25 April 2023: Original Paper
Levels of Procollagen Type I C-Terminal Pro-Peptide and Galectin-3, Arterial Stiffness Measured By Pulse Wave Velocity, and Cardiovascular Morbidity and Mortality in 44 Patients 2 Years After Kidney Transplantation
Madonna Salib1CDEFG, Nicolas Girerd1ADE, Arnaud Simon2BF, Anna Kearney-Schwartz34ADE, Kévin Duarte1BCD, Céline Leroy1B, Patrick Rossignol1DE, Athanase Benetos34BE, Luc Frimat2DE, Sophie Girerd12ADE*DOI: 10.12659/AOT.938137
Ann Transplant 2023; 28:e938137
Abstract
BACKGROUND: Cardiovascular (CV) mortality remains high despite the improvement of kidney function after kidney transplantation. In heart failure (HF), high concentrations of biomarkers of fibrosis, related to cardiac and/or vascular impairment, are associated with CV outcomes, but their significance in kidney transplantation is still unclear. Our aim was to investigate the association of procollagen type I C-terminal pro-peptide (PICP) and galectin-3 (Gal-3), markers of fibrosis, with arterial stiffness measured by pulse wave velocity (PWV) and CV morbi-mortality in kidney transplantation recipients from the prospective monocenter TRANSARTE study (Transplantation and Arteries), which compared the evolution of arterial stiffness in transplanted patients and patients remained on dialysis.
MATERIAL AND METHODS: PICP and Gal-3 were measured at 2 years after transplantation in 44 kidney transplantation patients. Spearman’s rank-order correlation analysis was conducted to assess the relationship between biomarkers and PWV. Association of biomarkers with CV morbi-mortality was evaluated using Cox regression analysis adjusted for age, renal function, and PWV.
RESULTS: There was no significant correlation between PWV and PICP (r=-0.16, P=0.3) or Gal-3 (r=0.03, P=0.85). Gal-3, after adjusting for key prognostic factors, including PWV, was significantly associated with CV morbi-mortality [HR (95% CI)=4.30 (1.01-18.22), P=0.048], whereas PICP was not significantly associated with outcome.
CONCLUSIONS: In multivariable adjusted analysis, elevated Gal-3 concentrations were associated with CV morbi-mortality in kidney transplantation patients, whereas PICP was not. As Gal-3 was not related to PWV, other sources of fibrosis (eg, cardiac fibrosis) may be underlying the prognostic value of Gal-3 in kidney transplantation.
Keywords: Cardiovascular Diseases, Fibrosis, Galectin-3, Human, Kidney Transplantation, Procollagen Type I Carboxy Terminal Peptide, Pulse Wave Analysis, Vascular Stiffness, Humans, Galectin 3, Prospective Studies, Heart Failure, Biomarkers
Background
The urgent need for a novel approach to better stratify cardiovascular (CV) risk in kidney transplantation recipients is derived from the high prevalence/incidence of CV diseases (CVD) in this population. Despite the restoration of renal function after kidney transplantation, CV-related death remains higher in kidney transplantation recipients compared with the general population [1].
Atherosclerosis, arteriosclerosis, and arterial stiffening are accelerated in end-stage renal disease (ESRD) [2,3]. Patients develop mechanical and physiological abnormalities of arteries due to fluid overload, bone disorder, chronic inflammation, hypertension, and endothelial dysfunction [3]. This subsequently can lead to CV fibrosis [2,4].
Improvement of renal function probably has beneficial effects on vascular structure and function, but kidney transplantation patients receive immunosuppressive therapy with deleterious effects on metabolic and vascular parameters [5]. The net output of these opposing factors is still insufficiently documented. Notably, the evolution of arterial stiffness in kidney transplantation is complex and has been reported to be associated with poor CV prognosis [6,7].
A number of CV markers can predict CV outcomes. Pulse wave velocity (PWV) has been shown to be a strong predictor of CV outcomes, independent of conventional prognostic factors such as age, left ventricular (LV) hypertrophy, and hypertension [8,9]. The biological factors underlying this association of PWV with outcome is currently not well understood. Arterial stiffness has been shown in other settings to be associated with fibrosis. Harvey et al [10] emphasized that molecular mechanisms underlying vascular stiffening follows vascular fibrosis and are related to increased expression and activation of matrix metalloproteinases, upregulation of galectin-3 (Gal-3), and activation of profibrotic pathways. Collagen peptides, which show circulating levels of collagen fibers degradation and/or production, and Gal-3 are commonly used biomarkers to explore biological fibrosis.
C-propeptide molecules control the assembly of collagen fibrils in the extracellular matrix (ECM) [11]. Serum procollagen type I C-terminal pro-peptide (PICP), a collagen type I synthesis biomarker, was suggested as a key marker in the development of myocardial fibrosis, as it was proven to be correlated with myocardial collagen in patients with hypertensive heart disease [12,13]. Moreover, PICP has been shown to be associated with higher rates of CV-related death in hemodialysis patients [14]. Notably, arterial stiffness, assessed by PWV, is significantly correlated with PICP in hypertensive patients with LV hypertrophy [15].
Gal-3 is a profibrotic molecule that has been shown to be involved in cardiac fibrosis, and is associated with CV outcomes in heart failure (HF) [16]. In dialysis patients, Gal-3 was found to be associated with a higher risk of all-cause mortality and CV death [14,17]. Importantly, a concomitant increase in Gal-3 concentration and PWV was demonstrated to be significantly associated with higher rates of all-cause mortality and major CV and cerebrovascular events in hemodialysis patients [18].
The relevance of PICP and Gal-3 and their relationship with PWV in kidney transplantation has not been studied yet. In this regard, we aimed to study the association of PICP and Gal-3, on top of PWV, with the composite outcome of death and CV events among kidney transplantation recipients in the TRANSARTE study (Transplantation and Arteries) [19], which characterized the evolution of arterial stiffness after kidney transplantation in ESRD patients.
Material and Methods
STUDY POPULATION:
The description of this single-center study of the prospective TRANSARTE (Transplantation and Arteries) has been previously published [19]. In short, 100 ESRD patients were recruited from the waiting list of the University Hospital of Nancy to characterize the evolution of arterial stiffness after kidney transplantation. Forty-four renal transplant recipients who had available after transplantation PWV measurement at 2-year follow-up were considered in this subsequent analysis. Informed consent was obtained from all subjects involved in the TRANSARTE study. The study protocol was reviewed and approved by the local research ethics committee of CPP-EST 3 (No. 06.10.03), France. This analysis was conducted under the umbrella of the sponsor of the study (Nancy University Hospital) under the supervision of the principal investigator of the study (LF).
FOLLOW-UP:
All patients included in the TRANSARTE study were routinely followed up at least once a year at the Nancy University Hospital. Clinical events occurring during follow-up were collected from the clinical charts until December 31, 2020. The following CV events were recorded from clinical chart reviews by a trained senior physician: acute coronary syndrome, myocardial infarction, angioplasty, vascular angioplasty, coronary bypass, ischemic or hemorrhagic stroke, angioplasty of lower extremity arteries, amputation, and atrial fibrillation.
MEASUREMENT OF PWV:
PWV was measured using the Complior® technique (ALAM Medical, Saint Quentin Fallavier, France), which simultaneously records arterial pulse waves at carotid and femoral sites. The propagation time (t) of the incident wave was calculated through mechanotransducer probes and the carotid–femoral distance (D) was determined with a tape measure. The time delay that represents the difference between the R-wave and the foot of the pressure pulse waveform at each site and the distance traveled by the pulse wave between the 2 recording sites, divided by the time, were used in the calculation of PWV. PWV (m/s) was calculated using the formula PWV=D×0.8/(t).
BIOMARKERS:
PICP and Gal-3 were measured in EDTA plasma. PICP was measured by ELISA (Quidel Corporation, Santa Clara, USA) and Gal-3 was measured by ELISA (BG MEDICINE, Foxboro, USA) at PWV assessment post kidney transplantation (~2-year).
STATISTICAL ANALYSIS:
Continuous data are presented as mean±standard deviation or median (25th and 75th percentiles), whereas categorical data are expressed as numbers (%). Spearman’s rank-order correlation (r) analysis was performed to assess the relationship between biomarkers and between PWV and biomarkers. In univariable and multivariable Cox proportional hazards models, we assessed associations of biomarkers (as log-transformed continuous variables) with the composite outcome death and CV events (CV events are defined by acute coronary syndrome, myocardial infarction, angioplasty, vascular angioplasty, coronary bypass, ischemic or hemorrhagic stroke, angioplasty of lower extremity arteries, amputation, and atrial fibrillation). In multivariable analysis, covariates included age at transplantation, estimated glomerular filtration rate (eGFR), and PWV. Moreover, associations between biomarkers and the composite outcome were assessed after adjusting for graft rejection as a time-dependent variable using Cox proportional hazards models. Analyses were performed using R version 4.0.2 (R Development Core Team, Vienna, Austria).
Results
BASELINE CHARACTERISTICS OF PATIENTS:
Of the 44 studied participants (median age 53 years [46–59], 43.2% female), 6 (13.6%) had diabetes mellitus, 1 (2.3%) had previous myocardial infarction or angina, and 2 (4.5%) had previous strokes. The majority of patients received a deceased donor allograft (90.9%) and calcineurin inhibitors (CNI) (97.7%), most of them cyclosporine (53.5%). PWV was 8.7±2.5 m/s and 8.6±2.2 at pre-transplantation and post-transplantation, respectively.
At mean follow-up time of 1.9 years, median levels of PICP and Gal-3 were 102.0 ng/mL [68.5–125.1] and 15.4 [13.1–20.3], respectively. Estimated glomerular filtration rate (eGFR) at time of biomarkers measurement was 42.2±14.2 mL/min/1.73 m2. During the median follow-up period of 10.8 years, 14 patients experienced a CV event, 5 patients returned in dialysis, and 13 patients died (Table 1).
CORRELATION AND ASSOCIATION OF PICP AND GAL-3 WITH PWV AND OUTCOME:
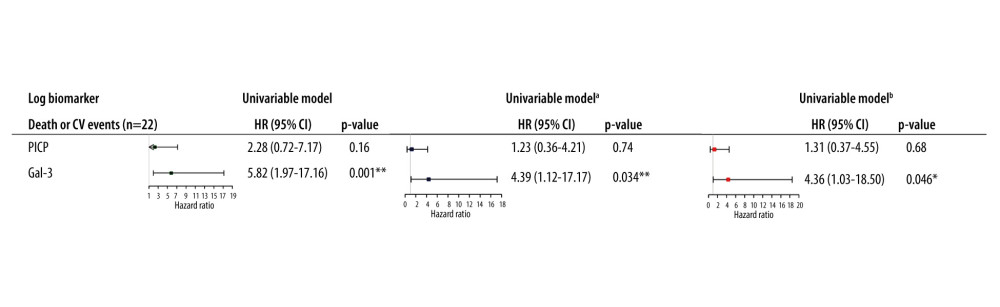
No correlation was found between PWV and PICP (r=−0.16, P=0.3) and between PWV and Gal-3 (r=0.03, P=0.85). There was no correlation between PICP and Gal-3 (r=−0.015, P=0.9). In continuous unadjusted analysis, PICP was not significantly associated with CV morbi-mortality, whereas Gal-3 was significantly associated with the outcome (HR per 1 log increase in Gal-3=5.82 (1.97–17.16), P=0.001). In the fully adjusted model for age at transplantation, eGFR, and PWV, a 1-log unit increase in Gal-3 was associated with a 4.36 fold greater risk of CV morbi-mortality (HR per 1-log increase in Gal-3=4.36 (1.03–18.50), P=0.046), whereas PICP was not significantly associated with outcome (Figure 1). Of note, further including diabetes mellitus in the multivariable models did not significantly change the association of biomarkers with the outcome (data not shown).
SENSITIVITY ANALYSIS:
When further adjusting for type of CNI (ie, cyclosporine or tacrolimus) or smoking in the multivariable models, we did not identify a significant modification of the associations of biomarkers with CV morbi-mortality (Supplementary Table 1). We also performed a forward variable selection for the Cox proportional hazards models with p-to-enter set at 15% for the following candidate variables: age at transplantation, eGFR at post-transplantation PWV measurement, diabetes mellitus, post-transplantation PWV, smoking, sex, previous CV events (ie, previous myocardial infarction or angina and previous stroke), and type of CNI. Only previous CV events was retained in the model. The association of biomarkers remained unchanged in this additional model adjusted for previous CV events (HR per 1-log increase in Gal-3=5.67 (1.42–22.65), P=0.014) (Supplementary Table 1).
We did not identify a significant modification of the associations between biomarkers, PICP and Gal-3, and CV morbi-mortality when adjusting for graft rejection as time-dependent variable (Supplementary Table 2).
Discussion
It is well established that ESRD patients develop structural and functional pathophysiological CV changes, including myocardial fibrosis, arterial stiffness, LVH, and HF [2]. Moreover, despite the improvement in renal function after kidney transplantation, CV mortality remains high in kidney transplantation recipients compared to the general population [1]. Furthermore, the impact of kidney transplantation on arterial stiffness is complex, considering the beneficial impact of the renal function improvement but the deleterious impact of immunosuppressive drugs [5,6]. The present study is the first study that aimed to investigate the association of CV biomarkers, PICP and Gal-3, on top of PWV, with CV morbi-mortality in kidney transplantation patients.
PICP is released into the blood as it cleaves from procollagen type I during the synthesis of fibril-forming collagen type I by the procollagen type I carboxy-terminal proteinase [20]. PICP is cleared from blood via the liver due to its high molecular weight (a 100 kDa procollagen) and therefore is considered a marker of collagen type I synthesis in the absence of liver cirrhosis [20,21]. Experimentally [22] and clinically [23], it was proven that high concentrations of collagen are related to myocardial stiffness and cardiac dysfunction/fibrosis.
Gal-3 is a β-galactoside-binding lectin that can bind to complex carbohydrates. The available evidence suggest that Gal-3 is a small protein (~30–35 kDA) that is expressed in several tissues such as the heart, blood vessels, and the kidneys. The upregulation of Gal-3 is secreted by activated macrophages and it binds with several ECM proteins [23–26]. In response to acute or chronic disease, Gal-3 binds to and activates myofibroblasts which, in turn, upregulate the formation of collagen into ECM, leading myocardial fibrosis [25].
Patients in the TRANSARTE study had a mean PICP of 105±42 ng/mL and mean Gal-3 of 18±10 ng/mL. These values are lower than those found in ESRD patients on chronic hemodialysis from the AURORA trial (mean PICP was 176±91 ng/mL and mean Gal-3 was 69±25 ng/mL) [14].
Arterial stiffness is an independent predictor of morbidity and mortality in ESRD [24]. There was no significant difference in PWV in transplanted patients (median PWV 9.2 [7.9–11.9] m/s) in comparison to those remaining on dialysis (median PWV 9.8 [7.7–12.1] m/s) in TRANSARTE study [19]. In a meta-analysis, Sidibe et al [25] showed that there is a significant reduction in overall PWV after successful kidney transplantation: a decrease of 1.20 m/s in central PWV (measured by carotid-femoral or aorta-femoral PWV), a decrease of 1.17 m/s in peripheral PWV (measured by carotid-radial PWV or femoral-distal PWV), and a decrease of 1.21 m/s in brachial-ankle PWV.
In the present study, there was no significant correlation between the biomarkers and PWV. Collagen type III was found to be the most abundant form in vascular walls and large arteries such as the aorta [26]. However, in the specific context of kidney transplantation, type I collagen expression may be related to other pathophysiological (possibly related to graft fibrosis) features [27,28]. PICP concentrations have been shown to be associated with myocardial fibrosis [12] but no direct evidence exists for vascular systemic damage. Actually, consistent with our study, plasma PICP concentrations were reported to be uncorrelated with arterial stiffness, measured by PWV, in hypertensive patients in 2 different studies [29,30]. Even if some interplay exists between the heart and vessels, in the setting of kidney transplantation, PICP may be more closely related to cardiac features than vascular ones, explaining the low correlation in our study between PICP and PWV.
Increased Gal-3 concentrations have been documented to be involved in cardiac modeling, such as myocardial fibrogenesis and the development of HF [31]. Based on these findings, we assessed the correlation between Gal-3 and PWV. Our study showed no correlation between Gal-3 concentrations and arterial stiffness in kidney transplantation patients, suggesting that cardiac disease more probably drives the association between Gal-3 and CV mortality than vascular damage.
Gal-3 acts as a marker of cardiac fibrosis in consequence of chronic inflammation and oxidative stress related to chronic kidney disease (CKD) [32]. Indeed, the current analysis showed that patients who were on dialysis before the transplantation had higher Gal-3 concentrations. This is likely related to the immune system being stimulated by the dialysis itself, leading to chronic inflammation and oxidative stress [33], but could be partly related to Gal-3 renal clearance [34]. Of note, chronic inflammation in kidney transplantation patients may lead to a prothrombotic state as well as chronic hypoxia associated with excessive accumulation of extracellular matrix in the vascular tree [33]. In the LURIC study, Gal-3 was reported to be associated with CV-related death and all-cause mortality in patients with impaired kidney function [17]. Moreover, previous studies reported an incremental prognostic value provided by Gal-3 in hemodialysis patients for all-cause mortality [35] or for CV morbi-mortality or all-cause mortality in hemodialysis patients [14]. Furthermore, Zhang et al [18] demonstrated that hemodialysis patients with both high PWV and Gal-3 had a significant risk increase of a composite of all-cause mortality and major adverse cerebrovascular and CV events.
In the present study, Gal-3 was significantly associated with the composite outcome CV morbi-mortality while adjusting for age at transplantation, eGFR, and PWV. This may suggest that despite the restoration of renal function following kidney transplantation, the histological CV modifications generated by uremic cardiomyopathy are not fully reversed [4] and continue to have an impact on patients’ clinical outcome. Higher concentrations of Gal-3 may also be a proxy for ongoing inflammation in the presence of arterial stiffness [36,37].
Our findings are promising in that they provide the basis for further development strategies to validate a panel of circulating markers in a large-scale of kidney transplantation population and to stratify patients according to their biomarker profiles, which could provide novel pathways for personalized treatment.
It should be noted that this was a single-center study with a small sample size. This moderate statistical power limited the number of covariates for which the model could be adjusted. However, it is the first study, to our knowledge, that assessed the association of PICP and Gal-3 with PWV and CV morbi-mortality in kidney transplantation patients.
Conclusions
In summary, higher Gal-3 concentrations conferred greater risk of CV morbi-mortality in kidney transplantation patients, while it was not associated with vascular modifications (as measured by PWV), thus suggesting that non-vascular (probably cardiac) fibrosis is an important predictor of outcome following kidney transplantation. These preliminary findings need further investigation to determine whether anti-Gal-3 treatment is be a path to consider in the setting of kidney transplantation.
Tables
Table 1. Baseline characteristics of patients.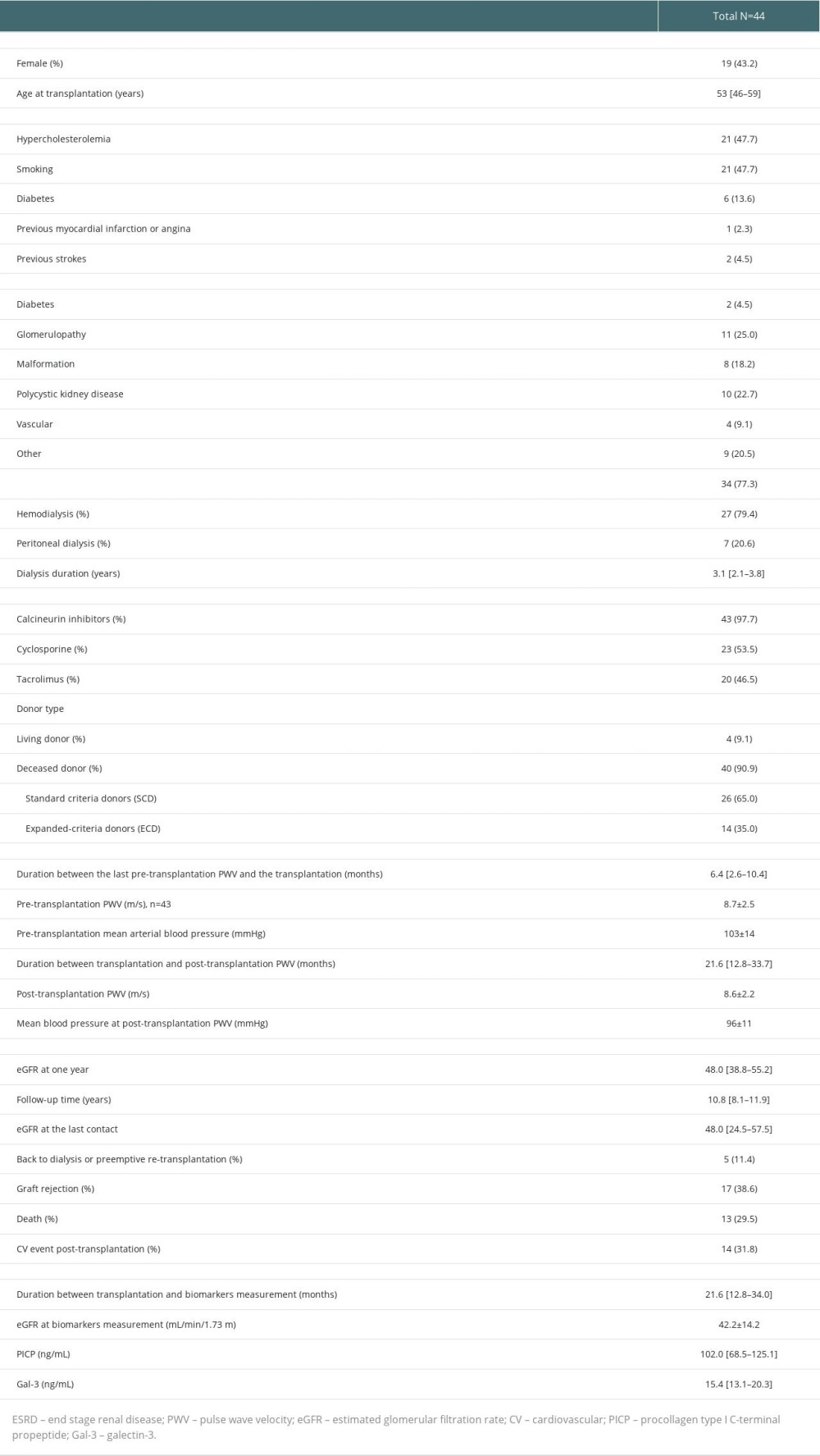 Supplementary Table 1. Association of biomarkers with cardiovascular morbi-mortality in multivariate cox models.
Supplementary Table 1. Association of biomarkers with cardiovascular morbi-mortality in multivariate cox models.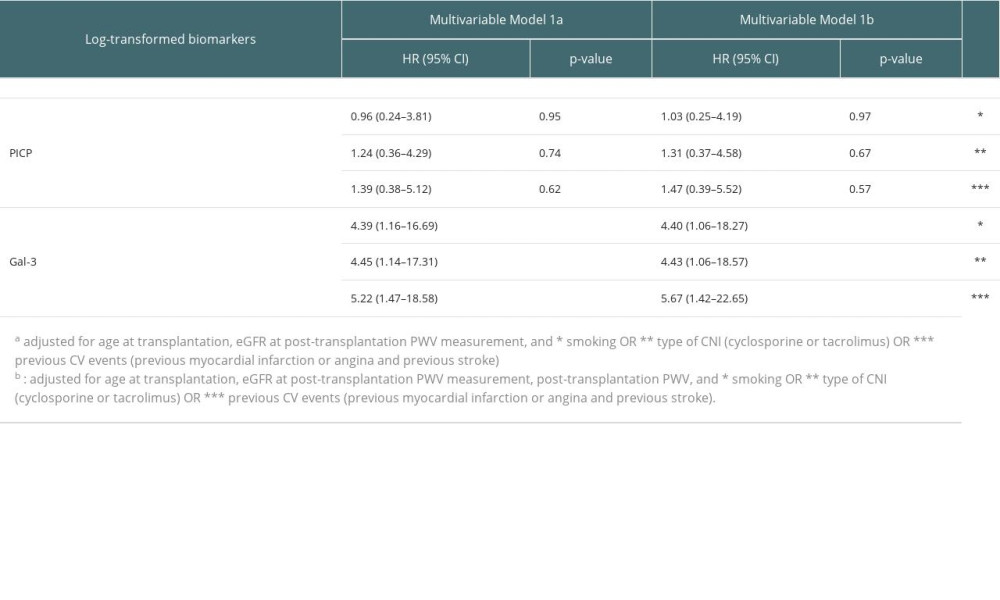 Supplementary Table 2. Association of biomarkers with CV morbi-mortality after adjusting for graft rejection as a time-dependent variable.
Supplementary Table 2. Association of biomarkers with CV morbi-mortality after adjusting for graft rejection as a time-dependent variable.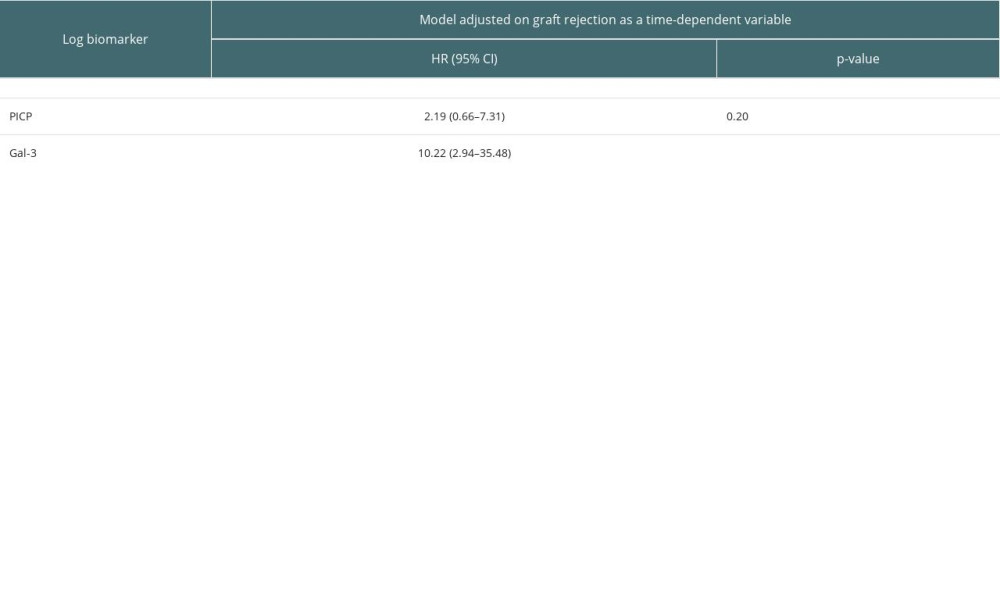
References
1. Rangaswami J, Mathew RO, Parasuraman R, Cardiovascular disease in the kidney transplant recipient: Epidemiology, diagnosis and management strategies: Nephrol Dial Transplant, 2019; 34(5); 760-73
2. Chirakarnjanakorn S, Navaneethan SD, Francis GS, Tang WH, Cardiovascular impact in patients undergoing maintenance hemodialysis: Clinical management considerations: Int J Cardiol, 2017; 232; 12-23
3. Briet M, Boutouyrie P, Laurent S, London GM, Arterial stiffness and pulse pressure in CKD and ESRD: Kidney Int, 2012; 82(4); 388-400
4. Wang X, Shapiro JI, Evolving concepts in the pathogenesis of uraemic cardiomyopathy: Nat Rev Nephrol, 2019; 15(3); 159-75
5. Melilli E, Manonelles A, Montero N, Impact of immunosuppressive therapy on arterial stiffness in kidney transplantation: Are all treatments the same?: Clin Kidney J, 2018; 11(3); 413-21
6. Desjardins MP, Sidibe A, Fortier C, Impact of kidney transplantation on aortic stiffness and aortic stiffness index β0: J Hypertens, 2019; 37(7); 1521-28
7. Salib M, Simon A, Girerd N, Association between long-term change in arterial stiffness and cardiovascular outcomes in kidney transplant recipients: Insights from the TRANSARTE study: Jouranl of Clinical Medicine, 2022; 11(5); 1410
8. Edwards NC, Ferro CJ, Townend JN, Steeds RP, Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: A pattern resembling heart failure with preserved ejection fraction: Heart, 2008; 94(8); 1038-43
9. Korogiannou M, Xagas E, Marinaki S, Arterial stiffness in patients with renal transplantation; Associations with co-morbid conditions, evolution, and prognostic importance for cardiovascular and renal outcomes: Front Cardiovasc Med, 2019; 6; 67
10. Harvey A, Montezano AC, Lopes RA, Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications: Can J Cardiol, 2016; 32(5); 659-68
11. Hulmes DJS, Roles of the procollagen C-propeptides in health and disease: Essays Biochem, 2019; 63(3); 313-23
12. Lopez B, Gonzalez A, Ravassa S, Circulating biomarkers of myocardial fibrosis: The need for a reappraisal: J Am Coll Cardiol, 2015; 65(22); 2449-56
13. Querejeta R, Lopez B, Gonzalez A, Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis: Circulation, 2004; 110(10); 1263-68
14. Salib M, Girerd S, Girerd N, Serum markers of fibrosis, cardiovascular and all-cause mortality in hemodialysis patients: The AURORA trial: Clin Res Cardiol, 2021; 111(6); 614-26
15. Ishikawa J, Kario K, Matsui Y, Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy: Hypertens Res, 2005; 28(12); 995-1001
16. Lopez-Andres N, Rossignol P, Iraqi W, Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial: Eur J Heart Fail, 2012; 14(1); 74-81
17. Drechsler C, Delgado G, Wanner C, Galectin-3, renal function, and clinical outcomes: Results from the LURIC and 4D studies: J Am Soc Nephrol, 2015; 26(9); 2213-21
18. Zhang Q, Yin K, Zhu M, Combining pulse wave velocity with galectin-3 to predict mortality and cerebrovascular and cardiovascular events in hemodialysis patients: Front Med (Lausanne), 2020; 7; 579021
19. Bachelet-Rousseau C, Kearney-Schwartz A, Frimat L, Evolution of arterial stiffness after kidney transplantation: Nephrol Dial Transplant, 2011; 26(10); 3386-91
20. Lopez B, Gonzalez A, Varo N, Biochemical assessment of myocardial fibrosis in hypertensive heart disease: Hypertension, 2001; 38(5); 1222-26
21. Coen G, Mazzaferro S, Ballanti P, Procollagen type I C-terminal extension peptide in predialysis chronic renal failure: Am J Nephrol, 1992; 12(4); 246-51
22. Jalil JE, Doering CW, Janicki JS, Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle: Circ Res, 1989; 64(6); 1041-50
23. Brilla CG, Funck RC, Rupp H, Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease: Circulation, 2000; 102(12); 1388-93
24. Boutouyrie P, Fliser D, Goldsmith D, Assessment of arterial stiffness for clinical and epidemiological studies: Methodological considerations for validation and entry into the European Renal and Cardiovascular Medicine registry: Nephrol Dial Transplant, 2014; 29(2); 232-39
25. Sidibe A, Fortier C, Desjardins MP, Reduction of arterial stiffness after kidney transplantation: A systematic review and meta-analysis: J Am Heart Assoc, 2017; 6(12); e007235
26. Dellegrottaglie S, Sands RL, Gillespie BW, Association between markers of collagen turnover, arterial stiffness and left ventricular hypertrophy in chronic kidney disease (CKD): The Renal Research Institute (RRI)-CKD study: Nephrol Dial Transplant, 2011; 26(9); 2891-98
27. Rasmussen DGK, Nielsen PM, Kasab-Oglo OY, A non-invasive biomarker of type III collagen degradation reflects ischaemia reperfusion injury in rats: Nephrol Dial Transplant, 2019; 34(8); 1301-9
28. Stribos EGD, Nielsen SH, Brix S, Non-invasive quantification of collagen turnover in renal transplant recipients: PLoS One, 2017; 12(4); e0175898
29. McNulty M, Mahmud A, Spiers P, Feely J, Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects: J Hum Hypertens, 2006; 20(11); 867-73
30. Skalska A, Gasowski J, Cwynar M, Grodzicki T, The relationship between pulse wave velocity and indexes of collagen synthesis in hypertensive patients, according to the level of systolic blood pressure: J Hum Hypertens, 2005; 19(9); 731-35
31. Zhong X, Qian X, Chen G, Song X, The role of galectin-3 in heart failure and cardiovascular disease: Clin Exp Pharmacol Physiol, 2019; 46(3); 197-203
32. Zhang T, Cao S, Yang H, Li J, Prognostic impact of galectin-3 in chronic kidney disease patients: A systematic review and meta-analysis: Int Urol Nephrol, 2019; 51(6); 1005-11
33. Ponticelli C, Campise MR, The inflammatory state is a risk factor for cardiovascular disease and graft fibrosis in kidney transplantation: Kidney Int, 2021; 100(3); 536-45
34. Meijers WC, van der Velde AR, Ruifrok WP, Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis: J Am Heart Assoc, 2014; 3(5); e000962
35. Obokata M, Sunaga H, Ishida H, Independent and incremental prognostic value of novel cardiac biomarkers in chronic hemodialysis patients: Am Heart J, 2016; 179; 29-41
36. Zanoli L, Lentini P, Briet M, Arterial stiffness in the heart disease of CKD: J Am Soc Nephrol, 2019; 30(6); 918-28
37. Tan R, Liu X, Wang J, Alternations of galectin levels after renal transplantation: Clin Biochem, 2014; 47(15); 83-88
Tables
 Table 1. Baseline characteristics of patients.
Table 1. Baseline characteristics of patients. Table 1. Baseline characteristics of patients.
Table 1. Baseline characteristics of patients. Supplementary Table 1. Association of biomarkers with cardiovascular morbi-mortality in multivariate cox models.
Supplementary Table 1. Association of biomarkers with cardiovascular morbi-mortality in multivariate cox models. Supplementary Table 2. Association of biomarkers with CV morbi-mortality after adjusting for graft rejection as a time-dependent variable.
Supplementary Table 2. Association of biomarkers with CV morbi-mortality after adjusting for graft rejection as a time-dependent variable. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860