27 September 2022: Original Paper
Using Postoperative Coagulation Parameters Within 24 h After Liver Transplantation to Predict Incidence of Stage 3 Acute Kidney Injury (AKI)
Chen Chen1BCD, Xiaolan Chen2BC, Xianyuan Zhao1D, Junqi Feng1E, Yuan Gao1E, Yuxiao DengDOI: 10.12659/AOT.937535
Ann Transplant 2022; 27:e937535
Abstract
BACKGROUND: This study aimed to investigate the relationship between coagulation function and the incidence of acute kidney injury (AKI) stage 3 within 24 h after liver transplantation (LT) and explore the predictive value of coagulation parameters for post-LT stage 3 AKI.
MATERIAL AND METHODS: A retrospective study was conducted on 241 patients who underwent LT at the Renji Hospital affiliated with Shanghai Jiao Tong University School of Medicine between February 2021 and February 2022. The coagulation parameters within 24 h after LT and the incidence of post-LT AKI within 7 days were recorded. The correlation between post-LT coagulation function and post-LT stage 3 AKI was determined using binary logistic regression analysis.
RESULTS: Post-LT AKI occurred in 99 cases (41.1%), 28 (28.3%) of which developed AKI stage 3. In univariate logistic regression analysis, multiple coagulation indexes of the AKI stage 3 group were worse than in the AKI stage 0-2 group. In multivariate logistic regression analysis, lower post-LT ADP-induced PLT aggregation rate (cut-off: 15.75%), higher D-dimer level (cut-off: 3.52 ug/ml), and prolonged R-value (cut-off: 7.5 min) within 24 h were independent risk factors for post-LT AKI stage 3. The AUROC value for predicting the incidence of post-LT AKI stage 3 combining the 3 indices was 0.835 (sensitivity: 83.3%, specificity: 76.9%). The decision curve showed that combining D-dimer, R-value, and ADP-induced PLT aggregation rate yielded the highest net benefit for predicting the incidence of stage 3 AKI.
CONCLUSIONS: Post-LT coagulation function within 24 h correlated with the incidence of post-LT AKI stage 3. Lower ADP-induced PLT aggregation rate, higher D-dimer level, and prolonged R-value from the TEG were independent risk factors for the incidence of post-LT AKI stage 3.
Keywords: Acute Kidney Injury, Blood Coagulation, Liver Transplantation, Adenosine Diphosphate, China, Humans, Incidence, Risk Factors
Background
Acute kidney injury (AKI) is one of the most common post-LT complications. The incidence of post-LT AKI is 12.3–56.6% [1]. Compared to patients with AKI stage1–2, post-LT stage 3 AKI has a greater impact on mortality and hospital stay [2,3]. However, studies on the prediction of post-LT stage 3 AKI are limited.
Previous studies have explored the risk factors for post-LT AKI incidence. These factors include the preoperative model for end-stage liver disease (MELD) score [4], intraoperative blood loss, infusion of blood products [5], and vasoactive drug use [6]. Furthermore, some clinical and mechanistic studies have suggested that extended-criteria donor grafts could lead to AKI through hepatic ischemia-reperfusion injury (HIRI). Nevertheless, most data were from single-center studies with limited patient samples, thus impeding the clinical application of these parameters. HIRI has been reported as an independent risk factor for post-LT AKI, and its severity is related to the severity of the AKI [7]. Pathophysiological changes induced by HIRI damage endothelial cells, ultimately impairing the coagulation function [8,9]. Furthermore, post-LT coagulation disorder is influenced by several parameters related to post-LT AKI and its severity, including preoperative MELD score, intraoperative bleeding, infusion of coagulation substances, and postoperative HIRI [10–13]. However, the relationship between post-LT coagulation function and the incidence of post-LT AKI and its severity has not been explored thus far. Hence, we hypothesized that post-LT coagulation function might be associated with post-LT AKI and could influence its severity. We conducted a retrospective study to evaluate the impact of miscellaneous coagulation parameters on the development of post-LT stage 3 AKI and to identify the parameters with high predictive value for post-LT stage 3 AKI.
Material and Methods
STUDY POPULATION AND ETHICAL APPROVAL:
Patients who underwent LT in the Department of Critical Care Medicine at the Renji Hospital between 2021.02 and 2022.02 were enrolled in this retrospective study. This study was approved by the Ethics Committee of the Renji Hospital, School of Medicine, Shanghai Jiao Tong University (KY2021-019).
The data of adult patients (>18 years) admitted to the intensive care unit (ICU) due to LT operation were screened. Patients with preoperative AKI, history of chronic kidney disease with a glomerular filtration rate less than 60 ml/min, combined liver and kidney transplantation, reoperation within 72 h, sudden cardiac arrest, and death within 72 h after the operation were excluded from the study. Additionally, the NYHA of all the patients finally included in this study was ≤2, and none of them had recently used anticoagulant and antiplatelet drugs. ADP or AA-induced platelet aggregation rate were measured using an ag800 automatic platelet aggregator. The reagent was AA and ADP activator provided by Shandong Tailixin Medical Co., Ltd. The quality of the reagent was qualified through sampling inspection at the factory and at the station. It is used within the valid time period. The reagent storage conditions meet the requirements
DIAGNOSTIC CRITERIA:
AKI is a syndrome that was defined using the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines [14]: (1) an increase in serum creatinine (Scr) by 26.5 mmol/L within 48 h; (2) an increase in Scr to 1.5 times baseline within the first 7 postoperative days; (3) the urine volume (UV) <0.5 mL/kg/h lasted more than 6 h after LT. Moreover, AKI was classified into 3 stages: stage 1, creatinine increased to 26.5 mmol/L or increased to 1.5–1.9 fold from baseline, or the UV <0.5 mL/(kg/h) lasting for 6–12 h; stage 2, increase by 2–2.9 fold or UV <0.5 mL/(kg/h) lasted >12 h; stage 3, increased >3-fold or increased in Scr to 354 umol/L or initiation of continuous renal replacement therapy (CRRT). Baseline Scr was defined as the lowest creatinine level within 1 month before transplantation. Patients were divided into the grade 0–2 AKI group and the grade 3 AKI group based on their AKI stage.
DATA COLLECTION:
The demographic data included pre-transplant data, sex, age, causes of LT, ascites volume, MELD score, laboratory data such as alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TB), and coagulation parameters namely international normalized ratio (INR), D-dimer, and fibrinogen degradation products (FDP). The whole blood cell analysis parameters included hemoglobin (HB) and platelet (PLT), while the intraoperative data included intraoperative blood loss and blood transfusion volume. Furthermore, the parameters measured within 24 h of admission to the ICU included liver function (ALT, AST, TB), platelet count, functional parameters (ADP or AA-induced platelet aggregation rate), various coagulation factors percentages (FII, FV, FVII, FVIII, FIX, FX, FXI, FXII, Protein S, Protein C, Antithrombin III, INR), and multiple parameters of TEG (R-value, k-value, α-angle, maximum amplitude (MA) value, Ly 30, coagulation time, and clot strength). Additionally, this study recorded creatinine and urine volume permutations within 7 days of surgery to diagnose AKI.
STATISTICAL ANALYSIS:
The continuous data are expressed as mean±standard deviation for normally distributed data and as median with interquartile range (IQR) for non-normally distributed data. The chi-square test or Fisher’s exact test was employed to compare the categorical variables. Continuous variables that did not have normal distribution were compared using the Mann-Whitney U test. Decision curve analysis was used to assess the net benefit. The AUROC expressed the predictive accuracy of the R-value, D-dimer level, and ADP-induced PLT aggregation rate. Moreover, the optimal cut-off values were obtained using Youden’s parameters. Statistical significance was set at
Results
DEMOGRAPHIC CHARACTERISTICS OF PATIENTS UNDERGOING LT:
The study population (n=241) comprised 175 men (72.6%) and 66 women (27.4%). The average age was 50.9±10.9 years. Demographic data are presented in Table 1. Among the 241 patients who underwent LT, the proportion of post-LT AKI was 41.1% (99/241). Out of the patients who developed AKI, the incidence of stage 1 AKI was 57.6% (57/99), stage 2 was 6.5% (14/99), and stage 3 was 28.3% (28/99). In this cohort, the post-LT mortality rate was 7.9% (19/241). The stage 3 mortality rate was 42.9% (12/28) among the patients with AKI, which was significantly higher than in the stage 0–2 patients (3.3%). Furthermore, patients with severe hepatic failure had a higher incidence of post-LT AKI stage 3 (43.9 vs 18.3, P=0.003). CRRT treatment was only performed in patients with stage 3 AKI, accounting for 21.4% (6/28) of patients, with a mortality rate of 66.7% (4/6).
COMPARING PRE- AND INTRA-OPERATIVE CLINICAL DATA BETWEEN THE POST-LT STAGE 0–2 AKI AND STAGE 3 AKI GROUPS:
The pre- and intraoperative clinical data are presented in Table 1. The preoperative MELD scores of patients with AKI stage 3 were higher than those in the stage 0–2 AKI group (17 vs 13, P=0.006). There was no significant difference in preoperative liver function (AST, ALT, ALB, and Scr) between the 2 groups. In contrast, the preoperative D-dimer and FDP levels were higher in patients with stage 3 AKI than in patients with stage 0–2 AKI (1.86 vs 0.85, P=0.002 and 12.4 vs 5.2, P=0.001, respectively). Regarding intraoperative markers, intraoperative bleeding in the stage 3 AKI group was greater than that in the stage 0–2 AKI group (800 vs 500, P=0.023). Moreover, patients with stage 3 AKI were infused with more red blood cell (RBC) suspension (8 vs 4,0.004) and plasma (850 vs 400, P=0.01).
COMPARISONS OF COAGULATION FUNCTION PARAMETERS WITHIN 24 H AFTER LT BETWEEN THE STAGE 0–2 AKI AND STAGE 3 GROUPS:
Table 2 shows the comparisons of coagulation parameters and other laboratory test within 24 h between the 2 groups. Compared with stage 0–2 AKI patients, patients in the stage 3 group had higher ALT level (815 vs 500, P<0.001) and AST level (2496 vs 962, P<0.001). Patients in the stage 3 group had higher Scr than those in the stage 0–2 group. When univariates were included in logistic regression (Table 3), patients in the stage 3 group had lower platelet counts (30.5 vs 52.0, P<0.001, OR=0.993), ADP (11.2 vs 38.2, OR=0.953, P<0.001), and AA (5.6 vs 34.4, OR=0.969, P=0.003)-induced platelet aggregation rates than in the stage 0–2 group. In addition, FV (16.0 vs 34.7, OR=0.950, P<0.001), FVIII (96.1 vs142.7, OR=0.989, P=0.005), and FIX (43.6 vs 51.8, OR=0.975, P=0.022) levels were markedly lower in the stage 3 group. The stage 3 AKI group had a higher INR (1.9 vs 1.5, OR=2.654, P<0.001) than the stage 0–2 group. Compared with stage 0–2, the stage 3 group had higher D-dimer levels (2.1 vs 4.9, OR=1.004, P<0.001) and significantly higher levels of FDP (13.3 vs 24.9, OR=1.007, P<0.001). However, the stage 3 group had lower FIB levels than the stage 0–2 group (0.9 vs 1.7, OR=0.231, P<0.001)
The stage 3 AKI group had a more heterogeneous TEG appearance; the R and K values were extended by 2.2 (6.8 vs 9.0, OR=1.204,
MULTIVARIATE LOGISTIC REGRESSION ANALYSIS OF THE INCIDENCE OF AKI STAGE 3 AFTER LT:
This study combined meaningful parameters from univariate and multivariate regression analyses, and the results determined that D-dimer, R-value, and ADP-induced platelet aggregation rates were independent risk factors for AKI stage 3 after LT. Table 3 shows the results from multivariate logistic regression analysis. Figure 2 displays how the incidence of stage 3 AKI increased with the number of independent risk factors. Indeed, when patients did not carry these 3 independent risk factors, the incidence rate of stage 3 AKI was 0%. Conversely, when patients had all 3 independent risk factors, the incidence rate of AKI stage 3 was 60%. Hence, the higher the number of risk factors, the greater the incidence of stage 3 AKI (R2=0.9213, P=0.04).
THE AUROC ANALYSIS OF D-DIMER, R-VALUE, AND ADP-INDUCED PLT AGGREGATION AS PARAMETERS FOR INCIDENCE OF STAGE 3 AKI:
Table 4 shows the result from AUROC analysis. The AUROC value of D-dimer was 0.723 (95% CI: 0.621–0.825), with a cut-off value of 3.52 ug/ml, a sensitivity of 60.7% and specificity of 74.5%, while that of the R-value was 0.815 (95% CI: 0.739–0.890), with a cut-off value of 7.5 min, a sensitivity of 92%, and specificity of 64.6%. The AUROC value of ADP-induced PLT aggregation was 0.758 (95% CI: 0.654–0.862), with a cut-off value of 15.75%, a sensitivity of 82.7% and a specificity of 65.4%. Notably, using these 3 parameters in combination to predict the incidence of AKI stage 3 could boost the AUROC score to 0.835, sensitivity to 83.3% and specificity to 76.9%. Figure 3 shows the sensitivity and specificity of each parameter and Figure 4 shows the ROC curve of combining D-dimer, R-value, and ADP-induced PLT aggregation rate for predicting the incidence of stage 3 AKI. The decision curve shows that combining D-dimer, R-value, and ADP-induced PLT aggregation rate yielded highest net benefit for predicting the incidence of stage 3 AKI (Figure 5).
COMBINING D-DIMER, R-VALUE, AND ADP-INDUCED PLT AGGREGATION RATE OF DIFFERENT SUBGROUPS FOR PREDICTING THE INCIDENCE OF STAGE 3 AKI AFTER LT:
Since the baseline data of the 2 groups were different, we performed subgroup analysis on hepatic failure, bleeding volume, MELD score, RBC suspension, and plasma infusion volume. We found that for different subgroups, the combination of the 3 indexes has good ability to predict the incidence of stage 3AKI after LT. There was no significant statistical difference in AUROC among different subgroups (Table 5).
Discussion
LIMITATIONS:
Firstly, this was a single-center, small-sample, retrospective study. Larger prospective studies are warranted to explore the association between coagulation function and post-LT stage 3 AKI. Secondly, the coagulation function within 24 h after LT was selected for earlier prediction of AKI incidence. In the future, several additional time points should be included in a specifically designed clinical study to predict the value of those coagulation parameters. Thirdly, VWF is an indicator of endothelial injury that was missing in our study. HIRI was a crucial mechanism related to post-LT coagulation functions and stage 3 AKI in our study. However, there is a lack of clinical indicators to diagnose HIRI. Hence, further laboratory and clinical research is required to elucidate this process. Finally, we lacked some key surgical data (eg, caval anastomosis technique), warm and cold ischemia times, and immunosuppression regimens, which have been related to postoperative renal injury.
Conclusions
The postoperative coagulation function within 24h correlated with the incidence of post-LT AKI stage 3. Specifically, lower ADP-induced PLT aggregation rate, higher D-dimer level, and prolonged R-value from the TEG were independent risk factors for the incidence of post-LT stage 3 AKI.
Figures
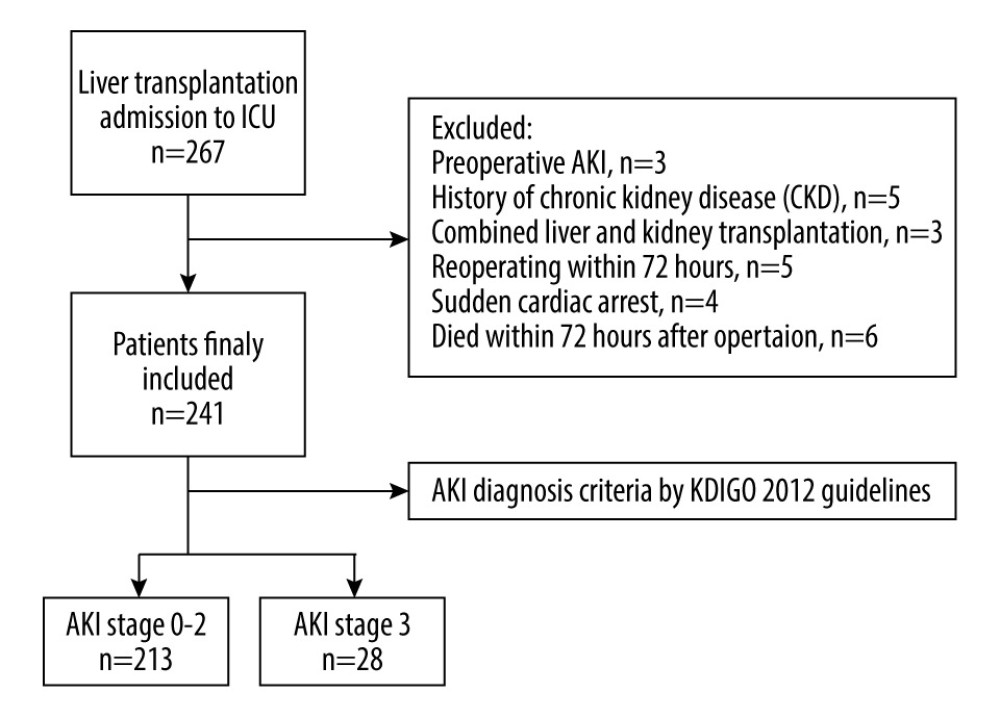 Figure 1. Flowchart of the study process.
Figure 1. Flowchart of the study process. 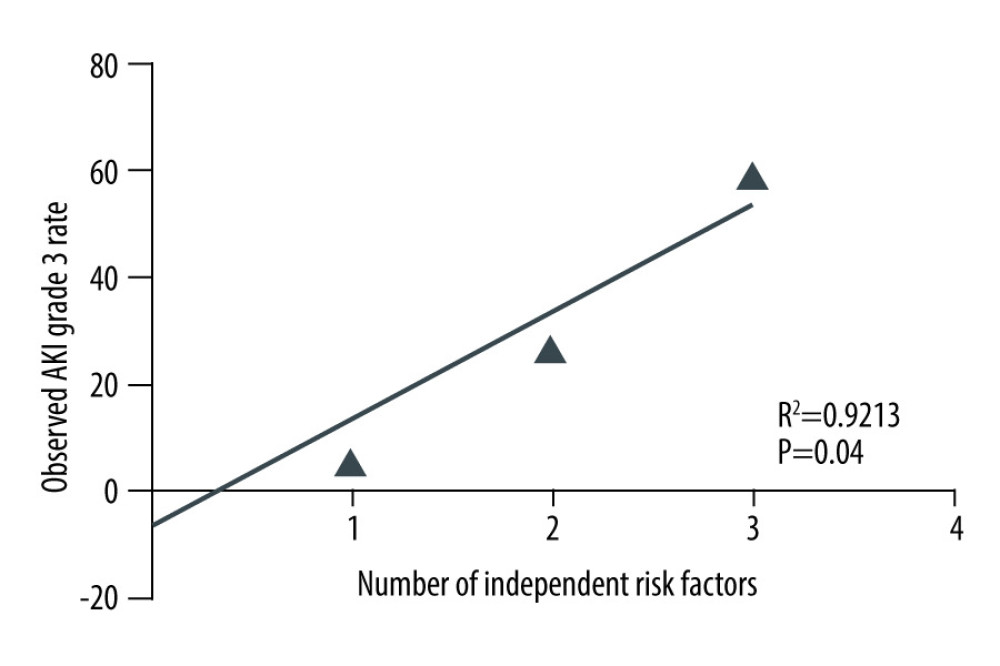 Figure 2. Relationship between the number of independent risk factors and the incidence of AKI stage 3.
Figure 2. Relationship between the number of independent risk factors and the incidence of AKI stage 3. 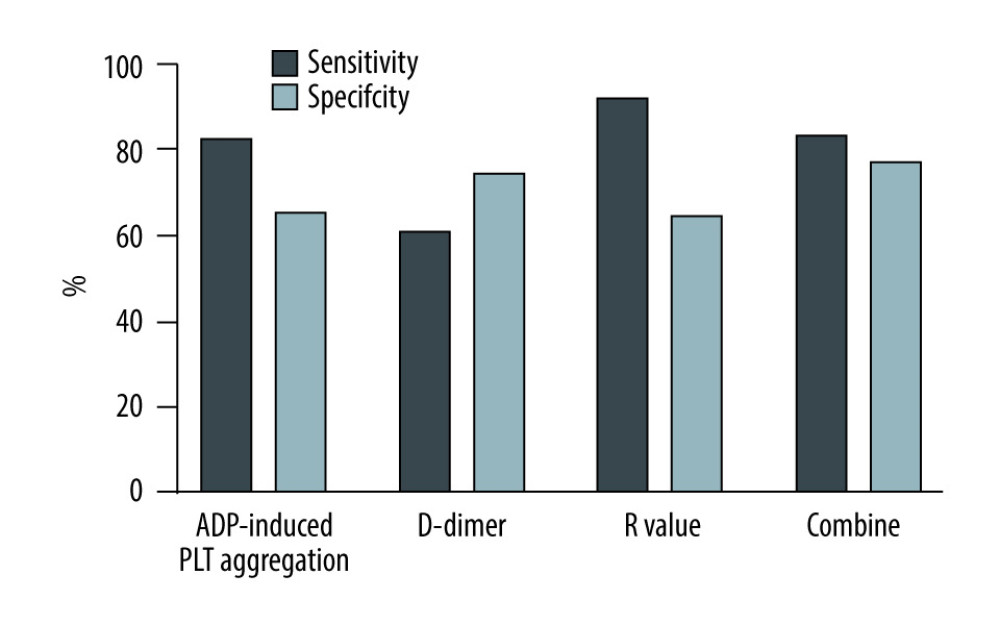 Figure 3. Sensitivity and specificity of different parameters in predicting the incidence of AKI stage 3.
Figure 3. Sensitivity and specificity of different parameters in predicting the incidence of AKI stage 3. 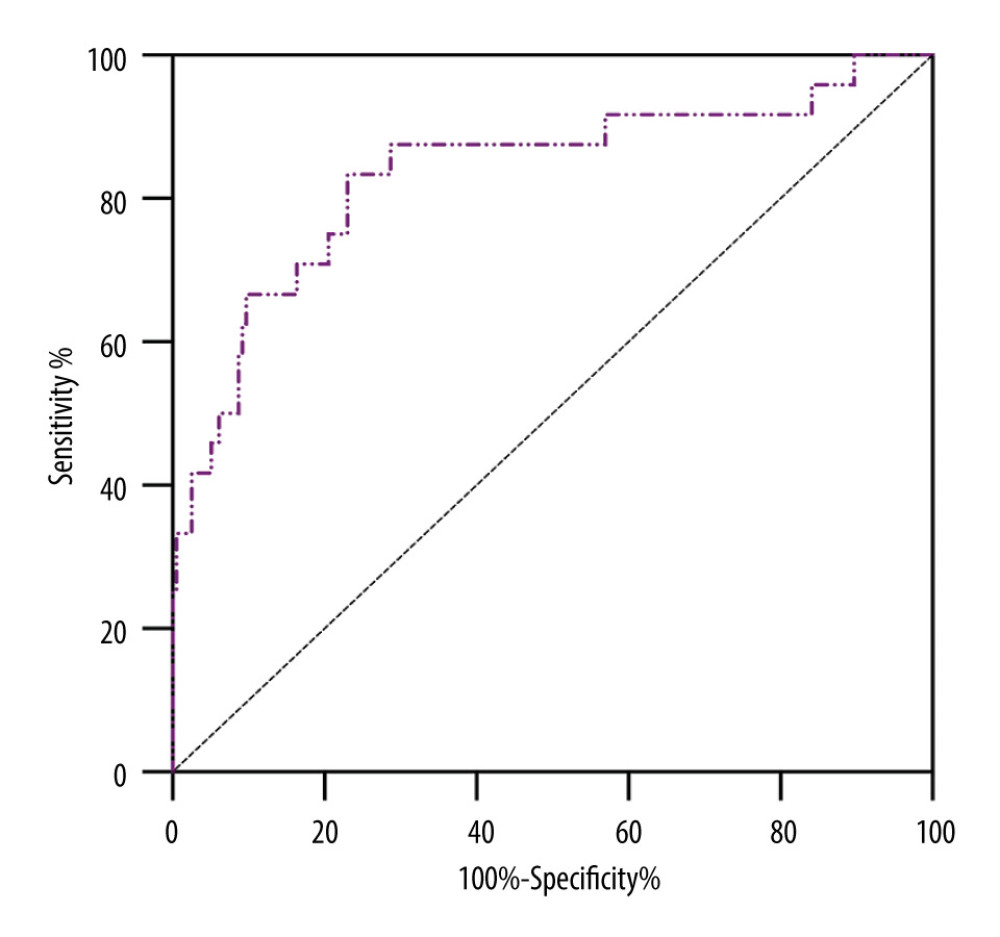 Figure 4. ROC curve of combining D-dimer, R-value and ADP-induced PLT aggregation rate for predicting the incidence of AKI stage 3.
Figure 4. ROC curve of combining D-dimer, R-value and ADP-induced PLT aggregation rate for predicting the incidence of AKI stage 3. 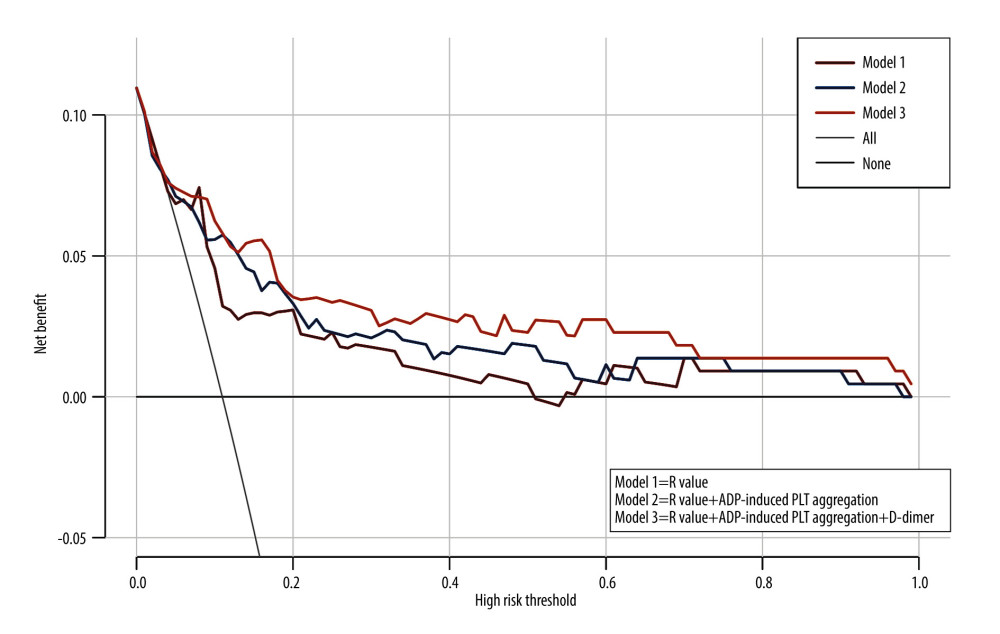 Figure 5. Decision curve of combining D-dimer, R-value, and ADP-induced PLT aggregation rate.
Figure 5. Decision curve of combining D-dimer, R-value, and ADP-induced PLT aggregation rate. Tables
Table 1. Demographic and pre-and intraoperative clinical data of the studied population (n=241).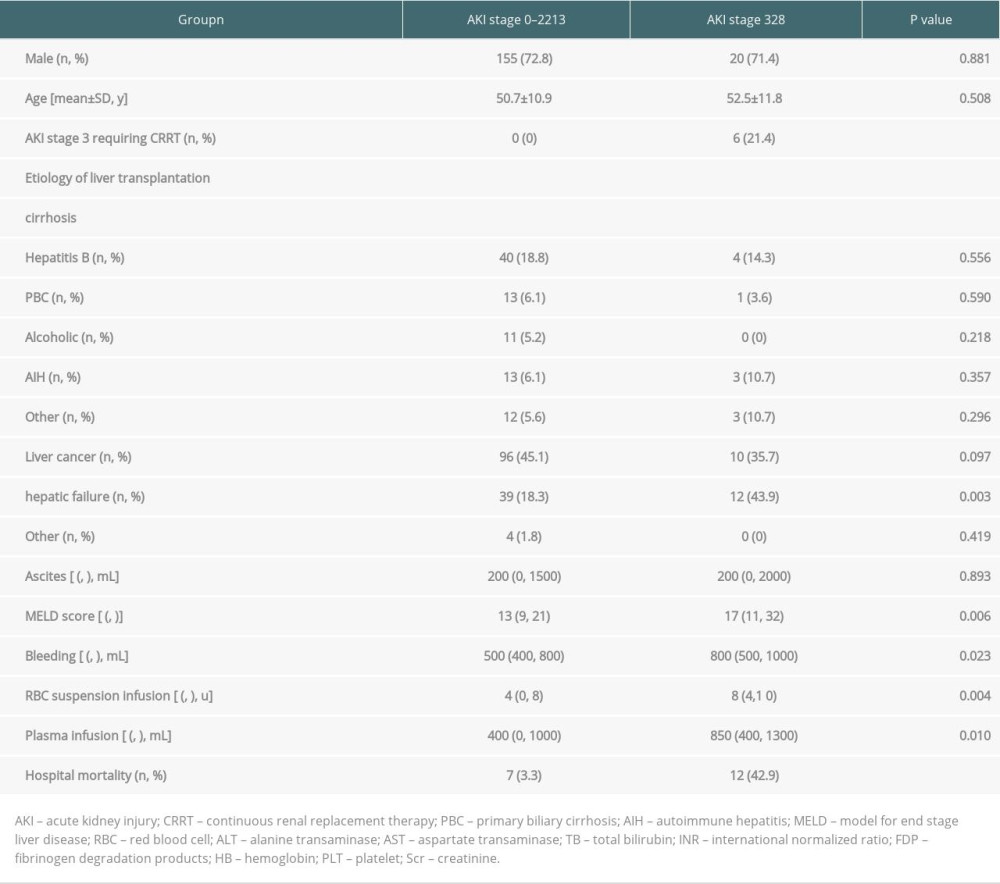 Table 2. Comparison of liver function and coagulation parameters between the 2 groups within 24 h of LT.
Table 2. Comparison of liver function and coagulation parameters between the 2 groups within 24 h of LT.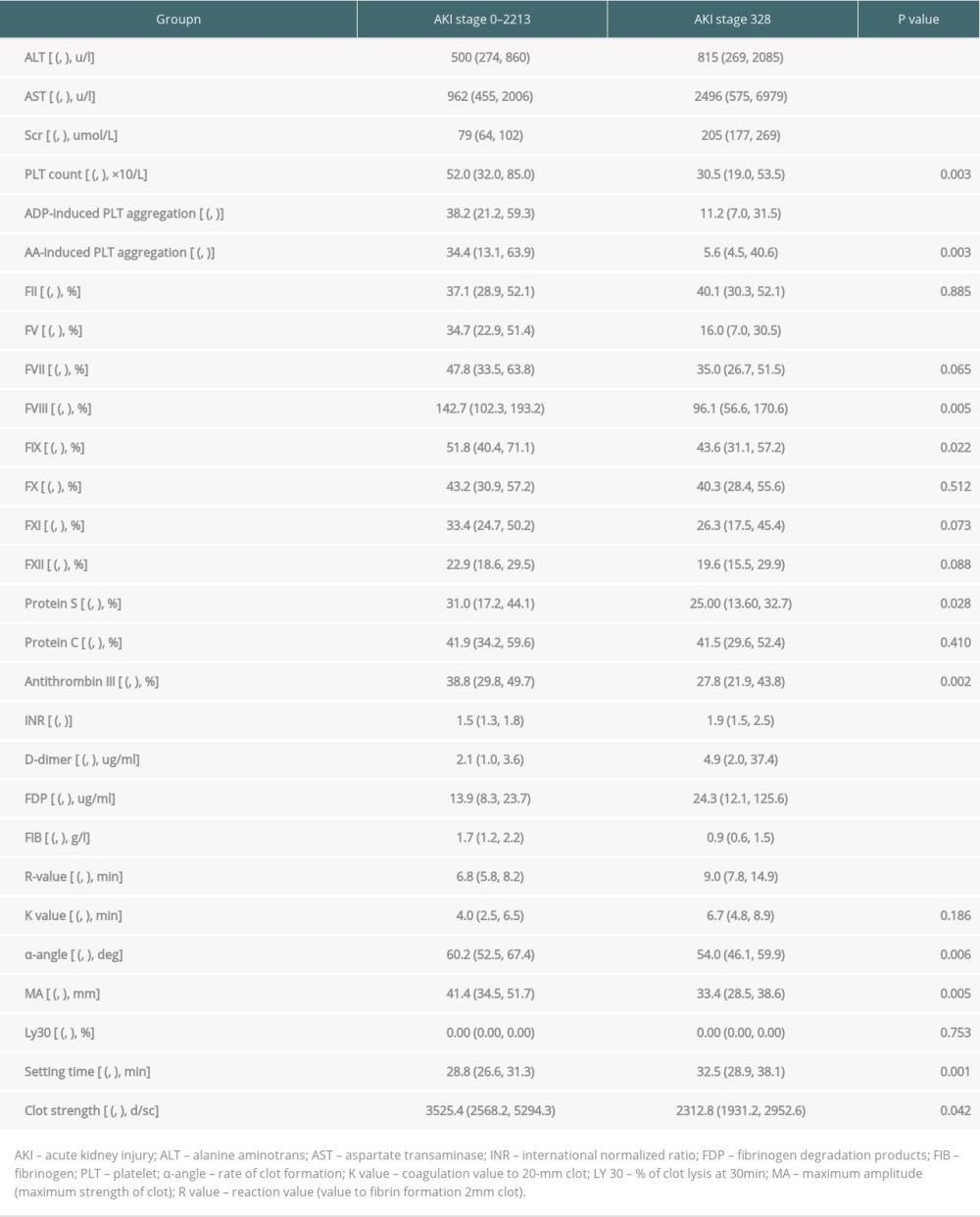 Table 3. Multivariate logistic regression analysis of the incidence of stage 3 AKI after LT.
Table 3. Multivariate logistic regression analysis of the incidence of stage 3 AKI after LT.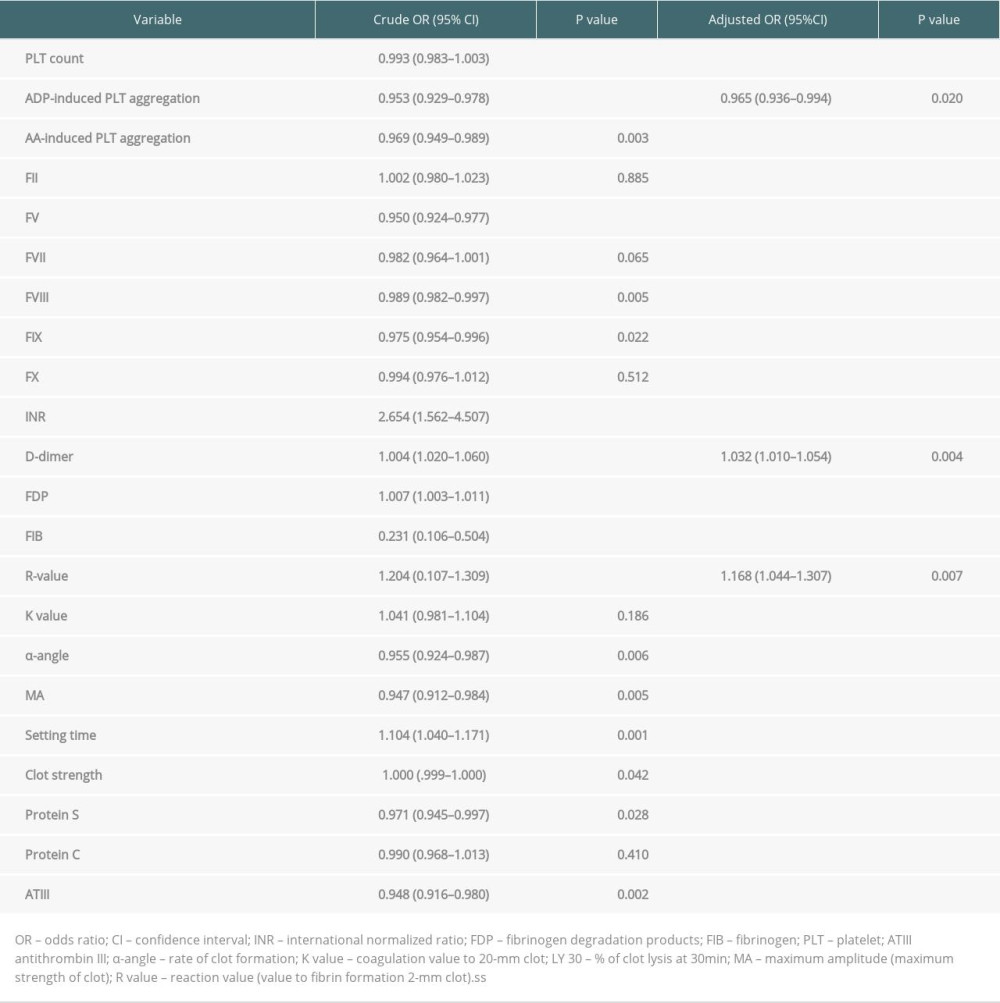 Table 4. Predictive values of D-dimer, R-value, and ADP-induced PLT aggregation for the incidence of stage 3 AKI after LT.
Table 4. Predictive values of D-dimer, R-value, and ADP-induced PLT aggregation for the incidence of stage 3 AKI after LT.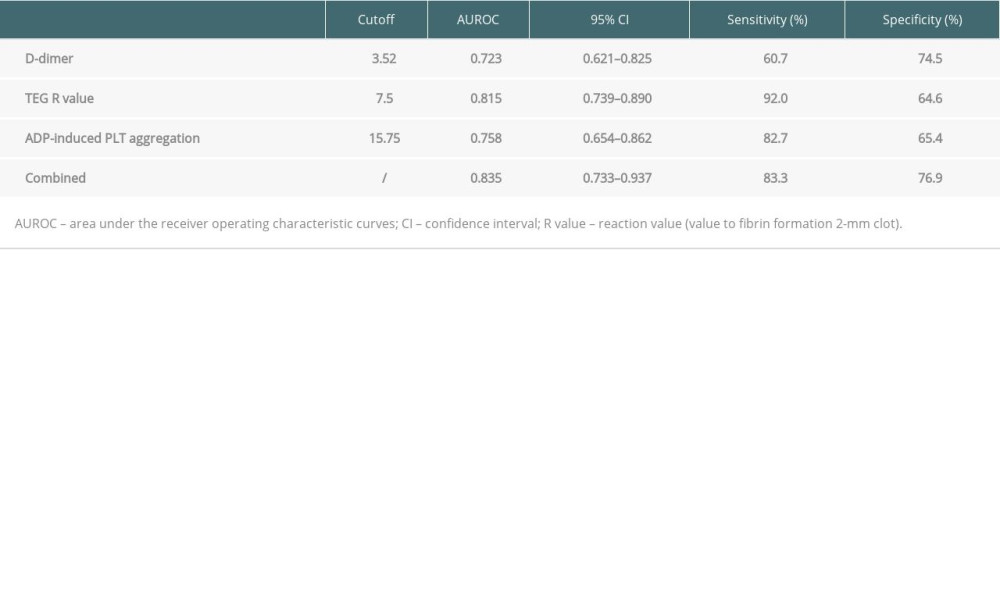 Table 5. Subgroup analysis of the combined 3 indexes for predicting the incidence of stage 3 AKI after LT.
Table 5. Subgroup analysis of the combined 3 indexes for predicting the incidence of stage 3 AKI after LT.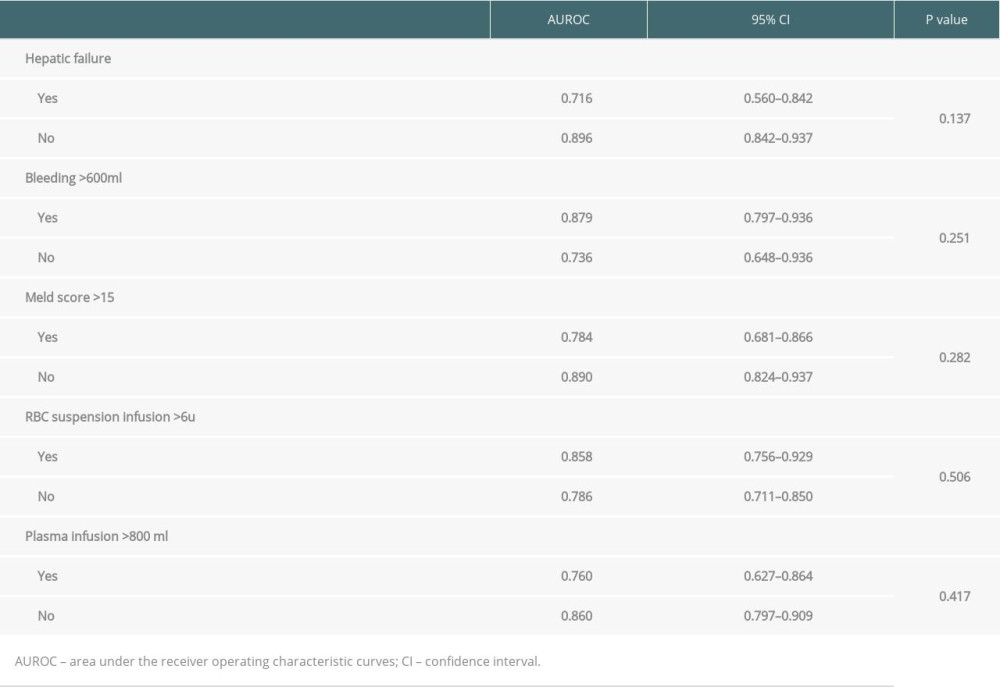
References
1. Dong V, Nadim MK, Karvellas CJ, Post-liver transplant acute kidney injury: Liver Transpl, 2021; 27(11); 1653-64
2. Joannidis M, Metnitz B, Bauer P, Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database: Intensive Care Med, 2009; 35(10); 1692-702
3. Sparrow HG, Swan JT, Moore LW, Disparate outcomes observed within Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury stage 1: Kidney Int, 2019; 95(4); 905-13
4. Tan L, Yang Y, Ma G, Early acute kidney injury after liver transplantation in patients with normal preoperative renal function: Clin Res Hepatol Gastroenterol, 2019; 43(4); 475-82
5. Cheng Y, Wei GQ, Cai QC, Prognostic value of model for end-stage liver disease incorporating with serum sodium score for development of acute kidney injury after liver transplantation: Chin Med J (Engl), 2018; 131(11); 1314-20
6. Yu JH, Kwon Y, Kim J, Influence of transfusion on the risk of acute kidney injury: ABO-compatible versus ABO-incompatible liver transplantation: J Clin Med, 2019; 8(11); 1785
7. Tokodai K, Lannsjö C, Kjaernet F, Association of post-reperfusion syndrome and ischemia-reperfusion injury with acute kidney injury after liver transplantation: Acta Anaesthesiol Scand, 2020; 64(6); 742-50
8. Granger DN, Kvietys PR, Reperfusion injury and reactive oxygen species: The evolution of a concept: Redox Biol, 2015; 6; 524-51
9. Liaw PC, Ito T, Iba T, DAMP and DIC: The role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC: Blood Rev, 2016; 30(4); 257-61
10. Jochmans I, Meurisse N, Neyrinck A, Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: Prospective cohort study: Liver Transpl, 2017; 23(5); 634-44
11. Rahman S, Davidson BR, Mallett SV, Early acute kidney injury after liver transplantation: Predisposing factors and clinical implications: World J Hepatol, 2017; 9(18); 823-32
12. Kalisvaart M, de Haan JE, Hesselink DA, The postreperfusion syndrome is associated with acute kidney injury following donation after brain death liver transplantation: Transpl Int, 2017; 30(7); 660-69
13. Durand F, Francoz C, Asrani SK, Acute kidney injury after liver transplantation: Transplantation, 2018; 102(10); 1636-49
14. Khwaja A, KDIGO clinical practice guidelines for acute kidney injury: Nephron Clin Pract, 2012; 120(4); c179-84
15. Feldkamp T, Bienholz A, Paul A, Renal damage after liver transplantation: Biosci Rep, 2019; 40(1); BSR20191187
16. Kollmann D, Neong SF, Rosales R, Renal dysfunction after liver transplantation: Effect of donor type: Liver Transpl, 2020; 26(6); 799-810
17. Thongprayoon C, Kaewput W, Thamcharoen N, Incidence and impact of acute kidney injury after liver transplantation: A meta-analysis: J Clin Med, 2019; 8(3); 372
18. Van Golen RF, Reiniers MJ, Vrisekoop N, The mechanisms and physiological relevance of glycocalyx degradation in hepatic ischemia/reperfusion injury: Antioxid Redox Signal, 2014; 21(7); 1098-118
19. Singhal AK, Symons JD, Boudina S, Role of endothelial cells in myocardial ischemia-reperfusion injury: Vasc Dis Prev, 2010; 7; 1-14
20. Agarwal B, Gatt A, Riddell A, Hemostasis in patients with acute kidney injury secondary to acute liver failure: Kidney Int, 2013; 84(1); 158-63
21. Lin KY, Chen HC, Jiang H, Predictive value of admission D-dimer for contrast induced acute kidney injury and poor outcomes post primary percutaneous coronary intervention: BMC Nep, 2020; 21(1); 90
22. Xu Z, Cheng B, Fu S, Coagulative biomarkers on admission to the ICU predict acute kidney injury and mortality in patients with septic shock caused by intraabdominal infection: Infect Drug Resist, 2019; 12; 2752-64
23. Hingorani SR, Seidel K, Pao E, Markers of coagulation activation and acute kidney injury in patients post hematopoietic cell transplantation: Bone Marrow Transplant, 2015; 50(5); 715-20
24. Park J, Kim Su, Choi HJ, Predictive role of the D-dimer level in acute kidney injury in living donor liver transplantation: A retrospective observational cohort study: J Clin Med, 2022; 11; 450
25. Cioni G, Cristani A, Mussini C, Incidence and clinical significance of elevated fifibrin(ogen) degradation product and/or D-dimer levels in liver cirrhosis patients: Ital J Gastroenterol, 1990; 22; 70-74
26. Gram J, Duscha H, Zurborn KH, Increased levels of fibrinolysis reaction products (D-dimer) in patients with decompensated alcoholic liver cirrhosis: Scand J Gastroenterol, 1991; 26; 1173-78
27. Li Y, Qi X, Li H, D-dimer level for predicting the in-hospital mortality in liver cirrhosis: A retrospective study: Exp Ther Med, 2017; 13(1); 285-89
28. Thai C, Oben C, Wagener G, Coagulation, hemostasis, and transfusion during liver transplantation: Best Pract Res Clin Anaesthesiol, 2020; 34(1); 79-87
29. Shorr AF, Thomas SJ, Alkins SA, D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients: Chest, 2002; 121(4); 12621268
30. Shebuski RJ, Kilgore KS, Role of inflammatory mediators in thrombogenesis: J Pharmacol Exp Ther, 2002; 300(3); 729-35
31. Jansen MPB, Florquin S, Roelofs JJTH, The role of platelets in acute kidney injury: Nat Rev Nephrol, 2018; 14(7); 457-71
32. Grommes J, Alard JE, Drechsler M, Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury: Am J Respir Crit Care Med, 2012; 185(6); 628-36
33. Lindemann S, Tolley ND, Dixon DA, Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis: J Cell Biol, 2001; 154(3); 485-90
34. Park J, Jeong J, Choi HJ, Role of thrombocytopenia in risk stratification for acute kidney injury after living donor liver transplantation: Platelets, 2021; 32(4); 453-62
35. Mallett SV, Cox DJ, Thrombelastography: Br J Anaesth, 1992; 69; 307-13
Figures
 Figure 1. Flowchart of the study process.
Figure 1. Flowchart of the study process. Figure 2. Relationship between the number of independent risk factors and the incidence of AKI stage 3.
Figure 2. Relationship between the number of independent risk factors and the incidence of AKI stage 3. Figure 3. Sensitivity and specificity of different parameters in predicting the incidence of AKI stage 3.
Figure 3. Sensitivity and specificity of different parameters in predicting the incidence of AKI stage 3. Figure 4. ROC curve of combining D-dimer, R-value and ADP-induced PLT aggregation rate for predicting the incidence of AKI stage 3.
Figure 4. ROC curve of combining D-dimer, R-value and ADP-induced PLT aggregation rate for predicting the incidence of AKI stage 3. Figure 5. Decision curve of combining D-dimer, R-value, and ADP-induced PLT aggregation rate.
Figure 5. Decision curve of combining D-dimer, R-value, and ADP-induced PLT aggregation rate. Tables
 Table 1. Demographic and pre-and intraoperative clinical data of the studied population (n=241).
Table 1. Demographic and pre-and intraoperative clinical data of the studied population (n=241). Table 2. Comparison of liver function and coagulation parameters between the 2 groups within 24 h of LT.
Table 2. Comparison of liver function and coagulation parameters between the 2 groups within 24 h of LT. Table 3. Multivariate logistic regression analysis of the incidence of stage 3 AKI after LT.
Table 3. Multivariate logistic regression analysis of the incidence of stage 3 AKI after LT. Table 4. Predictive values of D-dimer, R-value, and ADP-induced PLT aggregation for the incidence of stage 3 AKI after LT.
Table 4. Predictive values of D-dimer, R-value, and ADP-induced PLT aggregation for the incidence of stage 3 AKI after LT. Table 5. Subgroup analysis of the combined 3 indexes for predicting the incidence of stage 3 AKI after LT.
Table 5. Subgroup analysis of the combined 3 indexes for predicting the incidence of stage 3 AKI after LT. Table 1. Demographic and pre-and intraoperative clinical data of the studied population (n=241).
Table 1. Demographic and pre-and intraoperative clinical data of the studied population (n=241). Table 2. Comparison of liver function and coagulation parameters between the 2 groups within 24 h of LT.
Table 2. Comparison of liver function and coagulation parameters between the 2 groups within 24 h of LT. Table 3. Multivariate logistic regression analysis of the incidence of stage 3 AKI after LT.
Table 3. Multivariate logistic regression analysis of the incidence of stage 3 AKI after LT. Table 4. Predictive values of D-dimer, R-value, and ADP-induced PLT aggregation for the incidence of stage 3 AKI after LT.
Table 4. Predictive values of D-dimer, R-value, and ADP-induced PLT aggregation for the incidence of stage 3 AKI after LT. Table 5. Subgroup analysis of the combined 3 indexes for predicting the incidence of stage 3 AKI after LT.
Table 5. Subgroup analysis of the combined 3 indexes for predicting the incidence of stage 3 AKI after LT. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








