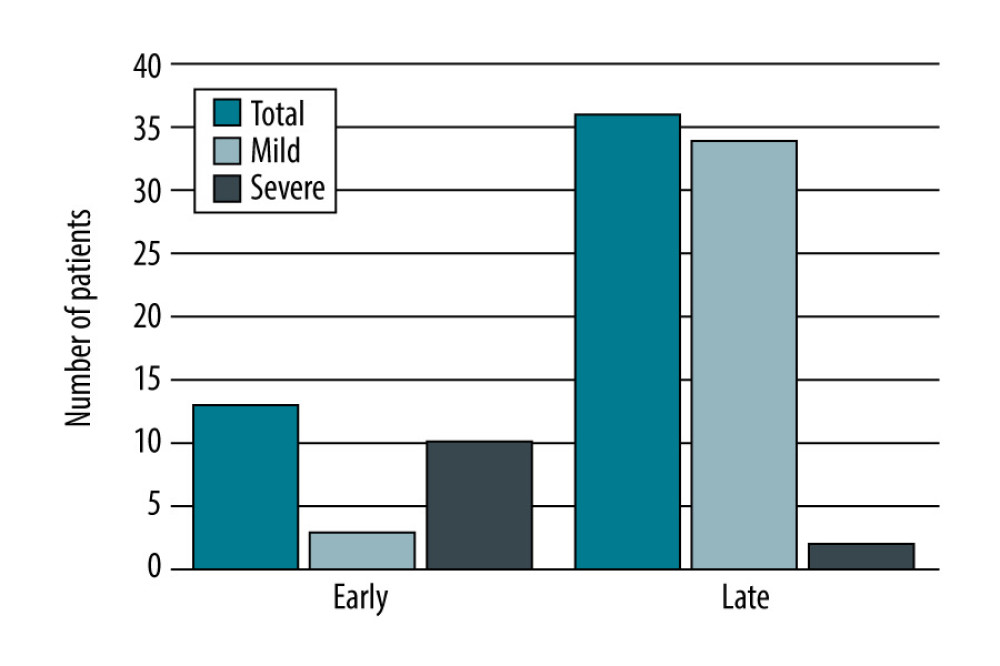 Figure 2. Number of patients with AP after liver transplantation based on the time from transplantation, created in Numbers (version 4.1.1, 4338).
Figure 2. Number of patients with AP after liver transplantation based on the time from transplantation, created in Numbers (version 4.1.1, 4338). 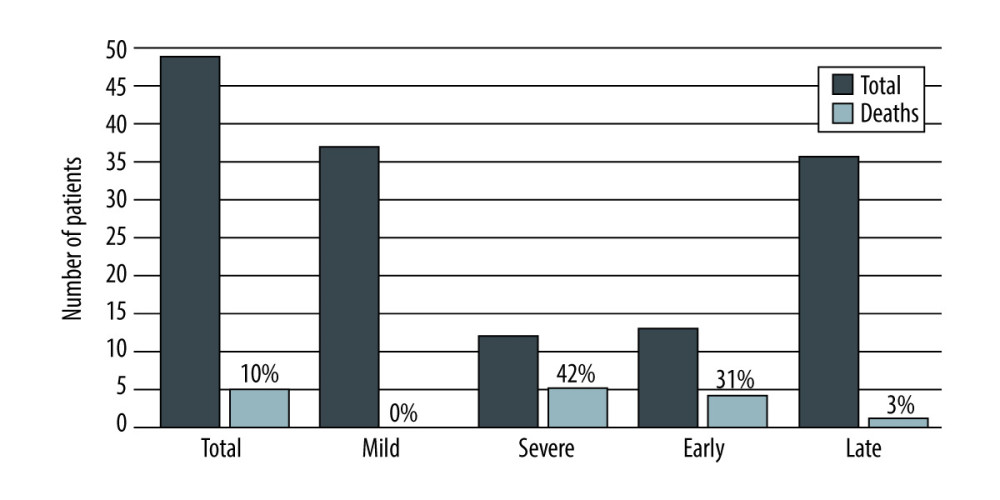 Figure 3. Mortality rate of acute pancreatitis after liver transplantation depending on the severity and time from the transplantation, created in Numbers (version 4.1.1, 4338).
Figure 3. Mortality rate of acute pancreatitis after liver transplantation depending on the severity and time from the transplantation, created in Numbers (version 4.1.1, 4338). Tables
Table 1. Characteristics of patients after liver transplantation in relation to AP.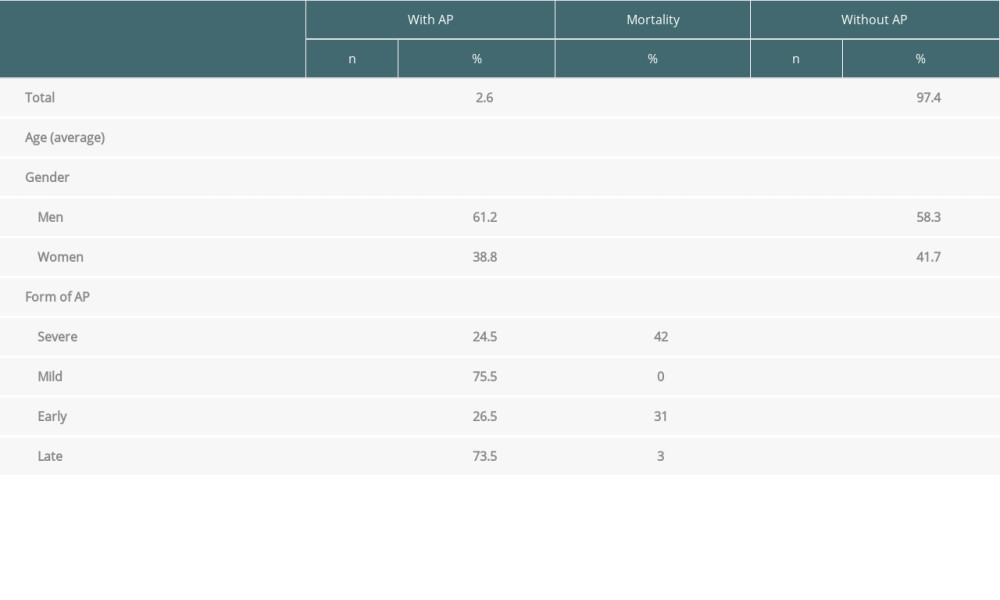 Table 2. Characteristics of liver transplant recipients with acute pancreatitis.
Table 2. Characteristics of liver transplant recipients with acute pancreatitis.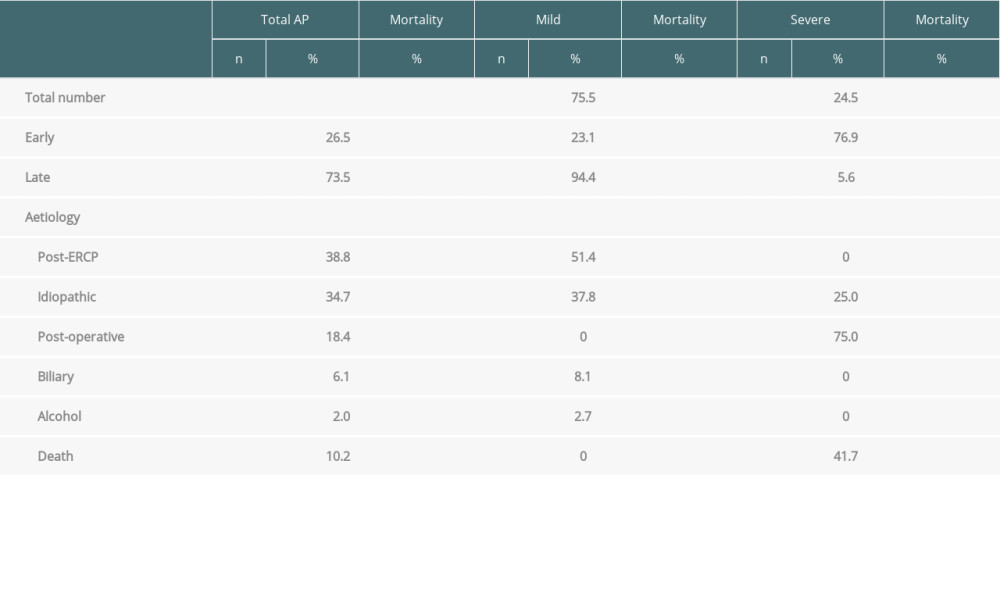 Table 3. Etiology of liver disease as an indication for OLTx in relation to AP.
Table 3. Etiology of liver disease as an indication for OLTx in relation to AP.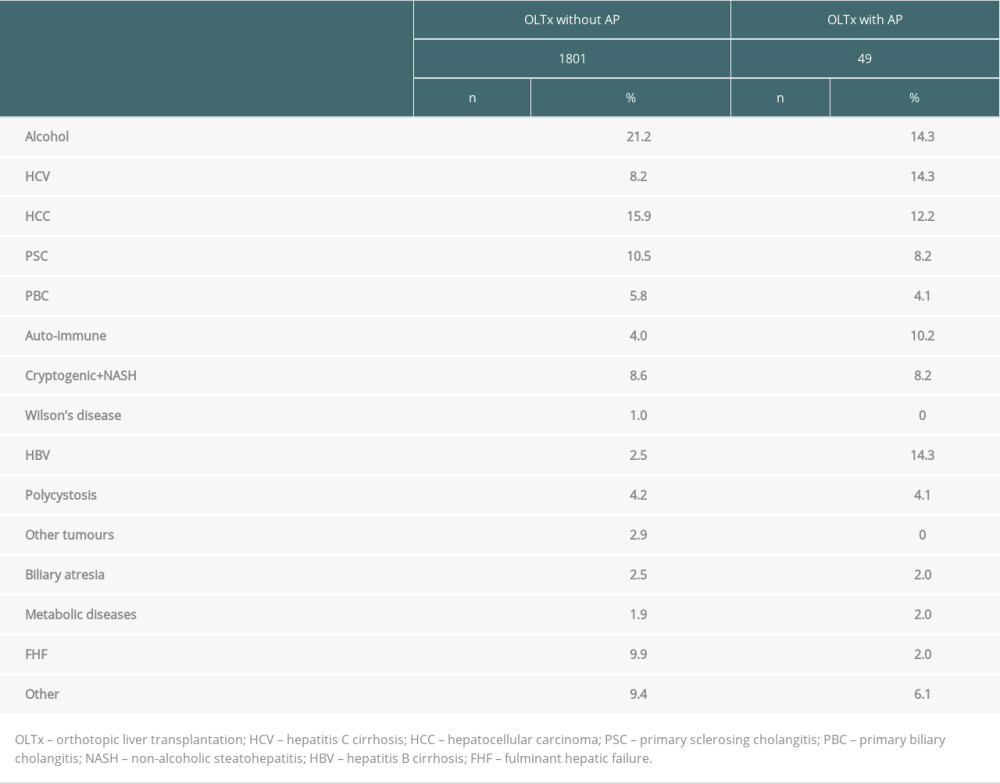 Table 4. Characteristics of liver transplant recipients who died due to AP.
Table 4. Characteristics of liver transplant recipients who died due to AP.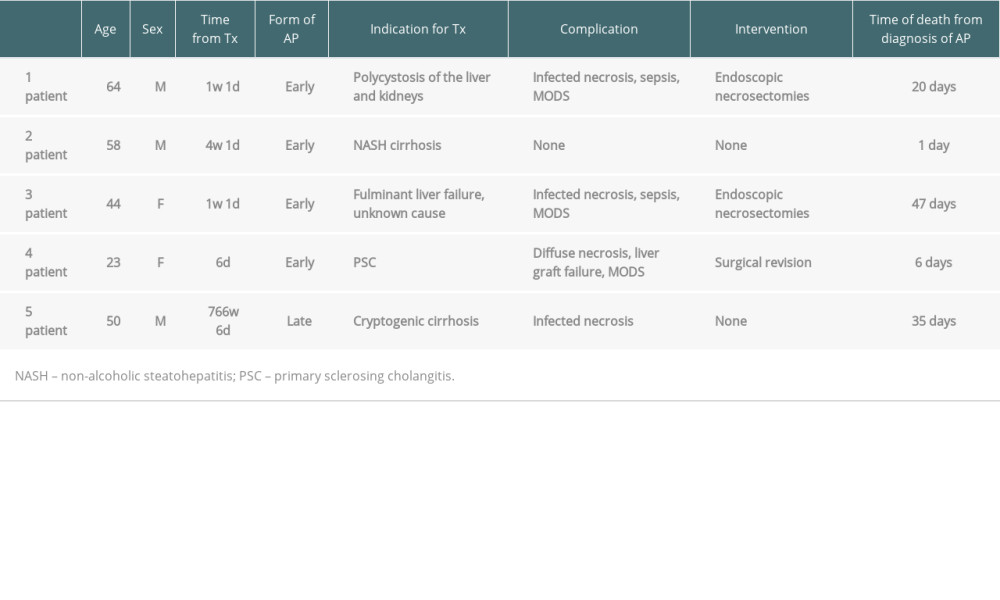
References
1. Arvanitakis M, Dumonceau JM, Albert J, Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines: Endoscopy, 2018; 50(5); 524-46
2. Xiao AY, Tan ML, Wu LM, Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies: Lancet Gastroenterol Hepatol, 2016; 1(1); 45-55
3. Ouyang G, Pan G, Liu Q, The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017: BMC Med, 2020; 18(1); 388
4. Graham D, Ito T, Busuttil R, Kaldas F, Pancreatitis in solid organ transplant patients: A review of the literature: OBM Hepatology and Gastroenterology, 2019; 3(3); 1-11
5. Bhadauria D, Thammishetti V, Goel A, An analysis of epidemiology, etiology, and outcomes of acute pancreatitis in renal transplant recipients: Transplant Proc, 2020; 52(3); 865-72
6. Tabakovic M, Salkic NN, Bosnjic J, Alibegovic E, Acute pancreatitis after kidney transplantation: Case Rep Transplant, 2012; 2012; 768193
7. Wang XL, Han W, Zhao P, Incidence, risk factors, outcomes, and risk score model of acute pancreatitis after allogeneic hematopoietic stem cell transplantation: Biol Blood Marrow Transplant, 2020; 26(6); 1171-78
8. Borhani AA, Dasyam AK, Papachristou G, Radiologic features of pancreatic and biliary complications following composite visceral transplantation: Abdom Imaging, 2015; 40(6); 1961-70
9. Lin TW, Tsai MT, Roan JN, Obscured hemorrhagic pancreatitis after orthotopic heart transplantation complicated with acute right heart failure and hepatic dysfunction: A case report: J Cardiothorac Surg, 2016; 11(1); 166
10. Nadalin S, Girotti P, Königsrainer A, Risk factors for and management of graft pancreatitis: Curr Opin Organ Transplant, 2013; 18(1); 89-96
11. Chuang YW, Huang ST, Yu TM, Acute pancreatitis risk after kidney transplantation: Propensity score matching analysis of a national cohort: PLoS One, 2019; 14(9); e0222169
12. Xu J, Xu L, Wei X, A case report: acute pancreatitis associated with tacrolimus in kidney transplantation: BMC Nephrol, 2019; 20(1); 209
13. Danalıoğlu A, Mitchell OJ, Singh VK, Acute pancreatitis following adult liver transplantation: A systematic review: Turk J Gastroenterol, 2015; 26(6); 450-55
14. Camargo CA, Greig PD, Levy GA, Clavien PA, Acute pancreatitis following liver transplantation: J Am Coll Surg, 1995; 181(3); 249-56
15. Krokos NV, Karavias D, Tzakis A, Acute pancreatitis after liver transplantation: incidence and contributing factors: Transpl Int, 1995; 8(1); 1-7
16. Russell TA, Park S, Agopian VG, Peritransplant pancreatitis: A marker of high mortality and graft failure in liver transplant patients: Liver Transpl, 2017; 23(7); 925-32
17. Alexander JA, Demetrius AJ, Gavaler JS, Pancreatitis following liver transplantation: Transplantation, 1988; 45(6); 1062-65
18. Xie Q, Liu J, Zhuang L, Report of two cases of acute pancreatitis after liver transplantation for HCC: Transplantation, 2018; 102; S871
19. Yanaga K, Shimada M, Gordon RD, Pancreatic complications following orthotopic liver transplantation: Clin Transplant, 1992; 6(2); 126-30
20. Banks PA, Bollen TL, Dervenis CAcute Pancreatitis Classification Working Group, Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus: Gut, 2013; 62(1); 102-11
21. , EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection: J Hepatol, 2017; 67(2); 370-98
22. Cavallari A, Vivarelli M, D’Errico A, Fatal necrotizing pancreatitis caused by hepatitis B virus infection in a liver transplant recipient: J Hepatol, 1995; 22(6); 685-90
23. Lankisch PG, Assmus C, Maisonneuve P, Lowenfels AB, Epidemiology of pancreatic diseases in Lüneburg County. A study in a defined german population: Pancreatology, 2002; 2(5); 469-77
24. Roberts SE, Morrison-Rees S, John A, The incidence and aetiology of acute pancreatitis across Europe: Pancreatology, 2017; 17(2); 155-65
25. Hujova A, Jarosova J, Janeckova J, Acute pancreatitis in patients after liver transplantation: Gastroenterologie a Hepatologie, 2020; 74(4); 311-18
26. Parenti DM, Steinberg W, Kang P, Infectious causes of acute pancreatitis: Pancreas, 1996; 13(4); 356-71
27. Jain P, Nijhawan S, Rai RR, Acute pancreatitis in acute viral hepatitis: World J Gastroenterol, 2007; 13(43); 5741-44
28. Panic N, Mihajlovic S, Vujasinovic M, Pancreatitis associated with viral hepatitis: Systematic review: J Clin Med, 2020; 9(10); 3309
29. Dumonceau JM, Kapral C, Aabakken L, ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline: Endoscopy, 2020; 52(2); 127-49
30. Balderramo D, Bordas JM, Sendino O, Complications after ERCP in liver transplant recipients: Gastrointest Endosc, 2011; 74(2); 285-94
31. Law R, Leal C, Dayyeh BA, Role of immunosuppression in post-endoscopic retrograde cholangiopancreatography pancreatitis after liver transplantation: A retrospective analysis: Liver Transpl, 2013; 19(12); 1354-60
32. Lupo L, Pirenne J, Gunson B, Acute-pancreatitis after orthotopic liver transplantation: Transplant Proc, 1997; 29(1–2); 473
33. Verran DJ, Gurkan A, Chui AK, Pancreatitis in adult orthotopic liver allograft recipients: risk factors and outcome: Liver Transpl, 2000; 6(3); 362-66
34. Fontana F, Cappelli G, Acute pancreatitis associated with everolimus after kidney transplantation: A case report: BMC Nephrology, 2016; 17(1); 163
35. Lorber MI, Van Buren CT, Flechner SM, Hepatobiliary and pancreatic complications of cyclosporine therapy in 466 renal transplant recipients: Transplantation, 1987; 43(1); 35-40
36. Ogunseinde BA, Wimmers E, Washington B, A case of tacrolimus (FK506)-induced pancreatitis and fatality 2 years postcadaveric renal transplant: Transplantation, 2003; 76(2); 448
37. Robinson DO, Pancreatitis and renal disease: Scand J Gastroenterol, 1977; 12(1); 17-20
38. Teich N, Mohl W, Bokemeyer BGerman IBD Study Group, Azathioprine-induced acute pancreatitis in patients with inflammatory bowel diseases-a prospective study on incidence and severity: J Crohns Colitis, 2016; 10(1); 61-68
39. Frick TW, Fryd DS, Goodale RL, Lack of association between azathioprine and acute pancreatitis in renal transplantation patients: Lancet, 1991; 337(8735); 251-52
40. Husu HL, Kuronen JA, Leppäniemi AK, Mentula PJ, Open necrosectomy in acute pancreatitis-obsolete or still useful?: World J Emerg Surg, 2020; 15(1); 21
41. Mejia J, Sucandy I, Steel J, Indications and outcomes of pancreatic surgery after liver transplantation: Clin Transplant, 2014; 28(3); 330-36
Figures
 Figure 1. Number of patients with AP after liver transplantation based on its severity, created in Numbers (version 4.1.1, 4338).
Figure 1. Number of patients with AP after liver transplantation based on its severity, created in Numbers (version 4.1.1, 4338). Figure 2. Number of patients with AP after liver transplantation based on the time from transplantation, created in Numbers (version 4.1.1, 4338).
Figure 2. Number of patients with AP after liver transplantation based on the time from transplantation, created in Numbers (version 4.1.1, 4338). Figure 3. Mortality rate of acute pancreatitis after liver transplantation depending on the severity and time from the transplantation, created in Numbers (version 4.1.1, 4338).
Figure 3. Mortality rate of acute pancreatitis after liver transplantation depending on the severity and time from the transplantation, created in Numbers (version 4.1.1, 4338). Tables
 Table 1. Characteristics of patients after liver transplantation in relation to AP.
Table 1. Characteristics of patients after liver transplantation in relation to AP. Table 2. Characteristics of liver transplant recipients with acute pancreatitis.
Table 2. Characteristics of liver transplant recipients with acute pancreatitis. Table 3. Etiology of liver disease as an indication for OLTx in relation to AP.
Table 3. Etiology of liver disease as an indication for OLTx in relation to AP. Table 4. Characteristics of liver transplant recipients who died due to AP.
Table 4. Characteristics of liver transplant recipients who died due to AP. Table 1. Characteristics of patients after liver transplantation in relation to AP.
Table 1. Characteristics of patients after liver transplantation in relation to AP. Table 2. Characteristics of liver transplant recipients with acute pancreatitis.
Table 2. Characteristics of liver transplant recipients with acute pancreatitis. Table 3. Etiology of liver disease as an indication for OLTx in relation to AP.
Table 3. Etiology of liver disease as an indication for OLTx in relation to AP. Table 4. Characteristics of liver transplant recipients who died due to AP.
Table 4. Characteristics of liver transplant recipients who died due to AP. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








