13 June 2023: Original Paper
Incidence of Thromboembolic Complications Following Kidney Transplantation with Short and Extended Aspirin Prophylaxis: A Retrospective Single-Center Study
Angus H. PeglerDOI: 10.12659/AOT.939143
Ann Transplant 2023; 28:e939143
Abstract
BACKGROUND: Aspirin prophylaxis has been associated with reduced graft-related thrombosis following kidney transplantation. Aspirin cessation, however, can increase risk of venous thromboembolic complications, including pulmonary thromboembolism and deep venous thrombosis. This single-center, retrospective, pre-post interventional study from Brisbane, Australia, aimed to compare the rate of thrombotic complications in 1208 adult kidney transplant recipients receiving postoperative aspirin for 5 days or >6 weeks.
MATERIAL AND METHODS: We enrolled1208 kidney transplant recipients who received 100 mg aspirin for 5 days (n=571) or >6 weeks (n=637) postoperatively. The primary outcome was venous thromboembolism (VTE) in the first 6 weeks after transplant, examined by multivariable logistic regression analysis. Secondary outcomes were renal vein/artery thrombosis, 1-month serum creatinine, rejection, myocardial infarction, stroke, blood transfusion, dialysis at day 5 and day 28, and mortality.
RESULTS: Sixteen (1.3%) patients experienced VTE (5-day n=8, 1.4%; >6-week n=8, 1.3%; P=0.8). Extended aspirin duration was not independently associated with a reduction in VTE (OR 0.91, 95% CI 0.32-2.57; P=0.9). Graft thrombosis was rare (n=3, 0.25%). Aspirin duration was not associated with cardiovascular events, blood transfusion, graft thrombosis, graft dysfunction, rejection, or mortality. VTE was independently associated with older age (OR 1.09, 95% CI 1.04-1.16; P=0.002), smoking (OR 3.59, 95% CI 1.20-13.2; P=0.032), younger donor age (OR 0.96, 95% CI 0.93-1.00; P=0.036), and thymoglobulin use (OR 10.5, 95% CI 3.09-32.1; P≥0.001).
CONCLUSIONS: Extended-duration aspirin use did not significantly reduce the incidence of VTE in the first 6 weeks following kidney transplantation. An association was identified between anti-human thymocyte immunoglobulin and VTE, which requires further assessment.
Keywords: Antilymphocyte Serum, Aspirin, Cardiovascular Diseases, Kidney Transplantation, renal insufficiency, venous thromboembolism, Adult, Humans, Incidence, Retrospective Studies
Background
Graft thrombosis is an important early complication following kidney transplantation and occurs in up to 2–3% of cases, usually resulting in graft loss [1]. This may be due to renal vein thrombosis or more rarely, arterial occlusion [1]. Risk of thrombosis is greatest in the first week after transplant [2] and may be caused by structural vascular abnormalities, rejection, or pre-existing prothrombotic states, including thrombophilias or diabetes mellitus [1]. A recent systematic review by Cheungpasitporn et al (2017) of 9 observational studies including 19 759 kidney transplant recipients suggested that postoperative aspirin use can reduce rates of allograft thrombosis, allograft failure, and adverse cardiac events or mortality [3]. Despite this, there is no clear consensus regarding the optimum protocol for use and duration of aspirin therapy in the early post-transplant period.
A subsequent review by Khalil et al (2019) of aspirin prophylaxis specifically for prevention of cardiovascular events in kidney transplantation recommended secondary prophylaxis in the setting of established coronary artery disease, but found insufficient evidence to recommend primary prophylaxis of cardiovascular events in all kidney transplant recipients [4]. The KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients (2009) recommends the use of aspirin prophylaxis in patients with diabetes and atherosclerotic cardiovascular disease, but acknowledges the need for further research to determine the efficacy and safety of aspirin primary prophylaxis in kidney transplant recipients [5]. In the Queensland Kidney Transplant Service, patients have routinely received subcutaneous heparin as VTE prophylaxis and a short (5-day) course of 100 mg aspirin daily as prophylaxis against graft thrombosis. This includes a single dose given pre-operatively with induction immunosuppression, either basiliximab (Simulect, Novartis Pharmaceuticals) or rabbit anti-thymocyte globulin (thymoglobulin, Genzyme) [6]. Ongoing immunosuppression was managed according to local guidelines and practice, and included the use of thymoglobulin for episodes of rejection as required.
In 2017, an increase in the number of postoperative venous thromboembolism (VTE) events was observed following kidney transplantation within the Queensland Kidney Transplant Service unit. The incidence of venous thromboembolism (VTE), encompassing both deep vein thrombosis (DVT) and pulmonary embolus (PE), is up to 8-fold higher in kidney transplant patients than in the general population [7,8]. This risk is greatest in the period immediately following transplantation [7,8]. Risk factors for VTE post-kidney transplant include prolonged hospitalization, medical multimorbidity, delayed graft function, the requirement for blood transfusion, and the immunosuppression regimen used [7–9]. VTE is associated with poor clinical outcomes in the transplant population, including increased rates of graft failure and death [8]. The short duration of postoperative aspirin prophylaxis used in the unit was identified as a plausible contributor to VTE risk, potentially compounded by a rebound hypercoagulability phenomenon after aspirin cessation. This led to a change in protocol to extend the duration of postoperative aspirin from 5 days to 3 months. Patients are routinely discharged to the care of their treating nephrologist after 6 weeks; therefore, the expected outcome was a demonstrated reduction in VTE risk in the first 6 weeks postoperatively.
Aspirin has been shown to be effective VTE prophylaxis following certain orthopedic procedures, in specific medical populations, and as secondary prophylaxis to prevent VTE recurrence [10]. Despite this, increased rates of coronary, cerebrovascular, and peripheral vascular events have been observed up to 4 weeks after cessation of aspirin [11–13], leading to suggestion of a rebound hypercoagulability phenomenon [14]. Rates of VTE following aspirin cessation have not been previously reported, but an increase in VTE due to rebound hypercoagulability after ceasing oral anticoagulation has been described [15], including in the kidney transplant population [16]. Prior research examining the effects of aspirin on VTE following kidney transplant is limited and there is currently insufficient evidence as to the optimal thromboprophylaxis in this population [17].
This retrospective study from a single center in Brisbane, Australia, aimed to compare rates of VTE in 1208 kidney transplant recipients receiving postoperative aspirin for 5 days or >6 weeks. Rates of graft thrombosis, cardiovascular events, bleeding, and graft function were also compared, as well as the incidence and risk factors for VTE in this population.
Material and Methods
STUDY DESIGN:
We performed a retrospective cohort study of all adult kidney transplants performed at Princess Alexandra Hospital, Brisbane, between 1 January 2014 and 30 June 2021. The cohort was stratified by protocol according to duration of postoperative aspirin prophylaxis as either 5 days (1 January 2014 to 29 August 2017) or >6 weeks (30 August 2017 to 30 June 2021). Additional VTE prevention measures remained unchanged throughout the cohort period, including subcutaneous heparin chemoprophylaxis, mechanical thromboprophylaxis with graduated compression stockings, and early postoperative mobilisation. Patients were followed up for 6 weeks before discharge to their treating nephrologist, where aspirin prophylaxis was recommended for a total of 3 months.
POPULATION:
The Queensland Kidney Transplant Service, based at Princess Alexandra Hospital in Brisbane, Australia, services a population of over 5 million people. The study included all kidney transplants in adult patients aged 18 years or older at Princess Alexandra Hospital between 1 January 2014 and 30 June 2021. Patients under the age of 18 years at the time of transplantation, and those who underwent simultaneous multiple organ transplants, were excluded.
DATA COLLECTION:
Patient demographical, clinical, and treatment data were obtained from the Princess Alexandra Hospital Integrated Nephrology Database. Data items collected included age, sex, coronary artery disease, peripheral vascular disease, cerebrovascular disease, hypertension, diabetes mellitus, chronic lung disease, smoking status, cause of chronic kidney disease, time from dialysis to transplantation, modality of dialysis, previous kidney transplantation, donor type, donor age, human leukocyte antigen (HLA) mismatches, ABO incompatibility, number of donor renal arteries and veins, operative time, cold and warm ischemic time, implantation biopsy, immunosuppression, thymoglobulin (induction or postoperative), intravenous immunoglobulin (IVIG) and plasma exchange use in the first 6 weeks post-transplant, and the initial admission duration.
OUTCOMES:
The primary outcome was VTE within 6 weeks, defined as DVT or PE confirmed on imaging (via ultrasound, computed tomography (CT), or ventilation-perfusion scan), excluding localized events associated with intravenous lines. VTE screening was performed upon clinical suspicion, not routinely in all patients. Secondary outcomes included renal vein/artery thrombosis causing graft loss, serum creatinine at 1 month, rejection (biopsy-proven or suspected), myocardial infarction and stroke (as identified in International Classification of Diseases (ICD)-10 codes), blood transfusion from date of transplant, dialysis at day 5 and day 28 (representing early and late graft dysfunction), and mortality.
STATISTICAL ANALYSIS:
Variables are represented as a median with interquartile range (IQR) for discrete and continuous variables, or as event numbers and proportions for categorical variables. Differences in the primary and secondary outcomes and baseline characteristics between the 5-day and >6-week aspirin groups were analysed by Wilcoxon rank sum test for continuous variables and by chi-squared test or Fisher’s exact test for categorical variables, as appropriate. P values less than 0.05 were considered statistically significant. Univariable and multivariable analysis was then performed to identify risk factors for VTE in this population. Variables included in this analysis were selected from the baseline characteristics using bidirectional stepwise elimination based on minimizing the Akaike information criterion (AIC), as well as aspirin duration (forced into model as the primary predictor of interest). After performing stepwise regression, variables included alongside aspirin duration were age at transplant, smoking status, donor age, and thymoglobulin use. Univariable and multivariable logistic regression was then performed for each of these variables with VTE as the outcome of interest. Imputation was not performed on missing data and missing data were excluded from the analyses. All analyses were conducted using the statistical program R version 4.1.2 (Boston, MA) [19].
Results
POPULATION CHARACTERISTICS:
During the study period, 1279 kidney transplants were performed. Of these, 71 patients were excluded due to age (n=56) or multi-organ transplantation (n=15), leaving 1208 cases included in the study (Figure 1). Of these, 571 transplants were performed under the 5-day aspirin protocol (2014–2017), and 637 under the >6-week protocol (2017–2021). Baseline characteristics for the study cohort are presented in Table 1. Compared with the 5-day aspirin group, the >6-week group had significantly higher proportions of coronary artery disease and diabetic kidney disease, longer operation durations, shorter hospitalization durations, and lower use of plasma exchange in the first 6 weeks after transplant. Fewer implantation biopsies were performed during the later period (2017–2021). All other baseline characteristics were comparable between groups.
PRIMARY OUTCOME: VTE WITHIN 6 WEEKS OF TRANSPLANT:
VTE occurred in 8 (1.4%) cases in the 5-day aspirin group and 8 (1.3%) cases in the >6-week aspirin group (P=0.8). Details of each VTE event are listed in Table 2. Fifteen of 16 patients (94%) who developed VTE had a PE, with only 1 case of DVT. PEs presented at a median time of 29.5 days after transplant (IQR 21.0, 35.0). No patients died from PE. Figure 2 depicts the timing of each event and demonstrates the peak in cases observed prior to the change in aspirin protocol in 2017.
RISK FACTORS FOR VTE:
Univariable and multivariable analysis of risk factors for VTE, including aspirin use duration and those determined via stepwise elimination, are displayed in Table 3. Extended aspirin administration after transplant was not associated with a reduced rate of VTE in the first 6 weeks compared with 5-day aspirin use on univariable or multivariable logistic regression (multivariable odds ratio (OR) 0.91, 95% confidence interval (CI) 0.32–2.57, P=0.9). Multivariable analysis of other selected variables showed increased risk of VTE with older age (OR 1.09 per year, 95% CI 1.04–1.16), current or previous smoking (OR 3.59, 95% CI 1.20–13.2), younger donor age (OR 0.96, 95% CI 0.93–1.00), and thymoglobulin use (OR 10.5, 95% CI 3.09–32.1). All VTE episodes occurred after thymoglobulin administration (mean 21.6 days after commencement, range 6–30 days).
SECONDARY OUTCOMES:
Overall rates of renal vein and renal artery thrombosis were low in the cohort (0.1% and 0.2%, respectively), No significant differences were observed between the 5-day and >6-week aspirin groups with respect to any of the secondary outcomes, including renal vein/artery thrombosis causing graft loss, serum creatinine at 1 month, rejection (biopsy-proven or suspected), myocardial infarction and stroke (as per ICD-10 codes), blood transfusion from date of transplant, dialysis dependant at day 5 and day 28 (representing early and late graft dysfunction), and mortality (Table 2).
Discussion
In this single-center pre-postoperative cohort study involving 1208 kidney transplants over an 8-year period, extending postoperative aspirin prophylaxis from 5 days to >6 weeks was not significantly associated with a change in the incidence of VTE in the first 6 weeks following kidney transplantation. The only independent predictors of VTE were older age, smoking history, younger donor age, and thymoglobulin use. No differences were observed in graft thrombosis, graft function, cardiovascular events, or blood transfusion requirements between the 5-day and >6-week aspirin groups.
The incidence of VTE within the first 6 weeks following kidney transplant in this study was 1.3%. Previous studies have reported an incidence of 2.4% at 3 months [8], and 3.0% at 1 year after kidney transplant [7], but no prior studies have specifically examined rates of VTE within the first 6 weeks. VTE risk in kidney transplant recipients compared to the general population is likely related to pelvic surgery, recurrent hospitalization, multimorbidity, immunosuppression, and impaired kidney function [8]. Our study was unable to demonstrate a reduction in rates of VTE after extending the duration of postoperative aspirin from 5 days to >6 weeks. Despite this, cardiovascular comorbidities were more prevalent in the extended aspirin group of our study, without a concordant rise in rates of VTE. It could be speculated that this may be related to a protective effect from increased aspirin duration, but greater patient numbers would be needed to assess this further. No clear cause was identified for the cluster of VTE cases observed in 2017, prior to the change in aspirin protocol at our institution. It is likely this rise reflected a regression to the mean, rather than an acute phenomenon contributing to VTE risk.
Previous research examining the effect of aspirin therapy on VTE following kidney transplantation is limited. A study by Verhave et al (2014) aimed to identify risk factors for thromboembolism in 913 kidney transplant recipients [7]. This included a nested case-control study comparing 68 cases of identified VTE with 260 controls, where no association was found between aspirin use (unknown duration) and VTE on univariable analysis (OR 0.5, 95% CI 0.2–1.1, nonsignificant) [7]. Patients were followed up from time of transplant (1990–2010) until 2012, a much longer time period than in this study, but the first postoperative year was found to present the highest risk period for VTE [7]. To the best of our knowledge, no other research has examined the effect of aspirin therapy, or its duration, on VTE following kidney transplantation.
Extending the duration of aspirin therapy was expected to reduce VTE risk and delay a potential rebound hypercoagulability following aspirin cessation to a time point when baseline risk is lower. While clustering of other cardiovascular events after antiplatelet withdrawal is well documented, the role of aspirin cessation in VTE has not been investigated before. The anti-thrombotic effects of aspirin occur through a reduction in tissue factor and thrombin expression, and upregulation of fibrinolysis [10,13]. Proposed mechanisms for a rebound hypercoagulability phenomenon following aspirin cessation relate to increased thromboxane production and decreased fibrinolysis [13], resulting in increased platelet activation [20–22]. A study by Alcock et al (2014), however, was unable to demonstrate an increase in platelet functional activity following aspirin cessation in healthy volunteers [14]. Further research is required to clarify the mechanism of this rebound hypercoagulability phenomenon and its role in venous thrombosis and thromboembolism risk.
Previous research has examined other VTE risk factors in this population. Similar to the findings of this study, a systematic review by Cicora et al (2018) reported that smoking and older age were predictors of VTE, but additionally found that previous peritoneal dialysis, prolonged cold ischemia time, deceased donation, donor age <6 or >60 years, delayed graft function, and use of mTOR inhibitors were associated with an increased risk of VTE [9]. These associations were not identified in the current study, although this may have been related to the low event rate and short follow-up period. Younger donor age was only weakly associated with increased VTE risk in this study and may not be clinically important. Thymoglobulin use was strongly associated with VTE in our population. This included both induction and postoperative use. The relationship between thymoglobulin and rates of VTE has not been directly examined following kidney transplantation. Verhave et al (2014) found no association between the use of induction therapy (including either thymoglobulin or anti-IL2 receptor blockade therapy) and VTE, but this apparent disparity may be due to the concurrent inclusion of anti-IL2 receptor blockade therapy or the inclusion of thymoglobulin use as induction therapy only, rather than for the treatment of rejection [7]. Our findings suggest that the elevated VTE risk with the use of thymoglobulin was not reduced by extending the duration of postoperative aspirin to >6 weeks. This warrants further study and patients undergoing thymoglobulin therapy may benefit from consideration of more intensive VTE prophylaxis throughout their treatment course.
The low incidence of graft thrombosis in this study (0.25%) is comparable with both previous literature measuring rates of graft thrombosis with the use of aspirin prophylaxis [2,23,24], and rates of graft loss from vascular causes in Australian transplant centers (0.95% between 2014 and 2019) [25]. Aspirin prophylaxis may contribute to this low rate [3]. For comparison, anecdotal reports of aspirin use in other Australian kidney transplant units was sought, showing a variety in local practice including no routine use, 1-month aspirin therapy, or ad-hoc use. A systematic review by Cheungpasitporn et al (2017), including 19 759 kidney transplant recipients, showed a pooled risk ratio with postoperative aspirin administration of 0.11 for allograft thrombosis (95% CI 0.02–0.53), 0.57 for allograft failure (95% CI 0.33–0.99) and 0.72 for adverse cardiac events or mortality (95% CI 0.59–0.88) [3]. This review, however, was limited to 9 cohort studies with no previous randomized controlled trials addressing this question. Comparatively, we found no change in graft function or failure, or rates of adverse cardiovascular events in the extended aspirin duration group. This may be due to the low event rate of this study, and the comparison with shorter duration of aspirin rather than no prophylaxis.
Importantly, no increase in bleeding events requiring transfusion was identified with an extended protocol of aspirin therapy postoperatively. Research regarding bleeding risk with aspirin use in kidney transplant recipients is limited [3]. Multiple systematic reviews of large-scale randomized controlled trials have established an increase in rates of major bleeding with aspirin compared to placebo [10]. In the peri-operative setting, the PeriOperative ISchemic Evaluation-2 (POISE-2) trial by Devereaux et al (2014) compared aspirin and placebo in 10 010 patients undergoing non-cardiac surgery [26]. An increased rate of major bleeding (hazard ratio (HR) 1.23, 95% CI 1.01–1.49), but not mortality, nonfatal myocardial infarction, or other secondary outcomes, was observed in the aspirin group [26]. These findings may not have been reflected in this study due to both groups receiving some duration of postoperative aspirin therapy.
This study is strengthened by its large sample size, prospective recording of data in a comprehensive transplant database prior to study design, and management of all patients according to a uniform protocol. The limitations of this study include its retrospective, single-center design, which risks recall bias and limits the generalizability of findings. The use of an observational methodology also reduces the ability to draw causal inferences. Despite the large sample size, the low event rate observed limits the power of the study and means that type 2 statistical errors could not be excluded. The low event rate limits the number of variables that can be included in the multivariable analysis, which may have led to overfitting and unstable estimates. The confidence intervals comparing VTE events in the 5-day and >6-week aspirin group are wide and include some values than may be clinically meaningful. Given the duration of this pre-post study, era or co-intervention biases with residual confounding may have occurred. Adherence to the unit protocol throughout this period was also unknown. Importantly, follow-up was limited to 6 weeks, meaning that safety and efficacy outcomes were unable to be evaluated beyond this time point. Data relating to the concurrent use of anticoagulation pre- and postoperatively, and prior history of coagulopathy or VTE, was not available to compare between the 2 groups which may have led to confounding.
Conclusions
In conclusion, extending the duration of aspirin prophylaxis from 5 days to >6 weeks did not significantly reduce the incidence of VTE events in the first 6 weeks following kidney transplantation. Despite this, no increase in bleeding events requiring blood transfusion was observed. Older age, smoking, and younger donor age were associated with an increased risk of VTE. This study also identified an association between the use of the therapeutic anti-rejection medication, anti-human thymocyte immunoglobulin, and VTE, which requires further research. Ongoing evaluation of the efficacy and optimal duration of aspirin prophylaxis following kidney transplantation by randomized controlled trial is warranted.
Figures
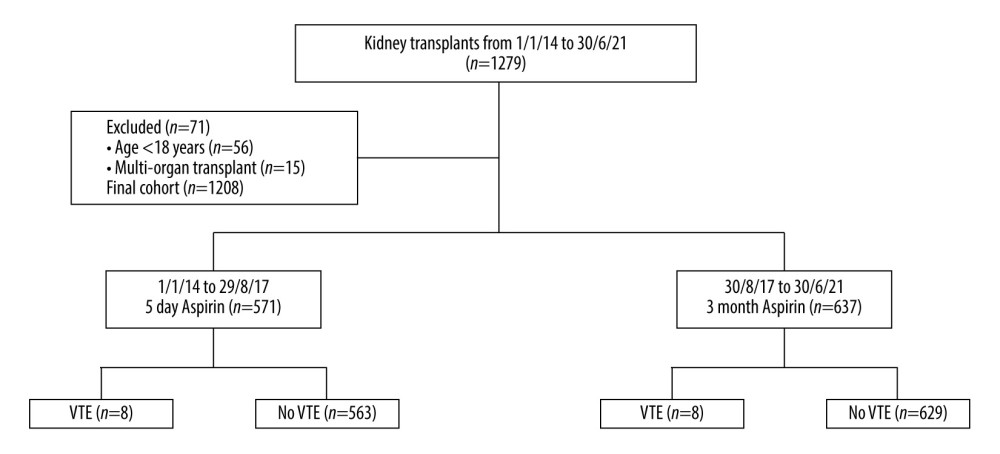 Figure 1. Flow diagram of study cohort, exclusion criteria and outcomes. VTE – venous thromboembolism. Generated using Microsoft Word, Version 16.36, Microsoft Corporation.
Figure 1. Flow diagram of study cohort, exclusion criteria and outcomes. VTE – venous thromboembolism. Generated using Microsoft Word, Version 16.36, Microsoft Corporation. 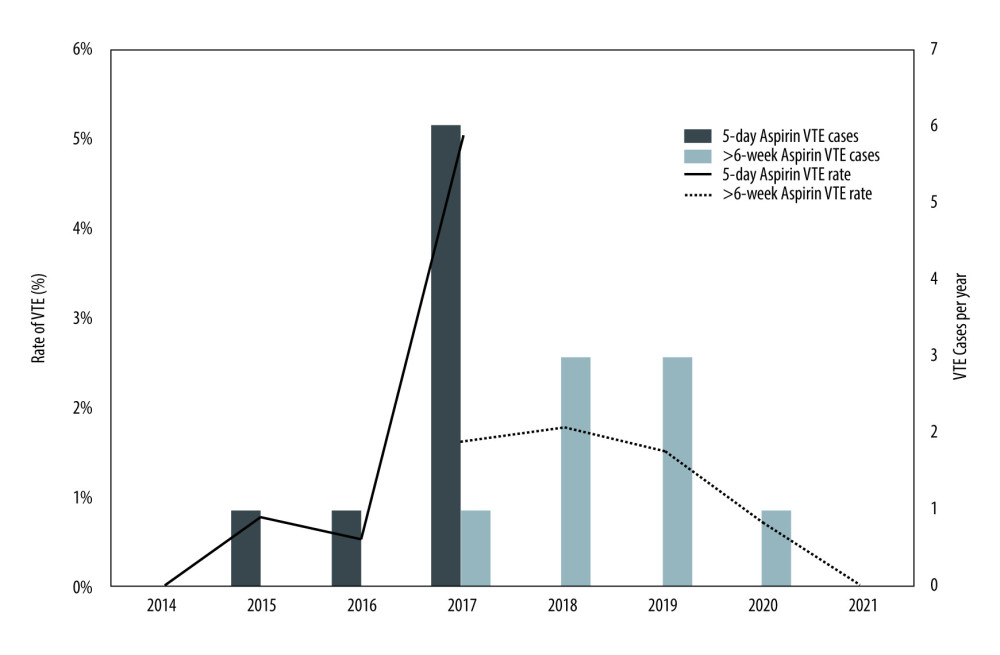 Figure 2. Rate and total cases of venous thromboembolism (VTE) per year from 2014–2021 under 5-day and >6-week aspirin duration protocols. VTE – venous thromboembolism. Generated using Microsoft Excel, Version 16.36, Microsoft Corporation.
Figure 2. Rate and total cases of venous thromboembolism (VTE) per year from 2014–2021 under 5-day and >6-week aspirin duration protocols. VTE – venous thromboembolism. Generated using Microsoft Excel, Version 16.36, Microsoft Corporation. Tables
Table 1. Baseline characteristics and outcomes 6 weeks post-transplant stratified by 5-day and >6-week aspirin protocol. Table 2. Details of venous thromboembolism (VTE) events during the study period.
Table 2. Details of venous thromboembolism (VTE) events during the study period.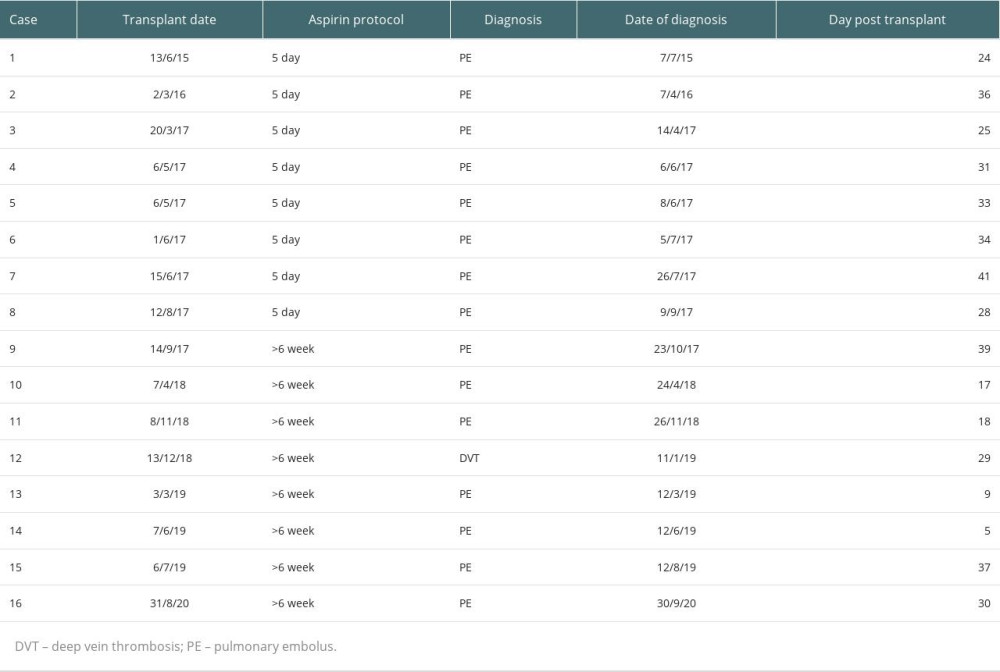 Table 3. Univariable and multivariable logistic regression analysis of effects of aspirin duration, age, smoking status, donor age and thymoglobulin use on venous thromboembolism (VTE).
Table 3. Univariable and multivariable logistic regression analysis of effects of aspirin duration, age, smoking status, donor age and thymoglobulin use on venous thromboembolism (VTE).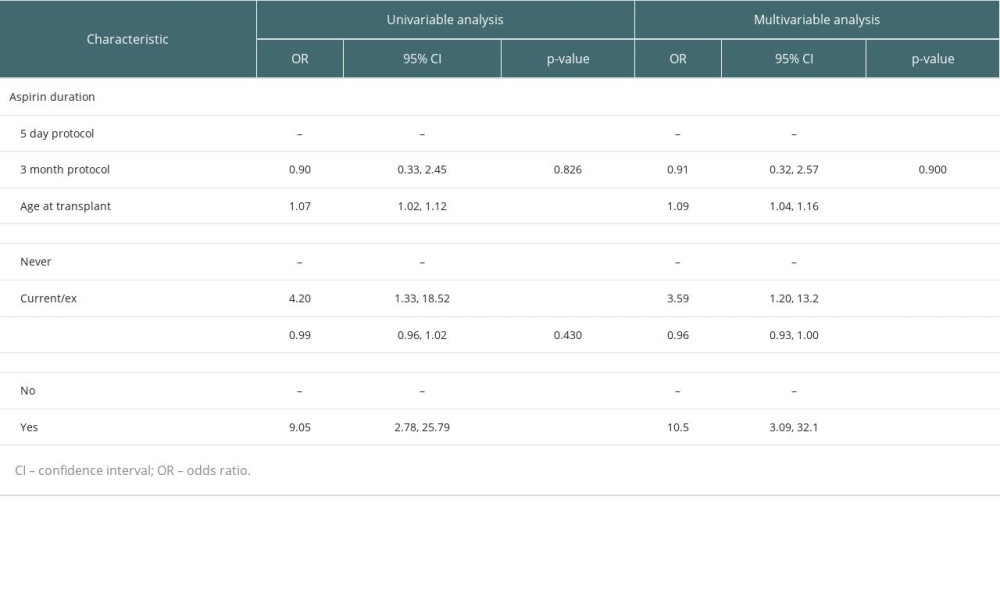
References
1. Surianarayanan V, Hoather TJ, Tingle SJ, Interventions for preventing thrombosis in solid organ transplant recipients: Cochrane Database Syst Rev, 2021; 3(3); CD011557
2. Robertson AJ, Nargund V, Gray DW, Morris PJ, Low dose aspirin as prophylaxis against renal-vein thrombosis in renal-transplant recipients: Nephrol Dial Transplant, 2000; 15(11); 1865-68
3. Cheungpasitporn W, Thongprayoon C, Mitema DG, The effect of aspirin on kidney allograft outcomes; A short review to current studies: J Nephropathol, 2017; 6(3); 110-17
4. Khalil MAM, Khalil M, Khamis SSA, Pros and cons of aspirin prophylaxis for prevention of cardiovascular events in kidney transplantation and review of evidence: Adv Prev Med, 2019; 20196139253
5. Eckardt K-U, Kasiske BL, Zeier MG, KDIGO clinical practice guideline for the care of kidney transplant recipients: Am J Transplant, 2009; 9; S1-S155
6. Mourad G, Morelon E, Noel C, The role of thymoglobulin induction in kidney transplantation: An update: Clin Transplant, 2012; 26(5); E450-64
7. Verhave JC, Tagalakis V, Suissa S, The risk of thromboembolic events in kidney transplant patients: Kidney Int, 2014; 85(6); 1454-60
8. Lam NN, Garg AX, Knoll GA, Venous thromboembolism and the risk of death and graft loss in kidney transplant recipients: Am J Nephrol, 2017; 46(4); 343-54
9. Cicora F, Petroni J, Roberti J, Prophylaxis of pulmonary embolism in kidney transplant recipients: Curr Urol Rep, 2018; 19(2); 17
10. Diep R, Garcia D, Does aspirin prevent venous thromboembolism?: Hematology 2014, the American Society of Hematology Education Program Book, 2020; 2020(1); 634-41
11. Biondi-Zoccai GG, Lotrionte M, Agostoni P, A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50 279 patients at risk for coronary artery disease: Eur Heart J, 2006; 27(22); 2667-74
12. Burger W, Chemnitius JM, Kneissl G, Rücker G, Low-dose aspirin for secondary cardiovascular prevention – cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysis: J Intern Med, 2005; 257(5); 399-414
13. Gerstein NS, Schulman PM, Gerstein WH, Should more patients continue aspirin therapy perioperatively?: Clinical impact of aspirin withdrawal syndrome: Ann Surg, 2012; 255(5); 811-19
14. Alcock RF, Reddel CJ, Pennings GJ, The rebound phenomenon after aspirin cessation: The biochemical evidence: Int J Cardiol, 2014; 174(2); 376-78
15. Cundiff DK, Clinical evidence for rebound hypercoagulability after discontinuing oral anticoagulants for venous thromboembolism: Medscape J Med, 2008; 10(11); 258
16. Poli D, Zanazzi M, Antonucci E, High rate of recurrence in renal transplant recipients after a first episode of venous thromboembolism: Transplantation, 2005; 80(6); 789-93
17. Kohli R, Estcourt L, Zaidi A, Efficacy and safety of chemical thromboprophylaxis in renal transplantation – a systematic review: Thromb Res, 2020; 192; 88-95
18. von Elm E, Altman DG, Egger M, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies: Ann Intern Med, 2007; 147(8); 573-77
19. : R: A language and enviornment for statistical computing, 2010, R Foundation for Statistical Computing http://www.R-project.org
20. Beving H, Zhao C, Albage A, Ivert T, Abnormally high platelet activity after discontinuation of acetylsalicylic acid treatment: Blood Coagul Fibrinolysis, 1996; 7(1); 80-84
21. Vial JH, Narkowicz C, Urinary prostanoid metabolites and serum thromboxane following recovery from cyclooxygenase inhibition with aspirin: Clin Appl Thromb Hemost, 1995; 1(1); 34-38
22. Doutremepuich C, Aguejouf O, Desplat V, Eizayaga FX, Aspirin therapy: An attempt to explain the events of prothrombotic complications after treatment discontinuation: Thromb Haemost, 2010; 103(1); 171-80
23. Stechman M, Charlwood N, Gray D, Handa A, Administration of 75 mg of aspirin daily for 28 days is sufficient prophylaxis against renal transplant vein thrombosis: Phlebology, 2007; 22(2); 83-85
24. Murphy GJ, Taha R, Windmill DC, Influence of aspirin on early allograft thrombosis and chronic allograft nephropathy following renal transplantation: Br J Surg, 2001; 88(2); 261-66
25. ANZDATA: Australia and New Zealand Dialysis and Transplant Registry, 2020 http://www.anzdata.org.au
26. Devereaux PJ, Mrkobrada M, Sessler DI, Aspirin in patients undergoing noncardiac surgery: N Engl J Med, 2014; 370(16); 1494-503
Figures
 Figure 1. Flow diagram of study cohort, exclusion criteria and outcomes. VTE – venous thromboembolism. Generated using Microsoft Word, Version 16.36, Microsoft Corporation.
Figure 1. Flow diagram of study cohort, exclusion criteria and outcomes. VTE – venous thromboembolism. Generated using Microsoft Word, Version 16.36, Microsoft Corporation. Figure 2. Rate and total cases of venous thromboembolism (VTE) per year from 2014–2021 under 5-day and >6-week aspirin duration protocols. VTE – venous thromboembolism. Generated using Microsoft Excel, Version 16.36, Microsoft Corporation.
Figure 2. Rate and total cases of venous thromboembolism (VTE) per year from 2014–2021 under 5-day and >6-week aspirin duration protocols. VTE – venous thromboembolism. Generated using Microsoft Excel, Version 16.36, Microsoft Corporation. Tables
 Table 1. Baseline characteristics and outcomes 6 weeks post-transplant stratified by 5-day and >6-week aspirin protocol.
Table 1. Baseline characteristics and outcomes 6 weeks post-transplant stratified by 5-day and >6-week aspirin protocol. Table 2. Details of venous thromboembolism (VTE) events during the study period.
Table 2. Details of venous thromboembolism (VTE) events during the study period. Table 3. Univariable and multivariable logistic regression analysis of effects of aspirin duration, age, smoking status, donor age and thymoglobulin use on venous thromboembolism (VTE).
Table 3. Univariable and multivariable logistic regression analysis of effects of aspirin duration, age, smoking status, donor age and thymoglobulin use on venous thromboembolism (VTE). Table 1. Baseline characteristics and outcomes 6 weeks post-transplant stratified by 5-day and >6-week aspirin protocol.
Table 1. Baseline characteristics and outcomes 6 weeks post-transplant stratified by 5-day and >6-week aspirin protocol. Table 2. Details of venous thromboembolism (VTE) events during the study period.
Table 2. Details of venous thromboembolism (VTE) events during the study period. Table 3. Univariable and multivariable logistic regression analysis of effects of aspirin duration, age, smoking status, donor age and thymoglobulin use on venous thromboembolism (VTE).
Table 3. Univariable and multivariable logistic regression analysis of effects of aspirin duration, age, smoking status, donor age and thymoglobulin use on venous thromboembolism (VTE). In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








