03 January 2023: Original Paper
Impact of Autologous Stem Cell Transplantation on Primary Central Nervous System Lymphoma in First-Line and Relapse Settings: A Retrospective Study in China
Jing Liu1BCDEF*, Haitao Wang1BCDF, Xiaohong Li1B, Yamei Wu1B, Yuanyuan Ma1B, Zhenyang Gu1B, Fei Li1B, Meng Li1BD, Jiayuan Guo2B, Yu Zhao1B, Quanshun Wang1B, Jian Bo1B, Wenrong Huang3B, Liping Dou1BD, Yuanbo Liu4AEF, Daihong Liu1BD, Xiaoxiong Wu1ADF, Chunji Gao1ADEFGDOI: 10.12659/AOT.938467
Ann Transplant 2023; 28:e938467
Abstract
BACKGROUND: Myeloablative chemotherapy supported by autologous stem cell transplantation (ASCT) is an option for primary central nervous system lymphoma (PCNSL) in both the relapse setting and as postremission consolidation, but the level of evidence in this field is still low.
MATERIAL AND METHODS: We retrospectively analyzed 47 HIV-negative PCNSL patients from 2010 to 2021. To assess the outcomes in patients undergoing ASCT.
RESULTS: Of the 47 patients, the median age was 51 (range, 21-77) years, and 28 (59.6%) were male. After induction, 33 (70.2%) patients achieved complete remission, and 6 (12.8%) patients achieved partial remission. At a median follow-up of 21.4 months (95% CI 8.86-33.95), the median progression-free survival (PFS) was 23.3 months (95% CI 14.87-31.73), and the 4-year PFS rate was 14.6%. The median overall survival (OS) time was 62.4 months (95% CI 41.93-82.87), and the 4-year OS rate was 71.5%. Among 20 patients who received ASCT (10 consolidation, 10 salvage), the 4-year PFS and 4-year OS rates were 57.3% and 71.2%, respectively. In the multivariate analysis, ASCT therapy (hazard ratio [HR] 0.16, P=0.016) and early remission (HR 0.12, p=0.003) were found to be independent prognostic factors for a longer PFS. Two treatment-related deaths occurred in patients with multiple relapses before ASCT. Pancytopenia and diarrhea were the most common adverse events.
CONCLUSIONS: ASCT offers potential long-term PFS with good tolerability for patients with PCNSL. Our retrospective cohort adds to the currently available literature and identifies disease status after induction as a significant factor affecting survival.
Keywords: Central Nervous System Neoplasms, Consolidation Chemotherapy, Hematopoietic Stem Cell Transplantation, Prognosis, Salvage Therapy, Humans, Male, Middle Aged, Female, Transplantation, Autologous, Antineoplastic Combined Chemotherapy Protocols, Recurrence, Lymphoma, Central Nervous System, Stem Cell Transplantation
Background
Primary central nervous system lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma (NHL) of the central nervous system, including the brain, spine, cerebrospinal fluid (CSF), and eyes [1,2]. It is predominantly diffuse large B-cell lymphoma of the activated B-cell subtype, but compared with that of systemic lymphoma, survival is usually inferior [3, 4]. High-dose methotrexate (HD-MTX)-based chemotherapy is the preferred standard induction regimen, but the remission period is short, and the disease often progresses and recurs. Therefore, subsequent consolidation therapy is proposed.
Consolidation strategies include whole-brain radiotherapy (WBRT), high-dose chemotherapy (HDC) supported by autologous stem cell transplantation (ASCT), and nonmyeloablative chemotherapy [5–8]. Currently, no clear consensus exists on the optimal choice for consolidation. To avoid the risk of severe neurotoxicity associated with WBRT in the management of PCNSL, as an alternative, ASCT is the most investigated strategy. A number of studies have reported that HDC supported by ASCT is highly efficacious with acceptable tolerability, mostly using thiotepa-based conditioning regimens [9–12]. However, the level of evidence in this field remains low, particularly that on the impact of conditioning regimens [13,14]. Additionally, no prospective randomized clinical trials involving patients with relapsed or refractory PCNSL have been published. Multiple therapies are in use today, but similar to the consolidation regimens described earlier, there is no consensus on the optimal salvage treatment of these patients [3].
In the current study, we summarized the characteristics of 47 PCNSL patients and reported the results of ASCT both in the first-line and relapse settings, addressing the efficacy and safety of different conditioning regimens.
Material and Methods
PATIENTS:
Data from 47 immunocompetent PCNSL patients from 3 centers with histologically confirmed newly diagnosed PCNSL between May 10, 2010 and June 3, 2021, were included in this analysis. Patients who achieved complete (CR) or partial remission (PR) after HD-MTX-based chemotherapy were selected for consolidation therapy with ASCT. For relapsed/refractory PCNSL, chemosensitive and fit patients were candidates for ASCT if they had not received this therapy previously. Additional criteria required to proceed to HDC followed by ASCT included age of 18 years or older, Eastern Cooperative Oncology Group (ECOG) performance status of 0–3, and adequate cardiac, pulmonary, hepatic, and renal functions. This was a retrospective study and all aspects of the study design were retrospective. All data were extracted from the medical records system. The study was approved by the Ethics Committee of Chinese People’s Liberation Army (PLA) General Hospital.
INDUCTION TREATMENT:
All patients received induction chemotherapy based on HD-MTX either as monotherapy (at least 3 g/m2 per infusion) or in combination. The HD-MTX-containing regimens mainly included HD-MTX with rituximab (RM), HD-MTX, cytarabine and rituximab (RMA), HD-MTX and temozolomide (MT) with or without rituximab, HD-MTX, cytarabine, idarubicin, and dexamethasone (MAID) with or without rituximab, HD-MTX with rituximab and lenalidomide (R2M), and HD-MTX, ibrutinib, and rituximab (IRM). Patient condition (age, performance status, and comorbidities) guided the clinician to select the most suitable regimen for an individual patient. Response was assessed after the second-to-third cycles of chemotherapy. Autologous stem cell mobilization and collection were performed during the fifth or sixth cycle of chemotherapy with daily colony stimulating factor (filgrastim, 7–10 μg/kg×4 days) administration.
SALVAGE TREATMENT:
Recurrent patients with remission times longer than 12 months after HD-MTX-based induction chemotherapy were retreated with HD-MTX-based chemotherapy. Patients with refractory disease or early recurrence (remission time less than 12 months) received several single-agent or combination therapy regimens, mainly drugs including liposomes, doxorubicin, temozolomide, pemetrexed, ibrutinib, etoposide, lenalidomide, and rituximab. For multiple relapses or rapid disease progression without better systemic therapy, WBRT was selected.
HIGH-DOSE CHEMOTHERAPY FOLLOWED BY AUTOLOGOUS STEM CELL TRANSPLANTATION:
The R-BEAM group received carmustine (300 mg/m2 IV) on day −7 (7 days before stem cell infusion), etoposide (200 mg/m2 IV) on day −6 to day −3, cytarabine (300 mg/m2 IV) on day −6 to day −3, melphalan (140 mg/m2 PO) on day −2, rituximab (375 mg/m2 IV) on day −1, and stem cell infusion on day 0. The TBC-like group received busulfan (3.2 mg/kg IV) on day −9 to day −7, thiotepa (250 mg/m2 IV) on day −6 to day −5, cyclophosphamide (200 mg/m2 IV) on day −4 to day −3, and stem cell infusion on day 0. The modified TBC group received cladribine (5 mg/m2 IV) on day −11 to day −8, cytarabine (1.5 g/m2 IV) on day −11 to day −8, thiotepa (250 mg/m2 IV) on day −11 to day −10, busulfan (3.2 mg/kg IV) on day −7 to day −5, etoposide (300 mg/m2 IV) on day −4, cyclophosphamide (40 mg/kg IV) on day −3 to day −2, and stem cell infusion on day 0 (Figure 1).
Patients received acyclovir as prophylaxis from day 1 and colony stimulating factor (filgrastim) from day 3 after stem cell infusion. Neutrophil engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count ≥0.5×109/L, and platelet engraftment was defined as the day at which the platelet count was ≥20×109/L and platelet transfusion was not needed.
EVALUATION OF TREATMENT RESPONSE AND DISEASE STATUS:
Responses were assessed by contrast-enhanced brain magnetic resonance imaging (MRI) according to the criteria of the International Workshop on Evaluation of Primary Central Nervous System Lymphoma [15]. CR was defined as resolution of tumor enhancement and normalization of the ocular fundi for at least 4 weeks in the absence of steroid treatment. PR was defined by at least a 50% reduction in the size of the measurable lesions or a reduction in vitreous infiltration. Stable disease (SD) was defined as no objective change in the lesions. Progressive disease (PD) was defined as a 25% increase in tumor size, vitreous infiltration, or the appearance of new lesions.
Relapse was defined as disease recurrence after CR lasting for at least 1 month after therapy. Refractory disease was defined as SD or PD during MTX-based first-line treatment or recurrence of the disease during therapy.
FOLLOW-UP:
Patients were assessed by contrast-enhanced MRI and a clinical evaluation every 2 months for the first year, every 4 months during the second and third years, every 6 months for years 4–5, and then every year thereafter.
STATISTICS:
Overall survival (OS) was calculated from the date of diagnosis to death by any cause, and progression-free survival (PFS) was calculated from the date of diagnosis to the date of treatment failure or death by any cause. To evaluate the role of ASCT in the relapse setting, PFS was calculated from the date of last recurrence before transplantation. OS1 and PFS1 were measured from the day of transplantation. Survival curves were computed using the Kaplan-Meier method and compared using the log-rank test. The chi-square test was conducted to compare variables between the ASCT and non-ASCT groups. A nonparametric test was conducted to compare the median time of neutrophil/platelet engraftment. The impact of clinical variables on survival was analyzed using a Cox proportional hazards model. All statistical analyses were performed using R software (version 4.2.0) and SPSS 23.0 software package, and
Results
CLINICAL CHARACTERISTICS:
A total of 47 patients with a diagnosis of PCNSL who received HD-MTX-based chemotherapy were included. The clinical characteristics of the patients are summarized in Table 1. The median age was 51 years (range 21–77), and 13 patients (27.7%) were ≥60 years of age. Twenty-eight patients (59.6%) were male. The diagnosis was obtained by stereotactic biopsy (68.1%), surgery (29.8%), and brain MRI (2.1%). Forty-five (95.7%) PCNSLs were proven to be DLBCL. Twenty patients received ASCT (10 as postremission consolidation and 10 as salvage treatment), and 27 patients did not. The comparison of baseline clinical characteristics between the 2 groups is summarized in Table 1. There were no statistically significant differences between the 2 groups. The 20 ASCT patients received 1 of 3 conditioning regimens: BEAM (n=2), TBC-like (n=5), or modified TBC (n=13) (Tables 2, 3).
RESPONSE TO THERAPY:
After induction chemotherapy, 33 (70.2%) out of 47 patients had already achieved CR, and 6 (12.8%) had achieved PR. Ten ASCT consolidation patients were in CR before and after ASCT. ASCT was used as salvage therapy for 10 relapsed/refractory patients, and 2 of the 3 patients in PR achieved CR after ASCT. The median number of days to neutrophil engraftment was 10.5 (range, 8–16) for ASCT as consolidation therapy and 14 (range, 10–31) for ASCT as salvage therapy (
SURVIVAL OUTCOME:
One patient was lost to follow-up after induction therapy and was not included in the survival evaluation. In addition, 2 patients were lost to follow-up after relapse and were not included in the overall survival evaluation. During a median follow-up of 21.4 months (95% confidence interval [CI] 8.86–33.95), 7 patients died, and the median OS was 62.4 months (95% CI 41.93–82.87). The 2- and 4-year OS rates were 95.3% and 71.5%, respectively. The median PFS was 23.3 months (95% CI 14.87–31.73), and the 2- and 4-year PFS rates were 43.8% and 14.6%, respectively (Figure 2A). For patients receiving ASCT therapy (n=20), the 2- and 4-year PFS rates were 89.1% and 57.3%, respectively, and the 2- and 4-year OS rates were 100% and 71.2%, respectively (Figure 2B). Treatment with ASCT resulted in longer PFS than treatment without ASCT (P=0.001) (Figure 2C). There were no significant differences in OS between the ASCT and without ASCT groups (P=0.52) (Figure 2D). In the multivariate analysis, treatment with ASCT (hazard ratio [HR] 0.16, 95% CI 0.04–0.71, P=0.016) and early response with CR/PR (HR 0.12, 95% CI 0.03–0.48, P =0.003) were significant prognostic factors for longer PFS (Table 4).
Among patients receiving ASCT, the 3-year OS1 and PFS1 probabilities from the day of transplantation were 76.3% and 50.9%, respectively. There were no significant differences in the PFS1 (P=0.21) or OS1 (P=0.069) between the ASCT consolidation and salvage groups (Figure 3).
Further analysis showed that among 33 CR patients, 16 received consolidation after induction therapy: ASCT (n=10), WBRT (n=1), and nonmyeloablative chemotherapy (n=5). The probabilities of 4-year PFS and OS were 43.0% and 59.3%, respectively. Patients who received consolidation had a longer PFS than patients without consolidation (P=0.0093). However, there was no significant difference in OS between the 2 groups (P=0.09) (Figure 4).
TOXICITY:
HD-MTX-based induction chemotherapy was well tolerated with no treatment-related deaths. Only 1 patient discontinued chemotherapy due to toxicity (consistently high methotrexate levels after methotrexate administration, elevated transaminase, and renal toxicity) and received WBRT. Treatment with ASCT both in the first-line and relapse settings was well tolerated. As expected, hematological and gastrointestinal toxicities were more common, and grade 4 nonhematological toxicity was uncommon. More mucositis and infections were seen in the TBC-based conditioning regimens than in the BEAM regimen. Two toxic deaths after ASCT as salvage treatment were due to infections (bacteremia and pneumonia) and occurred 0.5 and 4.5 months after ASCT. Of the 20 ASCT patients, 4 patients (20.0%) developed neurotoxicity, 3 of whom had previously received WBRT and 1 of whom had received WBRT after ASCT (Tables 2, 3).
Discussion
Induction therapy with HD-MTX-based chemotherapies (MTX dose of 3–8 g/m2) is the standard for PCNSL and can achieve an overall response rate (ORR) of more than 85% and a CR rate of 50% or higher [3,16]. Despite the high response rates with initial HD-MTX-based therapy, approximately 50% of responders are still at risk for progression or relapse. Moreover, approximately 25% of patients fail to respond to the initial treatment [17]. Some of the more aggressive regimens, such as consolidation or salvage, have been proposed, but there is currently no consensus on the optimal treatment. HDC-ASCT is the most investigated strategy [18].
In this study, 47 patients with PCNSL received HD-MTX-based induction chemotherapy. The dosage of MTX, MTX as monotherapy or combination, and the choice of additional antineoplastic medications to combine with HD-MTX depended on the age, performance status, and patient comorbidities. We mainly used drugs capable of crossing the blood–brain barrier, including Ara-C, idarubicin, rituximab, and temozolomide. Based on the reviewed literature and evidence, no single regimen is clearly the most effective [3]. After induction therapy, the ORR was 83.0%, with 33 (70.2%) patients achieving CR. Sixteen CR patients received further treatment as consolidation. Five and 12 relapses occurred in the post-consolidation and no consolidation groups, respectively. Patients who received consolidation had a longer PFS; however, there was no significant difference in OS between the 2 groups. The main goal of consolidation is to delay relapse and significantly improve PFS. Currently, no clear consensus exists on the optimal choice for consolidation. The consolidation therapy should be selected based on factors such as patient’s age, performance status, response to initial chemotherapy, and complications. In our previous meta-analysis, we compared ASCT with WBRT and found no significant differences in the CR rate, relapse rate, PFS, or OS. Cognitive functions were preserved or improved after ASCT, whereas cognitive impairment was observed after WBRT [19]. This study showed that ASCT was a feasible approach for consolidation, with good tolerability for newly diagnosed PCNSL patients. Hematological toxicity was more common without severe nonhematological toxicity. One of the 10 ASCT consolidation patients who had been subjected to WBRT after ASCT experienced epilepsy.
Considering that ASCT is an effective treatment for relapsed/refractory NHL [20], several studies have analyzed its use for patients with relapsed/refractory PCNSL [21–23]. In our study, 10 relapsed/refractory PCNSL patients received a TBC-based conditioning regimen followed by ASCT. Two of the 3 patients in PR achieved CR after ASCT. One PR patient before ASCT relapsed 19.2 months after ASCT. Three patients died after ASCT, and 2 patients (20%) were counted as having treatment-related mortality (TRM) with causes of death due to infection: 1 relapsed 2 times, and 1 relapsed 3 times before ASCT treatment. The 2-year PFS and OS estimates from the day of transplantation were both 52.5%. Six patients received prior WBRT, 3 (30.0%) of whom developed neurotoxicity. In several studies [21–23], the 2-year PFS estimate ranged from 56.1% to 58%, the 2-year OS estimate ranged from 59.6% to 69%, the 5-year OS estimate ranged from 51.4% to 55.6%, and the TRM ranged from 0% to 7.6%. Our study compared several studies, and the survival probability was lower with higher TRM. This difference may be explained by the fact that our sample size was small, with a short follow-up time and a different starting point for survival time (from diagnosis, from the day of transplantation, or from inclusion), 3 patients experienced multiple relapses, and 8 of 10 patients received a more intensive conditioning regimen (modified TBC). Second or later complete remission was associated with higher risks of relapse. Multiple relapses resulted in patients developing poor tolerance to treatment and death. In evaluating ASCT in the relapsed/refractory setting, 2 studies found that PFS was better for patients who received ASCT after salvage chemotherapy than for patients who received only salvage chemotherapy [21, 24]. In a study conducted by Soussain et al [21], the 2-year PFS probability was 43% among all the patients and 58% in TBC-ASCT patients. A study by Choi et al [24] showed that the median PFS of patients who received ASCT was 19.5 months but 6.7 months for those without ASCT. For refractory or recurrent patients who are good candidates for rechallenge with HD-MTX–based therapy, this may be the first option [25,26]. Usually, remission in these patients is longer than 12 months after initial HD-MTX–based therapy according to the NCCN Clinical Practice Guidelines in Oncology for CNS Cancers [27]. Chemosensitive patients in good condition could select ASCT if they have not received this therapy previously [11,22].
In our study, treatment with ASCT (10 consolidations and 10 salvage therapies) resulted in longer PFS than treatment without ASCT. This result may be related to the proportion of patients who received consolidation and treatment intensity. Half of the patients who received consolidation after induced remission and the dose-intensive conditioning regimen in the ASCT group had longer PFS than a lower number of patients (11%) who received consolidation and a milder consolidation with nonmyeloablative chemotherapy in the non-ASCT group. However, we did not find a difference in OS between those undergoing ASCT and those who did not. We observed a similar proportion of patients who received salvage treatment (WBRT or nonmyeloablative chemotherapy) in the non-ASCT and ASCT groups: 51.9% versus 50%, respectively. However, compared to that in the ASCT group, the proportion of consolidation was lower in the non-ASCT group (11% vs 50%). This may be the reason for the same OS in both groups. Because the main goal of consolidation is to improve PFS, no clear consensus exists on the optimal choice for salvage therapies.
Currently, most recommendations and treatment decisions for conditioning regimens are based on single-arm phase II trials and retrospective studies, and there have been few randomized prospective clinical trials. In our study, 20 patients with PCNSL underwent ASCT with 1 of 3 conditioning regimens: BEAM (n=2), TBC-like (n=5), and modified TBC (n=13). Considering that the BEAM regimen is effective with good tolerability for systemic lymphomas and is a feasible approach for elderly patients with DLBCL [28,29], several early studies have analyzed its use in patients with newly diagnosed PCNSL [7,30,31]. These studies showed that TRM as a result of BEAM followed by ASCT ranged from 0% to 7.1%, and the relapse rate was 17.6% to 57.1%, with more common toxicities including neutropenia and infections. However, these drugs have poor blood–brain barrier permeability and are associated with a higher relapse rate. Thiotepa, busulfan, and cyclophosphamide can penetrate the blood–brain barrier [32]. Thiotepa distributes into the CSF after administration; 15 minutes later, the CSF levels of the drug are equivalent to its plasma concentrations [32,33]. The CSF concentration of busulfan appears to be approximately equivalent to the plasma concentration [34,35]. Cyclophosphamide is the most commonly used treatment in a variety of bone marrow transplantation conditioning regimens requiring immunosuppression and/or antineoplastic activity. It is distributed throughout the body, including in the CSF [33]. Theoretically, TBC drugs should produce better effects than the BEAM regimen because of their good blood–brain barrier penetration. The reviewed literature indicates that the relapse rate ranged from 0% to 14.3% with the TBC regimen [10,36–38], which is much lower than that of the BEAM regimens (17.6–57.1%). In our study, the relapse rates were 50% and 0% in the BEAM and TBC-like groups, respectively. TBC combined with cytarabine, cladribine, and etoposide was defined as the modified TBC regimen, which was proposed for the first time. The cytarabine CSF concentration rises linearly with increasing doses (1–3 g/m2) [39]. Thirteen patients received the modified TBC regimen; 1 relapse occurred after 19.2 months, and 2 patients died 4.5 and 0.53 months after ASCT due to infections. TBC-like drugs, especially the modified TBC conditioning regimen, appeared to be more toxic than BEAM drugs. This finding suggests that TBC-based treatment may be more suitable for patients who are younger and in good condition, particularly those with their first complete remission, and that BEAM may be more suitable for patients who are older with more comorbid conditions. In our study, due to the small number of cases in each group, we did not conduct statistical analysis to compare the survival rates. One observational cohort study indicated that the thiotepa-based conditioning regimen was associated with higher rates of survival compared with BEAM, despite higher rates of early toxic effects and non-relapse mortality [40]. One meta-analysis comparing different condition regimens indicated that although BCNU/TT (carmustine/thiotepa) carried the lower risk of transplant-related mortality and equivalent response rate to TBC, TBC showed a lower relapse rate and superior overall survival (OS) and progression-free survival (PFS) rates [41].
Regarding prognostic factors, the multivariate Cox regression analyses conducted in our study showed that treatment with ASCT and early response with CR/PR were significant prognostic factors for longer PFS. These results are inconsistent with the 2 commonly used prognostic scoring systems, the International Extranodal Lymphoma Study Group (IELSG) and the Memorial Sloan Kettering Cancer Center (MSKCC) score [42,43]. The IELSG score was based on data from patients diagnosed over 30 years ago and followed up for a median of only 24 months. Furthermore, patients with increased intracranial pressure may be unable to undergo lumbar puncture to obtain CSF protein concentrations. In the MSKCC score, the assessment of performance status is fairly subjective [44]. We did not evaluate the LDH and CSF protein levels because of a lack of data. Recently, a study by Sun et al [8] proposed early response with CR/PR as a prognostic factor, consistent with our results.
This study has several limitations: (1) This study was retrospective in nature and had a small sample size. (2) Selection bias may have occurred. The most common criterion for ASCT eligibility is age, and patients aged <60 years are more often considered for this modality. (3) The induction therapy or intensive chemotherapy regimens used among different centers were significantly different. (4) Formal neuropsychiatric testing was not performed in all patients before induction therapy, after induction therapy, before ASCT, after ASCT, or during disease progression.
Conclusions
Sequential HD-MTX-based chemotherapy followed by HDC/ASCT is a promising therapy option leading to remarkable long-term survival in eligible patients. Two randomized clinical trials (IELSG 32 trial and PRECIS trial) compared HDC/ASCT to WBRT as consolidation in first-line therapy, showing that no significant differences in 2-year PFS; cognitive impairment was observed after WBRT, whereas cognitive functions were preserved or improved after ASCT. Upcoming results of the NCT01511562 and NCT02531841 studies will help to decide between nonmyeloablative chemotherapy and HDC/ASCT consolidation. Reduced-dose irradiation is an option for patients who are not candidates for aggressive chemotherapy, and we hope that currently ongoing clinical trials (NCT02313389, NCT02623010, NCT02498951, and NCT0412035) that evaluate maintenance treatment will prove its efficacy. Further studies are needed to determine the optimal conditioning regimen followed by ASCT, the optimal strategy for relapse after ASCT, and the optimal strategy for patients with multiple relapses.
Figures
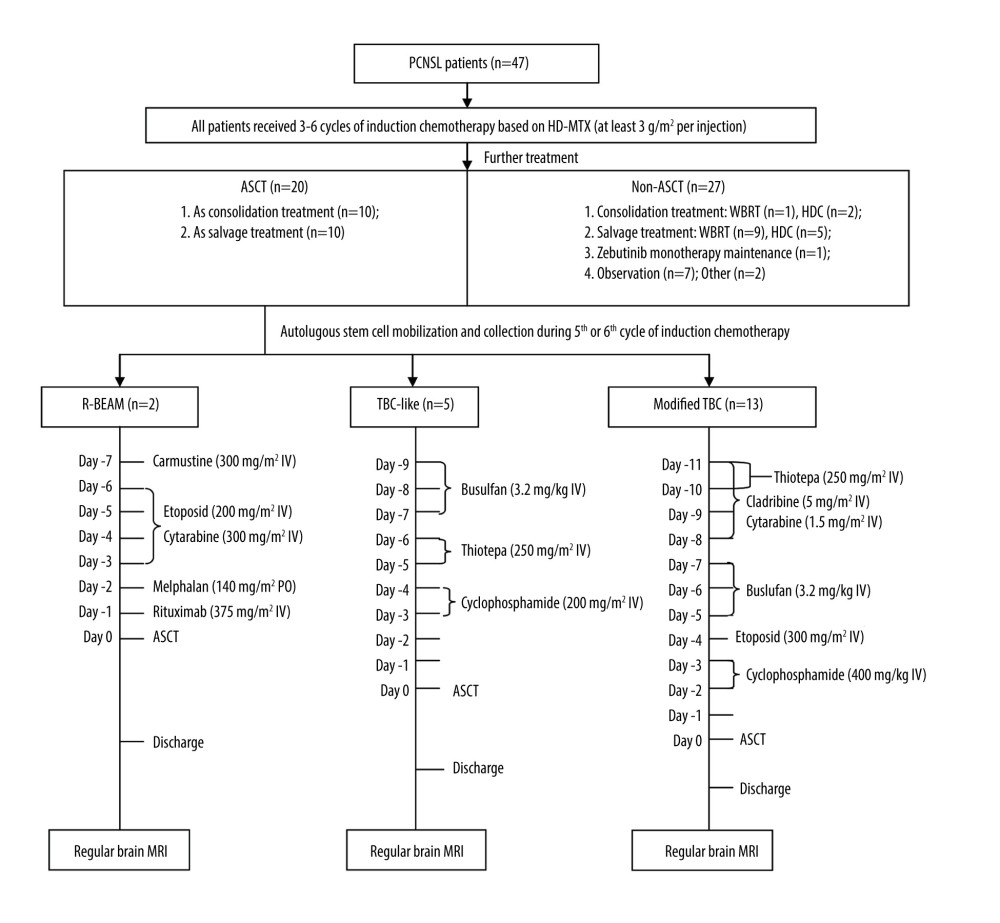 Figure 1. Treatment procedures. (Adobe Illustrator 2022).
Figure 1. Treatment procedures. (Adobe Illustrator 2022). 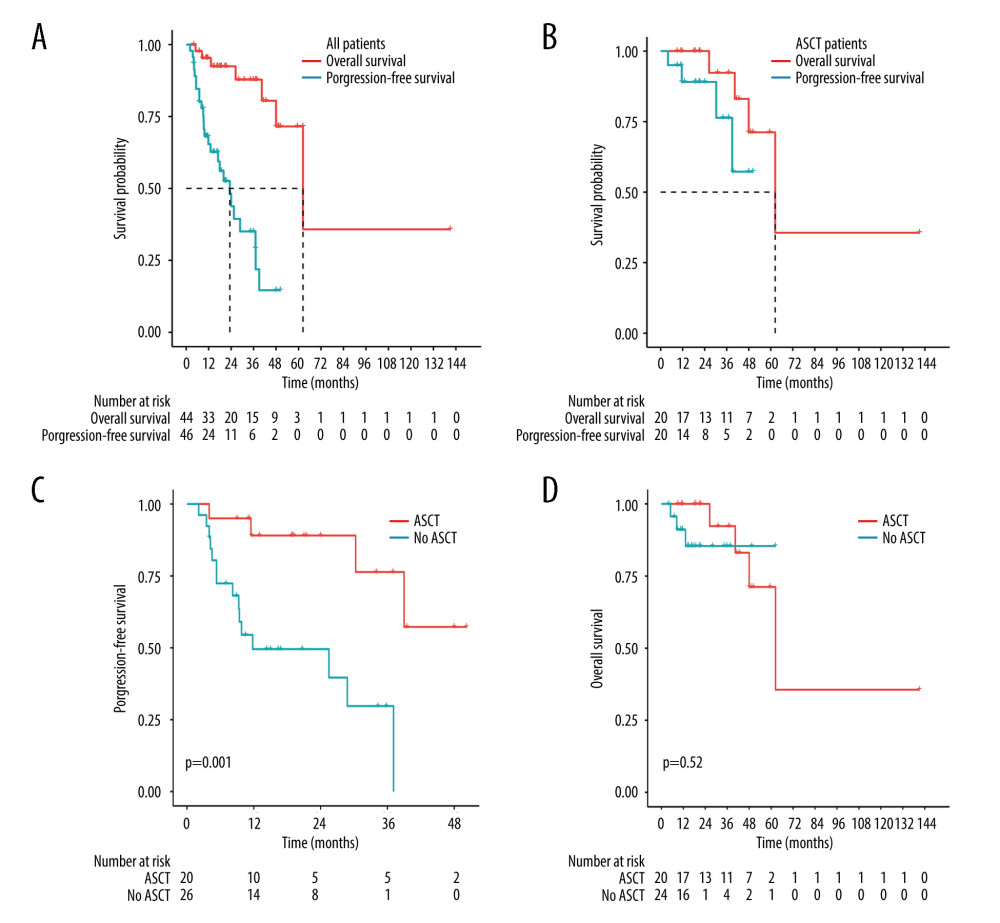 Figure 2. Kaplan-Meier analysis of survival in PCNSL patients. (A) PFS and OS for all PCNSL patients. (B) PFS and OS for patients who received ASCT. (C) PFS for treatment with ASCT compared to without ASCT (P=0.001). (D) OS for treatment with ASCT compared to without ASCT (P=0.52). (R software, version 4.2.0).
Figure 2. Kaplan-Meier analysis of survival in PCNSL patients. (A) PFS and OS for all PCNSL patients. (B) PFS and OS for patients who received ASCT. (C) PFS for treatment with ASCT compared to without ASCT (P=0.001). (D) OS for treatment with ASCT compared to without ASCT (P=0.52). (R software, version 4.2.0). 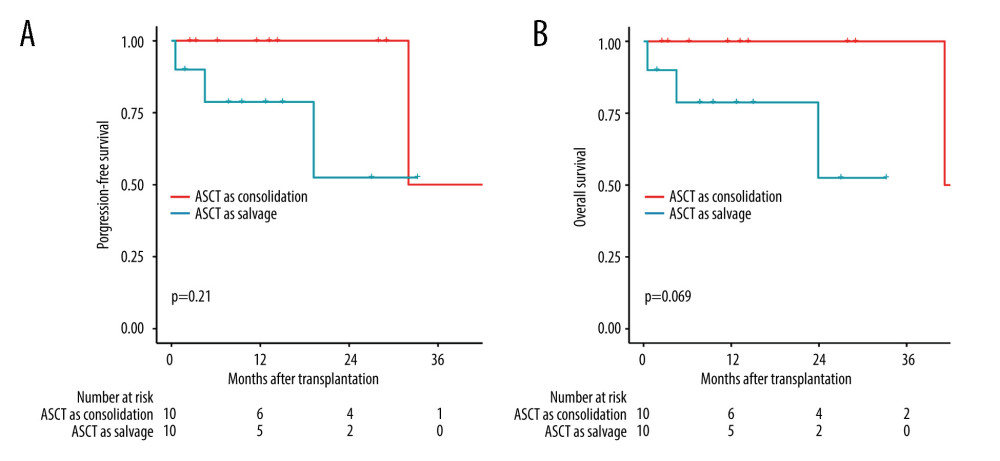 Figure 3. Comparison of survival between groups ASCT as consolidation and ASCT as salvage treatment by the log-rank test. (A) Comparison of progression-free survival (P=0.21). (B) Comparison of overall survival (P=0.069). (R software, version 4.2.0).
Figure 3. Comparison of survival between groups ASCT as consolidation and ASCT as salvage treatment by the log-rank test. (A) Comparison of progression-free survival (P=0.21). (B) Comparison of overall survival (P=0.069). (R software, version 4.2.0). 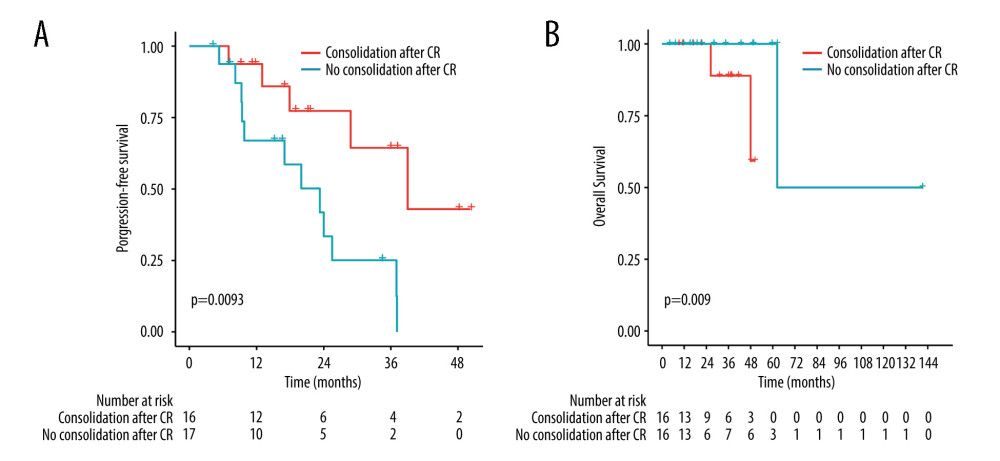 Figure 4. Comparison of survival between groups treatment with consolidation and without consolidation after complete remission by the log-rank test. (A) Comparison of PFS (P=0.0093). (B) Comparison of OS (P=0.09). (R software, version 4.2.0).
Figure 4. Comparison of survival between groups treatment with consolidation and without consolidation after complete remission by the log-rank test. (A) Comparison of PFS (P=0.0093). (B) Comparison of OS (P=0.09). (R software, version 4.2.0). Tables
Table 1. Clinical Characteristics of PCNSL patients.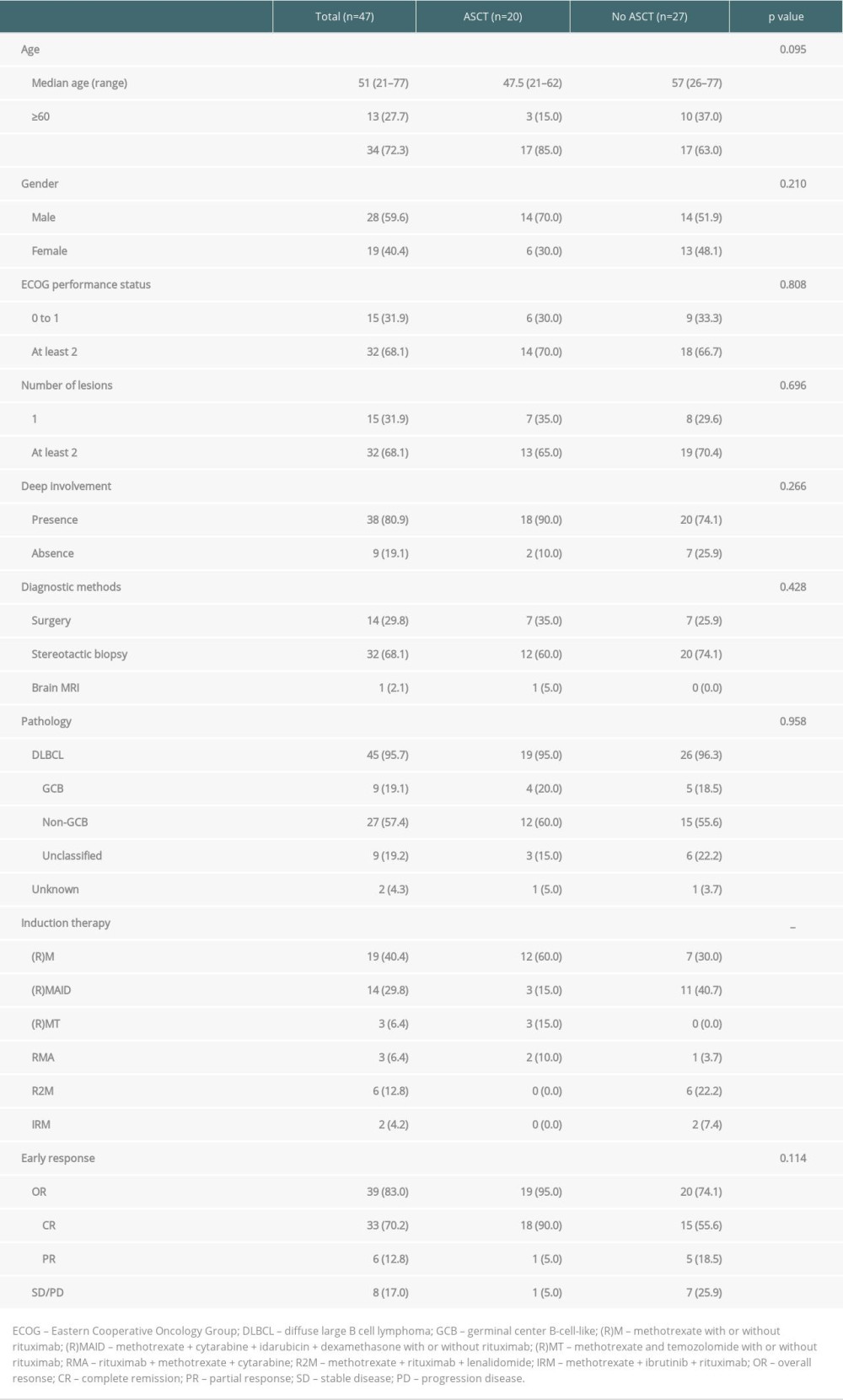 Table 2. ASCT as consolidation treatment for patients newly diagnosed with PCNSL.
Table 2. ASCT as consolidation treatment for patients newly diagnosed with PCNSL.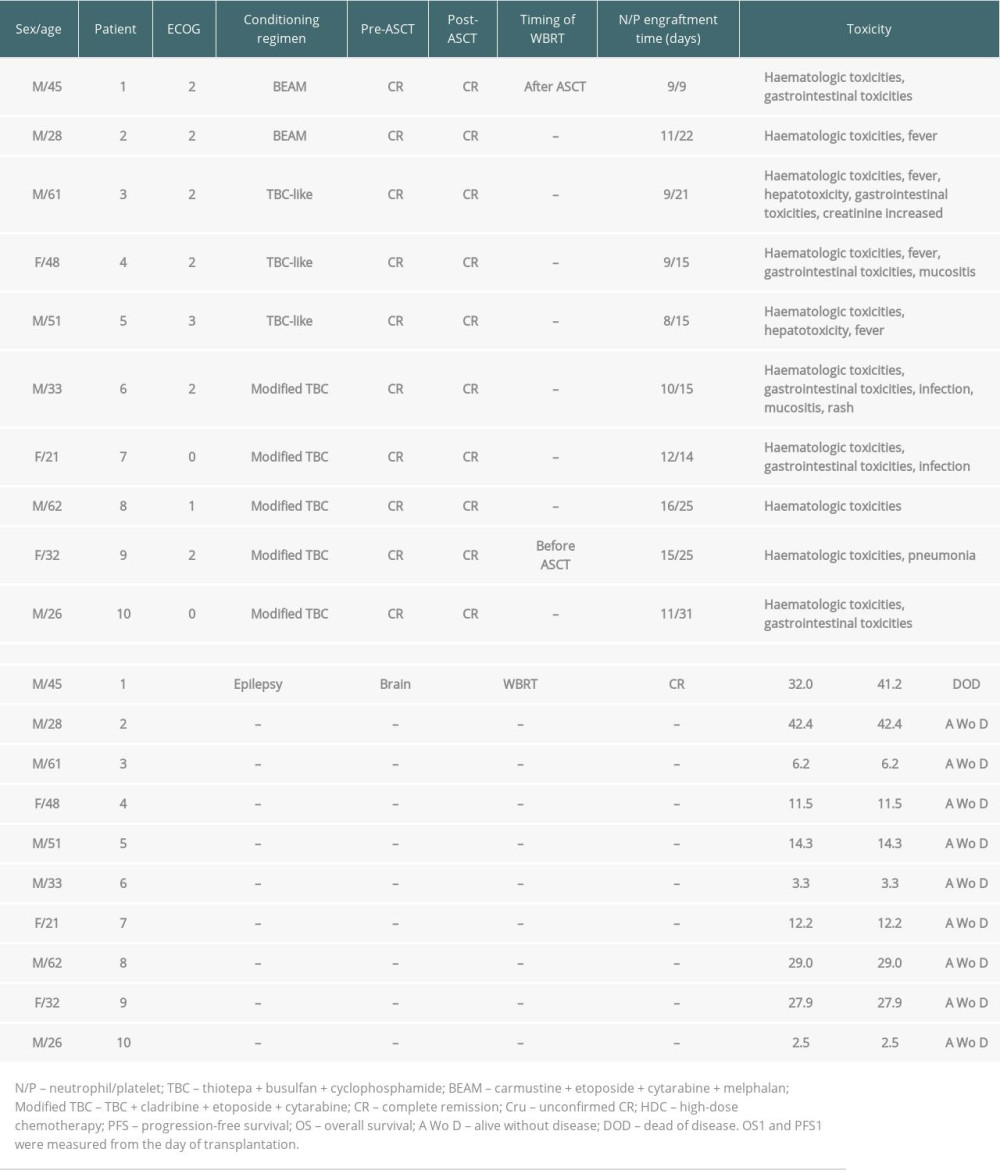 Table 3. ASCT as salvage treatment for relapsed/refractory patients with PCNSL.
Table 3. ASCT as salvage treatment for relapsed/refractory patients with PCNSL.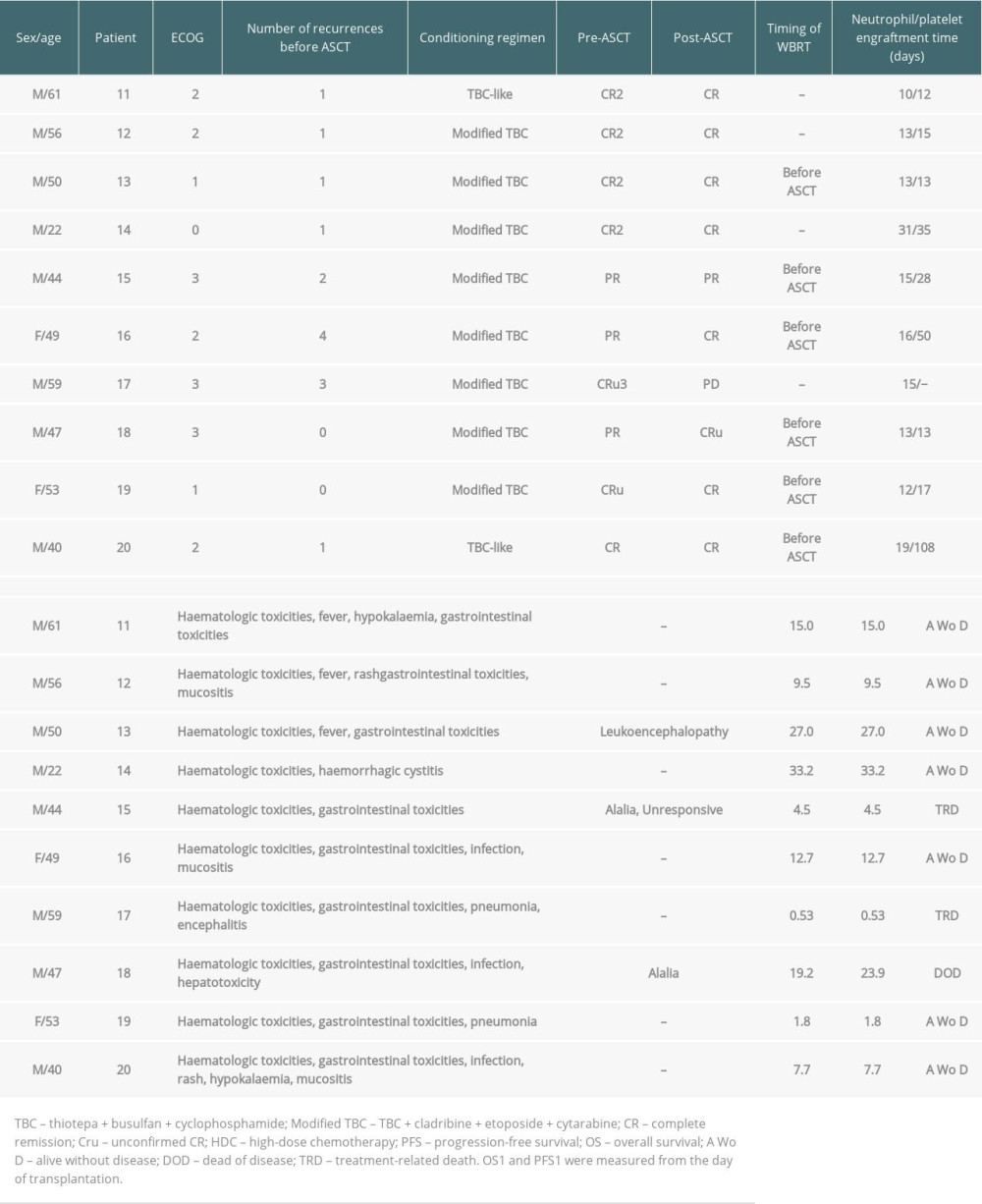 Table 4. Univariate and multivariate analyses of OS and PFS for patients (Cox test).
Table 4. Univariate and multivariate analyses of OS and PFS for patients (Cox test).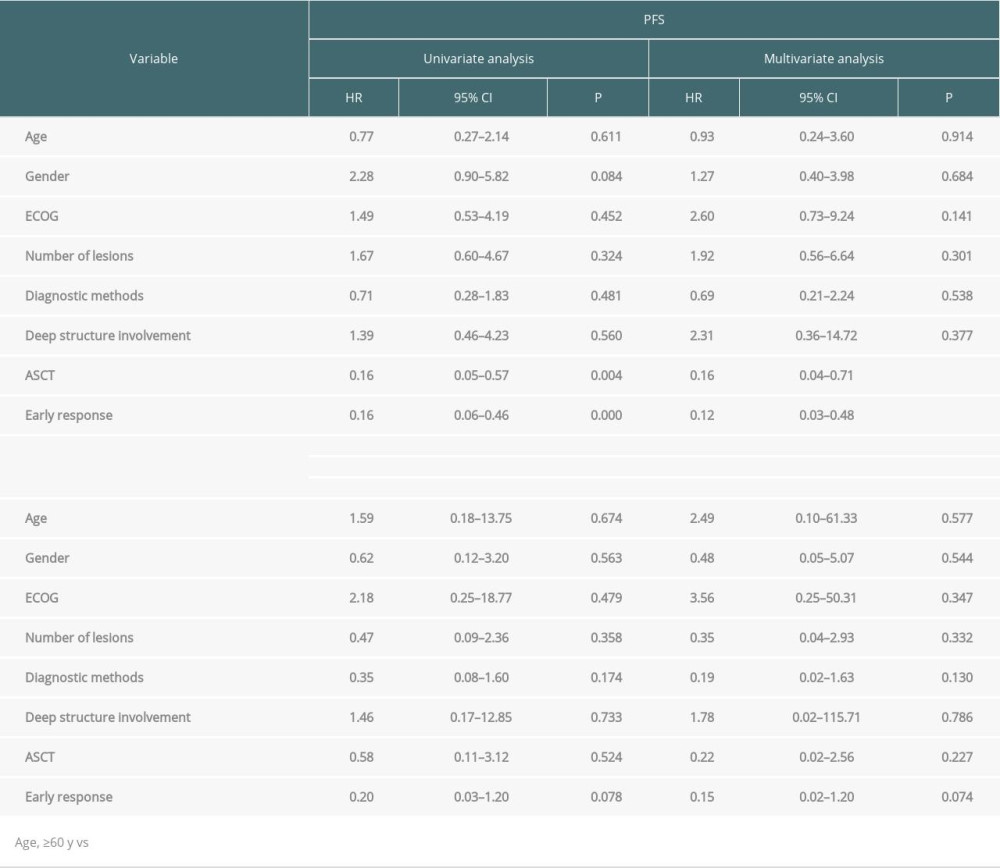
References
1. Batchelor T, Loeffler JS, Primary CNS lymphoma: J Clin Oncol, 2006; 24(8); 1281-88
2. Wang CC, Carnevale J, Rubenstein JL, Progress in central nervous system lymphomas: Br J Haematol, 2014; 166(3); 311-25
3. Holdhoff M, Mrugala MM, Grommes C, Challenges in the treatment of newly diagnosed and recurrent primary central nervous system lymphoma: J Natl Compr Canc Netw, 2020; 18(11); 1571-78
4. Grommes C, DeAngelis LM, Primary CNS lymphoma: J Clin Oncol, 2017; 35(21); 2410-18
5. Thiel E, Korfel A, Martus P, High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial: Lancet Oncol, 2010; 11(11); 1036-47
6. Birsen R, Willems L, Pallud J, Efficacy and safety of high-dose etoposide cytarabine as consolidation following rituximab methotrexate temozolomide induction in newly diagnosed primary central nervous system lymphoma in immunocompetent patients: Haematologica, 2018; 103(7); e296-e99
7. Abrey LE, Moskowitz CH, Mason WP, Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: An intent-to-treat analysis: J Clin Oncol, 2003; 21(22); 4151-56
8. Sun X, Wu Y, Xing R, Non-myeloablative chemotherapy as consolidation strategy after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: A retrospective single center study in China: Front Oncol, 2022; 12; 792274
9. Ferreri AJM, Cwynarski K, Pulczynski E, Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial: Lancet Haematol, 2017; 4(11); e510-e23
10. Houillier C, Taillandier L, Dureau S, Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: Results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study: J Clin Oncol, 2019; 37(10); 823-33
11. Welch MR, Sauter CS, Matasar MJ, Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide: Leuk Lymphoma, 2015; 56(2); 361-67
12. Illerhaus G, Muller F, Feuerhake F, High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system: Haematologica, 2008; 93(1); 147-48
13. Gaut D, Schiller GJ, Hematopoietic stem cell transplantation in primary central nervous system lymphoma: A review of the literature: Int J Hematol, 2019; 109(3); 260-77
14. Hill JM, Meehan KR, Should thiotepa-based regimens be the new transplant conditioning strategy for primary central nervous system lymphoma?: JAMA Oncol, 2021; 7(7); 1003-4
15. Abrey LE, Batchelor TT, Ferreri AJ, Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma: J Clin Oncol, 2005; 23(22); 5034-43
16. Ferreri AJ, Cwynarski K, Pulczynski E, Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial: Lancet Haematol, 2016; 3(5); e217-e27
17. Han CH, Batchelor TT, Diagnosis and management of primary central nervous system lymphoma: Cancer, 2017; 123(22); 4314-24
18. Ferreri AJ, Illerhaus G, The role of autologous stem cell transplantation in primary central nervous system lymphoma: Blood, 2016; 127(13); 1642-49
19. Liu J, Guo J, Sun X, Efficacy and safety of autologous stem-cell transplantation as part of first-line treatment for newly diagnosed primary central nervous system lymphoma: A systematic review and meta-analysis: Front Oncol, 2021; 11; 799721
20. Philip T, Guglielmi C, Hagenbeek A, Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma: N Engl J Med, 1995; 333(23); 1540-45
21. Soussain C, Hoang-Xuan K, Taillandier L, Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire: J Clin Oncol, 2008; 26(15); 2512-18
22. Kasenda B, Ihorst G, Schroers R, High-dose chemotherapy with autologous haematopoietic stem cell support for relapsed or refractory primary CNS lymphoma: A prospective multicentre trial by the German Cooperative PCNSL study group: Leukemia, 2017; 31(12); 2623-29
23. Soussain C, Choquet S, Fourme E, Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: A retrospective study of 79 cases: Haematologica, 2012; 97(11); 1751-56
24. Choi MK, Kang ES, Kim DW, Treatment outcome of relapsed/refractory primary central nervous system diffuse large B-cell lymphoma: A single-center experience of autologous stem cell transplantation: Int J Hematol, 2013; 98(3); 346-54
25. Plotkin SR, Betensky RA, Hochberg FH, Treatment of relapsed central nervous system lymphoma with high-dose methotrexate: Clin Cancer Res, 2004; 10(17); 5643-46
26. Pentsova E, Deangelis LM, Omuro A, Methotrexate re-challenge for recurrent primary central nervous system lymphoma: J Neurooncol, 2014; 117(1); 161-65
27. Nabors LB, Portnow J, Ahluwalia M, Central nervous system Cancers, version 3.2020, NCCN clinical practice guidelines in oncology: J Natl Compr Canc Netw, 2020; 18(11); 1537-70
28. Lahoud OB, Sauter CS, Hamlin PA, High-dose chemotherapy and autologous stem cell transplant in older patients with lymphoma: Curr Oncol Rep, 2015; 17(9); 42
29. William BM, Loberiza FR, Whalen V, Impact of conditioning regimen on outcome of 2-year disease-free survivors of autologous stem cell transplantation for Hodgkin lymphoma: Clin Lymphoma Myeloma Leuk, 2013; 13(4); 417-23
30. Brevet M, Garidi R, Gruson B, First-line autologous stem cell transplantation in primary CNS lymphoma: Eur J Haematol, 2005; 75(4); 288-92
31. Colombat P, Lemevel A, Bertrand P, High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: A multicenter phase II study of the GOELAMS group: Bone Marrow Transplant, 2006; 38(6); 417-20
32. Heideman RL, Cole DE, Balis F, Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: Evidence for dose-dependent plasma clearance of thiotepa: Cancer Res, 1989; 49(3); 736-41
33. Wiebe VJ, Smith BR, DeGregorio MW, Pharmacology of agents used in bone marrow transplant conditioning regimens: Crit Rev Oncol Hematol, 1992; 13(3); 241-70
34. Hassan M, Oberg G, Ehrsson H, Pharmacokinetic and metabolic studies of high-dose busulphan in adults: Eur J Clin Pharmacol, 1989; 36(5); 525-30
35. Hassan M, Ehrsson H, Smedmyr B, Cerebrospinal fluid and plasma concentrations of busulfan during high-dose therapy: Bone Marrow Transplant, 1989; 4(1); 113-14
36. Chen YB, Batchelor T, Li S, Phase 2 trial of high-dose rituximab with high-dose cytarabine mobilization therapy and high-dose thiotepa, busulfan, and cyclophosphamide autologous stem cell transplantation in patients with central nervous system involvement by non-Hodgkin lymphoma: Cancer, 2015; 121(2); 226-33
37. Omuro A, Correa DD, DeAngelis LM, R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma: Blood, 2015; 125(9); 1403-10
38. Cheng T, Forsyth P, Chaudhry A, High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma: Bone Marrow Transplant, 2003; 31(8); 679-85
39. Slevin ML, Piall EM, Aherne GW, Effect of dose and schedule on pharmacokinetics of high-dose cytosine arabinoside in plasma and cerebrospinal fluid: J Clin Oncol, 1983; 1(9); 546-51
40. Scordo M, Wang TP, Ahn KW, Outcomes associated with Thiotepa-Based conditioning in patients with primary central nervous system lymphoma after autologous hematopoietic cell transplant: JAMA Oncol, 2021; 7(7); 993-1003
41. Alnahhas I, Jawish M, Alsawas M, Autologous stem-cell transplantation for primary central nervous system lymphoma: Systematic review and meta-analysis: Clin Lymphoma Myeloma Leuk, 2019; 19; e129-41
42. Ferreri AJ, Blay JY, Reni M, Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience: J Clin Oncol, 2003; 21(2); 266-72
43. Abrey LE, Ben-Porat L, Panageas KS, Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model: J Clin Oncol, 2006; 24(36); 5711-15
44. Jang JE, Kim YR, Kim SJ, A new prognostic model using absolute lymphocyte count in patients with primary central nervous system lymphoma: Eur J Cancer, 2016; 57; 127-35
Figures
 Figure 1. Treatment procedures. (Adobe Illustrator 2022).
Figure 1. Treatment procedures. (Adobe Illustrator 2022). Figure 2. Kaplan-Meier analysis of survival in PCNSL patients. (A) PFS and OS for all PCNSL patients. (B) PFS and OS for patients who received ASCT. (C) PFS for treatment with ASCT compared to without ASCT (P=0.001). (D) OS for treatment with ASCT compared to without ASCT (P=0.52). (R software, version 4.2.0).
Figure 2. Kaplan-Meier analysis of survival in PCNSL patients. (A) PFS and OS for all PCNSL patients. (B) PFS and OS for patients who received ASCT. (C) PFS for treatment with ASCT compared to without ASCT (P=0.001). (D) OS for treatment with ASCT compared to without ASCT (P=0.52). (R software, version 4.2.0). Figure 3. Comparison of survival between groups ASCT as consolidation and ASCT as salvage treatment by the log-rank test. (A) Comparison of progression-free survival (P=0.21). (B) Comparison of overall survival (P=0.069). (R software, version 4.2.0).
Figure 3. Comparison of survival between groups ASCT as consolidation and ASCT as salvage treatment by the log-rank test. (A) Comparison of progression-free survival (P=0.21). (B) Comparison of overall survival (P=0.069). (R software, version 4.2.0). Figure 4. Comparison of survival between groups treatment with consolidation and without consolidation after complete remission by the log-rank test. (A) Comparison of PFS (P=0.0093). (B) Comparison of OS (P=0.09). (R software, version 4.2.0).
Figure 4. Comparison of survival between groups treatment with consolidation and without consolidation after complete remission by the log-rank test. (A) Comparison of PFS (P=0.0093). (B) Comparison of OS (P=0.09). (R software, version 4.2.0). Tables
 Table 1. Clinical Characteristics of PCNSL patients.
Table 1. Clinical Characteristics of PCNSL patients. Table 2. ASCT as consolidation treatment for patients newly diagnosed with PCNSL.
Table 2. ASCT as consolidation treatment for patients newly diagnosed with PCNSL. Table 3. ASCT as salvage treatment for relapsed/refractory patients with PCNSL.
Table 3. ASCT as salvage treatment for relapsed/refractory patients with PCNSL. Table 4. Univariate and multivariate analyses of OS and PFS for patients (Cox test).
Table 4. Univariate and multivariate analyses of OS and PFS for patients (Cox test). Table 1. Clinical Characteristics of PCNSL patients.
Table 1. Clinical Characteristics of PCNSL patients. Table 2. ASCT as consolidation treatment for patients newly diagnosed with PCNSL.
Table 2. ASCT as consolidation treatment for patients newly diagnosed with PCNSL. Table 3. ASCT as salvage treatment for relapsed/refractory patients with PCNSL.
Table 3. ASCT as salvage treatment for relapsed/refractory patients with PCNSL. Table 4. Univariate and multivariate analyses of OS and PFS for patients (Cox test).
Table 4. Univariate and multivariate analyses of OS and PFS for patients (Cox test). In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








