27 June 2023: Original Paper
A 15-Year Retrospective Study of Supportive Extracorporeal Therapies Including Plasma Exchange and Continuous Venovenous Hemodiafiltration of 114 Adults with Acute Liver Failure Awaiting Liver Transplantation
Ilhan OcakDOI: 10.12659/AOT.939745
Ann Transplant 2023; 28:e939745
Abstract
BACKGROUND: Recently, there has been a recommendation to utilize a combination of supportive extracorporeal therapies, specifically plasma exchange and continuous venovenous hemodiafiltration, in patients with acute liver failure. This 15-year retrospective study aimed to evaluate supportive extracorporeal therapy, including plasma exchange and continuous venovenous hemodiafiltration, for 114 adults with acute liver failure awaiting liver transplant.
MATERIAL AND METHODS: In this retrospective study, the medical records of 1288 adult patients who underwent liver transplantation and 161 adult patients who received alternative therapy were analyzed; 114 patients who received combined supportive extracorporeal therapy for acute liver failure were included in the study. Biochemical laboratory data were compared before and after therapy.
RESULTS: The study included 50 male and 64 female patients. The first group (34 patients) recovered with liver transplantation, and 4 patients died in the first year after liver transplantation. In the second group (80 patients), 66 patients recovered without liver transplantation, while 14 patients died within the first 2 weeks after therapy. All patients showed significant reductions in serum hepatic function tests (alanine transaminase, aspartate transaminase, and total bilirubin), ammonia, and prothrombin time/international normalized ratio after discontinuation of combined supportive extracorporeal therapy (P<0.01). There was also a significant improvement in the hemodynamic parameter.
CONCLUSIONS: This combined extracorporeal therapy can be used as a supportive treatment for both recovery and bridge to liver transplantation in patients with acute liver failure. In addition, treatment can be continued until liver regeneration and until a usable donor is found.
Keywords: Acute-On-Chronic Liver Failure, Liver Diseases, Liver Transplantation, Plasmapheresis
Background
Technical Aspects
PLASMA EXCHANGE:
Plasma exchange is a therapeutic method that involves the extraction of a patient’s plasma and substitution with a suitable fluid. This procedure can be accomplished through either centrifugation or filtration techniques. Centrifugation segregates blood components based on their density, while filtration separates them using a porous membrane, taking into account their size. Both methods are equally reliable, effective, and impactful.
During plasma exchange, the withdrawn plasma is substituted with cryoprecipitate, fresh-frozen plasma, or an alternative suitable fluid. This substitution proves especially advantageous as it restores crucial components like albumin, coagulation factors, and inflammatory mediators. The amount of plasma replaced usually falls within the range of 1 to 1.5 times the anticipated plasma volume of the individual. To approximate the plasma volume based on the total blood volume, physiological factors such as sex, height, weight, body composition, and hematocrit are factored in. A plasma exchange procedure that replaces 1–1.5 times the plasma volume can effectively eliminate approximately 70% of the substances from the intravascular space.
CONTINUOUS VENOVENOUS HEMODIAFILTRATION:
Continuous venovenous hemodiafiltration is a specialized renal replacement therapy that combines 2 techniques: hemodialysis and hemofiltration. This innovative method effectively eliminates substances with varying molecular weights by utilizing both diffusion and convection processes. Diffusion refers to the movement of solutes across a partially permeable membrane in response to concentration gradients.
The removal of solutes is affected by various elements, including differences in concentration, the size of the solute, its interaction with proteins, and the surface area of the filter. Tiny substances like urea and creatinine are effectively eliminated through the process of diffusion. Conversely, convection enables the movement of water and dissolved solutes across a partially permeable membrane, driven by variances in pressure. This mechanism facilitates the elimination of molecules of medium to large size, such as cytokines, depending on the permeability of the membrane. In summary, continuous venovenous hemodiafiltration offers an effective and comprehensive approach to remove a wide range of molecules by utilizing both diffusion and convection techniques.
COMBINED SUPPORTIVE EXTRACORPOREAL THERAPY:
Combined supportive extracorporeal therapy is a combination of plasma exchange and continuous venovenous hemodiafiltration. It is a combination of 1 or 2 sessions of plasma exchange per day while continuing continuous venovenous hemodiafiltration.
Material and Methods
Technical Aspects
ETHICS APPROVAL:
The retrospective study was approved by the Institutional Review Board of Memorial Sisli Hospital, with the date 03/06/2022 and number 520/22.
Patient data were obtained from the hospital information system. No funding was received for this study, and the author declared no conflicts of interest. The study was conducted at the Liver Transplant Intensive Care Unit of Memorial Sisli Hospital, Turkey.
Informed consent was waived due to the retrospective nature of the study. However, consent for all procedures and applications was obtained from these patients hospitalized in the Intensive Care Unit. Patient data were obtained from the hospital information system.
PATIENTS:
This study was a retrospective analysis of the medical records of 1288 adult patients who underwent liver transplantation and 161 adult patients who received alternative treatments, between January 2006 and January 2021, at Memorial Sisli Hospital in Istanbul, Turkey. The hospital conducted an average of 100 liver transplant procedures annually during this period, and the Intensive Care Unit provided pre- and post-transplant care to the patients.
This study focused on 2 groups of 114 adult patients with acute liver failure who received combined supportive extracorporeal therapy of continuous venovenous hemodiafiltration and plasma exchange. The first group consisted of 34 adult patients undergoing combined supportive extracorporeal therapy for acute liver failure who were on the waiting list for liver transplantation. The first group consisted of patients who were candidates for liver transplantation according to the End-Stage Liver Disease Model-Na score and were in the process of finding a living donor. During their waiting period, their condition deteriorated from chronic liver failure to acute liver failure, also known as acute-on-chronic liver failure. Therapy was applied to this group for bridge to liver transplantation. The second group consisted of 80 patients who underwent combined supportive extracorporeal therapy for acute liver failure due to different causes. Therapy was applied to this group for recovery or bridge to liver transplantation.
Patients who received mono-supportive extracorporeal therapy or did not undergo supportive extracorporeal therapy were excluded from the study (Figure 1, Table 1). The supportive extracorporeal therapy protocol used in our Intensive Care Unit was applied (Figure 2) [14].
SUPPORTIVE EXTRACORPOREAL THERAPY PROTOCOL: The algorithm depicted in Figure 2 was developed and converted into a protocol for the indication of mono- or combined supportive extracorporeal therapy and liver transplantation. Our apheresis technicians and nurses performed the supportive extracorporeal therapy application in our unit following this protocol. The algorithm was subsequently published [14]. The primary aim of this study was to evaluate the efficacy of combined supportive extracorporeal therapy in patients with acute liver failure or acute liver failure who were on the waiting list for liver transplantation. The study specifically focused on the outcomes of patients receiving this combined therapy, as there is limited information available on its effectiveness in treating acute liver failure. The retrospective study excluded mono-supportive extracorporeal therapy, and non-supportive extracorporeal therapy patients to maintain the focus on evaluating outcomes of the combined supportive extracorporeal therapy. Including mono-supportive extracorporeal therapy patients would add another variable to the analysis, while non-supportive extracorporeal therapy patients did not receive any supportive extracorporeal therapy, making their outcomes less relevant to evaluation of the combined therapy.
The grade of hepatic encephalopathy was evaluated using the West Haven classification, and magnetic resonance imaging was used to verify the grade of encephalopathy.
CONTINUOUS VENOVENOUS HEMODIAFILTRATION PROTOCOL:
A renal replacement device and hemodiafiltration kit manufactured by Fresenius Medical Care, Bad Homburg, Germany, were used to perform continuous venovenous hemodiafiltration. Continuous citrate-calcium anticoagulation was administered throughout the day, with blood flow rates ranging from 3 to 5 mL/kg/min, and dialysate flow rates ranging from 180 to 300 mL/kg/h. The dialysate and replacement solutions used were multibic, multiplus, citrate-calcium dialysate, citrate-calcium dialysate-potassium 2 plus, citrate-calcium dialysate-potassium 4 plus, and 4% sodium citrate.
PLASMA EXCHANGE PROTOCOL:
Plasma exchange was performed using a continuous renal replacement device and Fresenius plasma exchange kit. The therapy was administered twice daily for 2–4 h per session, with 30 cc/min and 50 cc/kg of sessions calculated, and 1.5 volumes for a similar volume of fresh-frozen plasma replacement without anticoagulant treatment. After each plasma exchange session, the plasma exchange circuit was disconnected from the continuous venovenous hemodiafiltration circuit, which had been attached as a side flow. The study included laboratory values and vital sign recordings before and after the combined supportive extracorporeal therapy, as well as the duration and frequency of plasma exchange treatments.
STATISTICAL ANALYSES:
The statistical analyses were performed using SPSS version 26.0 (IBM Corp.; Armonk, New York, USA). The normality of the study data was assessed using the Kolmogorov-Smirnoff test. Mean values were utilized for data that followed a normal distribution, whereas median (IQR) values were employed for data that did not follow a normal distribution. The Wilcoxon test was applied to compare pre- and post-session laboratory values for combined supportive extracorporeal therapy. The Mann-Whitney U test was used to compare the data of patients who underwent liver transplant with those who did not. A significance level of less than 0.05 (
Results
COMBINED SUPPORTIVE EXTRACORPOREAL THERAPY RESULTS:
The adult patients received combined supportive extracorporeal therapy for an average of 8 days. All patients had twice-daily plasma exchange treatments. With a minimum of 6 and a high of 28, the average number of sessions was 14.24. The average length of the continuous venovenous hemodiafiltration was 199 hours (8.29 days), ranging from 79 to 343 h.
LABORATORY VALUES OF PATIENTS BEFORE AND AFTER COMBINED SUPPORTIVE EXTRACORPOREAL THERAPY:
Before the first combined supportive extracorporeal therapy, the liver transplant candidates’ serum total bilirubin levels were noticeably higher than those of the survivors (patients recovered from acute liver failure). Alanine and aspartate transaminase readings, however, were much lower. After combined supportive extracorporeal therapy, the survivors considerably outperformed the liver transplant candidates in terms of serum ammonia, and prothrombin time/international normalized ratio, and total bilirubin values (Table 2). Serum levels of the hepatic function tests (total bilirubin, alanine, and aspartate transaminase), ammonia, and prothrombin time/international normalized ratio were all significantly lower in all patients (34 with liver transplants and 80 without), compared to the initial levels following combined supportive extracorporeal therapy. Mean arterial pressure and other hemodynamic indicators saw a considerable improvement as well (Table 3). After combined supportive extracorporeal therapy, patients with and without liver transplant had significantly decreased serum levels of prothrombin time/international normalized ratio, ammonia, and the hepatic function test results (total bilirubin, alanine, and aspartate transaminase). Mean arterial pressure and other hemodynamic indicators were considerably improved as well (Table 4). Before the first combined supportive extracorporeal therapy, survivors had considerably lower alanine, and aspartate transaminase readings, ammonia, and prothrombin time/international normalized ratio levels than did ex-patients (deaths). The mean arterial pressure and other hemodynamic indicators were noticeably closer to normal. Serum hepatic function test results (total bilirubin, alanine, and aspartate transaminase), ammonia, prothrombin time/international normalized ratio levels, and hemodynamic parameters (eg, mean arterial pressure) of survivors following the last combined supportive extracorporeal therapy were not statistically different from those in ex-patients (Table 5).
COMPLICATIONS OF COMBINED SUPPORTIVE EXTRACORPOREAL THERAPY:
During plasma exchange in the combined supportive extracorporeal therapy, hypernatremia (5 individuals) and metabolic acidosis (4 patients) developed. Hemodiafiltration in supportive extracorporeal therapy made it better. Four individuals also had a slight skin rash as a result of the fresh-frozen plasma utilized during plasma exchange. The action was suspended. In the process, fresh-frozen plasma was replaced with a new one. After the skin rashes subsided, the procedure went on.
MORTALITY:
Four of 34 patients who underwent liver transplant by performing combined supportive extracorporeal therapy died within the first year. Of the 80 non-transplanted patients who underwent combined supportive extracorporeal therapy, 14 died within the first 14 days while the combined supportive extracorporeal therapy was in progress (Figure 1), with 12.28% mortality and 57.8% transplant-free survival rates.
Discussion
This medical study assessed the first group of 34 liver transplantation candidates and the second group of 80 non-transplantation patients who received combined supportive extracorporeal therapy and were monitored in the Liver Transplant Intensive Care Unit.
A total of 34 patients who underwent liver transplantation using combined supportive extracorporeal therapy experienced a mortality rate of 4% within the first year. Among the 80 non-transplanted patients who received combined support extracorporeal therapy, 14 individuals (17.5%) died within the initial 14 days of treatment. After completion of combined support extracorporeal therapy, the mortality rate was 12.28%, the bridge-to-transplant rate was 29.8%, and the survival rate without liver transplantation was 57.8%. Additionally, an 87.7% rate of improvement or regression in encephalopathy grade was observed. These findings appear to be consistent with the results of a recent study conducted by Gün et al [13]; in a similar study of pediatric patients with a comparable etiology, they reported a mortality rate of 32.5%, a bridge-to-transplant rate of 16.6%, and a recovery rate of 67.5% without liver transplantation at the completion of combined support extracorporeal therapy. They also observed improvement or regression in encephalopathy grade. Overall, their study results agree with the present study, demonstrating the effectiveness of combined support extracorporeal therapy in patients with similar etiology. The present study revealed that encephalopathy in liver transplant candidates could not be permanently reduced below grade 3, but its progression varied and did not lead to hepatic coma or worsening severity. This demonstrates the precise and tightly regulated control of fluid and electrolyte balance necessary to manage intracranial pressure, as discussed in the literature. Hence, combined supportive extracorporeal therapy can be utilized to stabilize liver transplant candidates. Out of the 66 adult patients who clinically recovered, encephalopathy improved to grade 1 or below. Unfortunately, 14 adult patients who were unsuitable for transplantation or unable to find a suitable donor died, and it was not possible to permanently decrease their encephalopathy grade below grade 3. All adult patients who received combined supportive extracorporeal therapy and were closely monitored in the Intensive Care Unit demonstrated significant improvement in laboratory values and vital signs. However, those who did not show permanent reduction in encephalopathy grade did not exhibit clinical improvement. The present results show that the key prognostic factor is encephalopathy, which must be permanently reduced to grade 1 or lower using combined supportive extracorporeal therapy. The surviving patients who clinically recovered experienced long-lasting clinical improvement, including reduced encephalopathy grade and low PT/INR values. This was achieved through consistent high clearance of ammonia, as documented in the literature, likely due to a well-functioning liver or liver tissue regeneration [15]. Moreover, the recovery of patients with acute liver failure induced by paracetamol without the need for liver transplantation aligns with findings in the literature [16]. Numerous case reports of liver failure depict rapid progression to multiple organ failure, accompanied by systemic infection, brain swelling, circulatory collapse, abnormal coagulation, and metabolic disturbances [17]. Several clinical studies have demonstrated the potential of continuous renal replacement therapy techniques, such as continuous venovenous hemodiafiltration, in reducing inflammation and inflammatory mediators in septic or multiple organ failure patients [17,18]. Even in the most severe cases involving circulatory failure, such as cerebral edema, continuous renal replacement therapy can be utilized safely [19]. When performed continuously, continuous renal replacement therapy can effectively eliminate highly concentrated substances from the intravascular and extravascular spaces. Acute kidney injury necessitating continuous renal replacement thearapy is a common complication of multiple organ failure in acute liver failure, occurring in 30% to 50% of such patients. Continuous renal replacement therapy helps remove excessive fluid volume from the body and ensures the elimination of endogenous toxins that cannot be expelled by the kidneys. It facilitates clinical improvement in patients who are experiencing hemodynamic instability, have developed encephalopathy, and are undergoing a substantial inflammatory process due to heightened and uncleared cytokines. Additionally, it is extensively utilized to reduce the risk of elevated intracranial pressure and cerebral edema in individuals with severe hyperammonemia [20]. Plasma exchange is employed to eliminate circulating inflammatory cytokines, endotoxins, and neurotoxins that arise in patients with acute liver failure. This procedure decreases bilirubin and ammonia levels, corrects coagulopathy, and regulates the antimicrobial response, resulting in improved hemodynamics and increased likelihood of recovery without the need for a transplant [20,21]. These eliminated substances have been reported to play a significant role in the development of both hepatic encephalopathy and mutiple organ failure in acute liver failure. Many researchers demonstrated that plasma exchange treatment can correct bilirubin, coagulopathy, and ammonia levels [22,23]. Simultaneous dialysis and plasma exchange performed together, also known as combined supportive extracorporeal therapy, have been in practice since 1999 and have been documented in the literature as tandem procedures [24]. Studies have shown that an artificial liver support system, such as continuous hemofiltration with plasma exchange, can effectively remove medium-molecular-weight substances and improve hepatic coma [25–27]. In fact, combining plasma exchange and hemodiafiltration has been shown to improve hepatic coma in many patients, with a 56% survival rate reported in 67 patients with fulminant hepatitis [28]. Although transplant-free survival rates are similar to previous reports on combined supportive extracorporeal therapy, patients should still be closely monitored and treated in the Intensive Care Unit until their liver function is restored or transplantation is performed, with various types of monitoring and measures employed to ensure the best possible outcome. Our team’s collaborative approach to liver transplantation, implemented at the appropriate timing, demonstrates effectiveness in preventing mortality and ensuring neurological stability. In the present study, the low mortality rate was due to the early and proactive utilization of artificial liver support. Previous research has indicated that the rapid and substantial administration of fresh-frozen plasma during plasma exchange can lead to adverse effects like hypernatremia, metabolic alkalosis, and colloid osmotic pressure. Furthermore, administering large volumes of fresh-frozen plasma can result in complications such as brain and pulmonary edema [29]. The advantages of plasma exchange in patients with acute liver failure were initially discovered in a randomized controlled trial conducted by Larsen et al in 2016 [30]. The occurrence of persistent cerebral edema and hepatic coma in acute liver failure patients is a cause for concern as it indicates the sudden onset of irreversible cerebral damage. Williams and Wendon [31] reported this as a contraindication for liver transplantation. Since 2007, our medical unit has used a combined approach involving plasma exchange and continuous venovenous hemodiafiltration to effectively eliminate hepatic toxins and minimize the adverse effects associated with the sole administration of plasma exchange, such as hypernatremia, colloid osmotic pressure, and metabolic alkalosis [32,33]. The present study has some limitations. Since it was conducted at a single center, and was retrospective and lacking a control group, randomized controlled studies are necessary to further explore this topic. Additionally, excluding mono-supportive extracorporeal therapy and non-supportive extracorporeal therapy patient groups from the study is a weakness of the study.
Conclusions
The combined use of continuous venovenous hemodiafiltration and plasma exchange has shown a significant improvement in both clinical and biochemical parameters, particularly in encephalopathy cases. The therapy has also been found to have a substantial impact on patient survival, with a low mortality rate of 12.28% and a high transplant-free survival rate of 57.8%. Hence, this combined extractorporeal therapy can be used as a supportive treatment for both recovery and bridge to liver transplantation in patients with acute liver failure. In addition, treatment can be continued until liver regeneration and until a suitable donor is found.
Figures
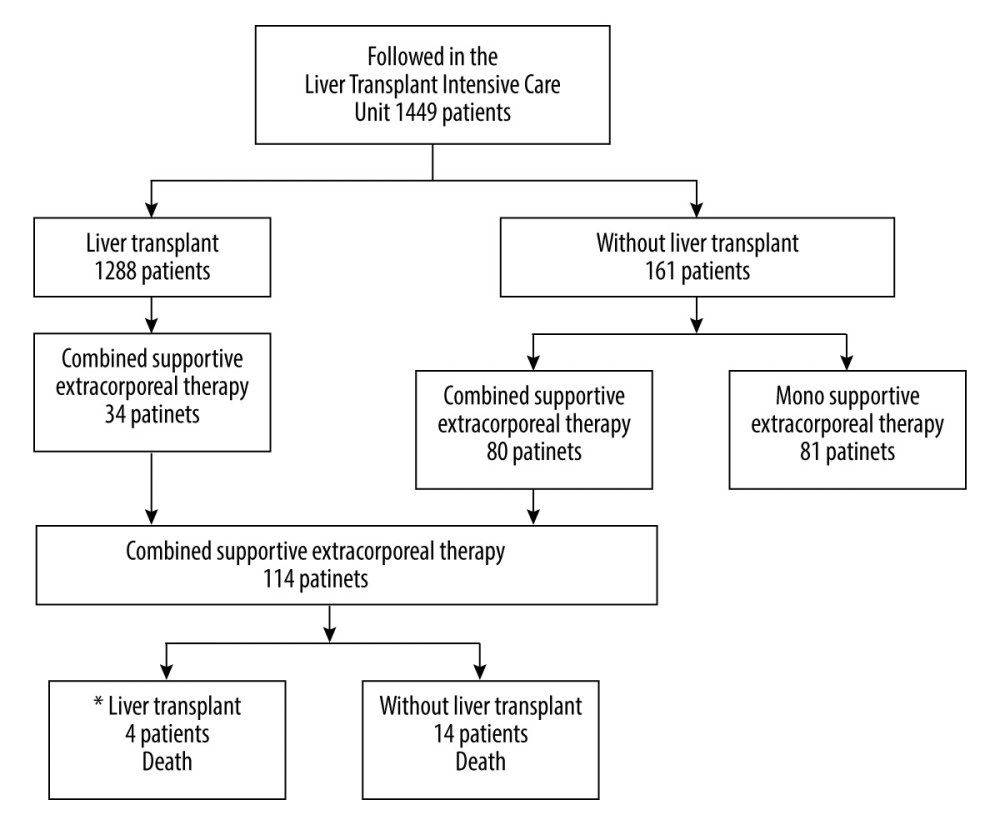 Figure 1. Flow diagram of patients in the study. * 1 year motrality. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation of Figure 1.
Figure 1. Flow diagram of patients in the study. * 1 year motrality. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation of Figure 1. 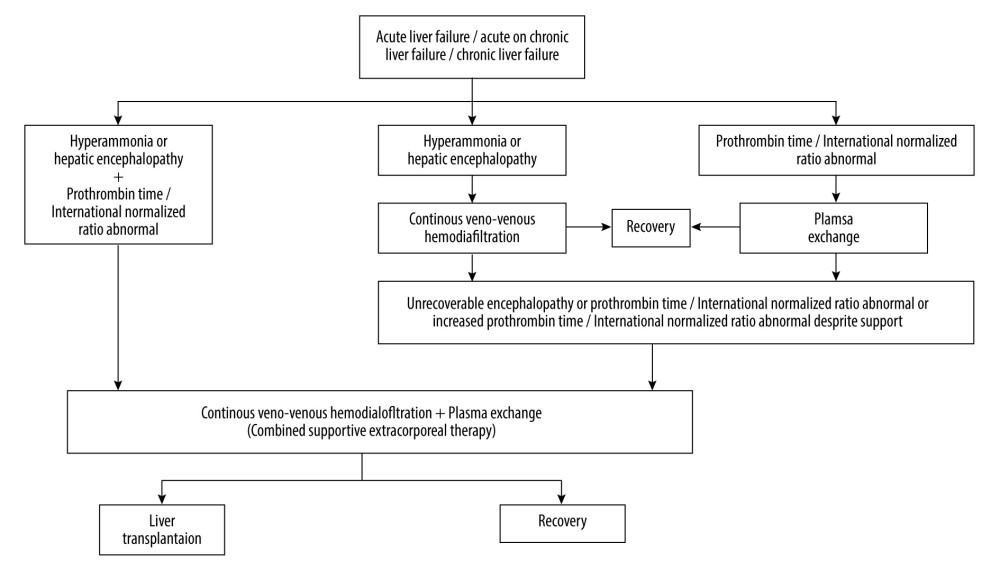 Figure 2. Extracorporeal therapy protocol for acute liver failure or acute-on-chronic liver failure or chronic liver failure. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation ofFigure 2.
Figure 2. Extracorporeal therapy protocol for acute liver failure or acute-on-chronic liver failure or chronic liver failure. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation ofFigure 2. Tables
Table 1. Data of patients at the time of admission to the Intensive Care Unit.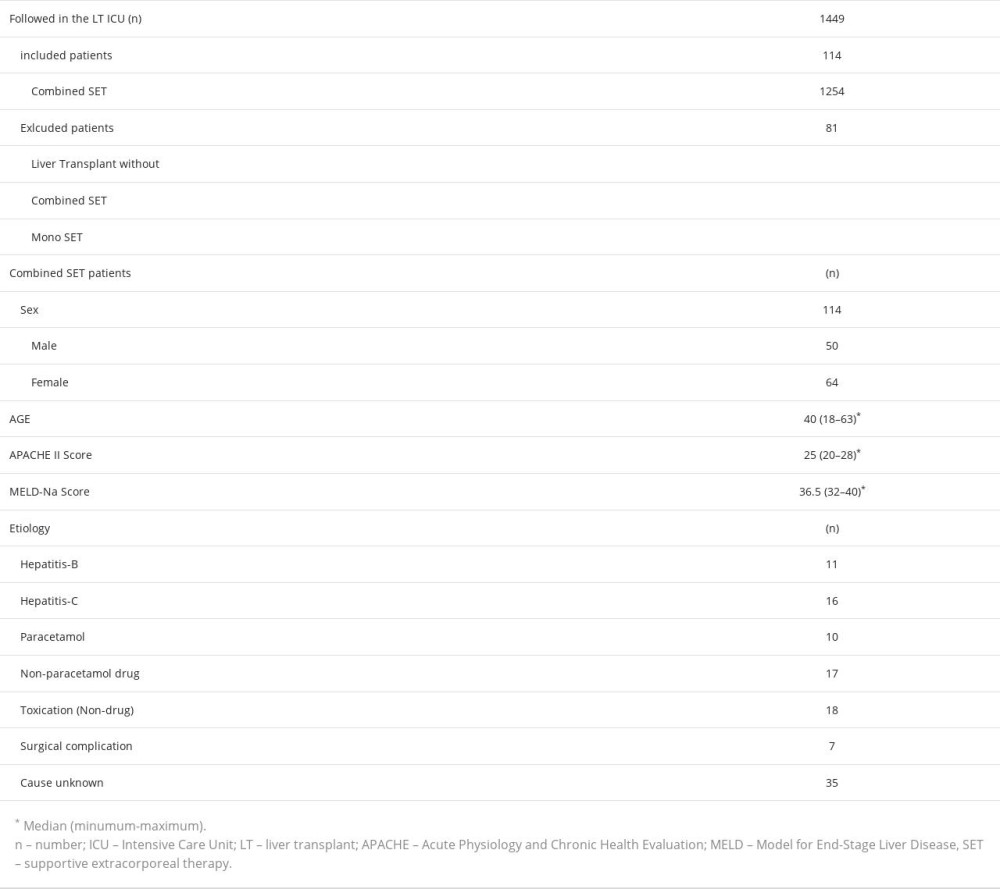 Table 2. Laboratory values of patients before the first combined supportive extracorporeal therapy and after the last combined supportive extracorporeal therapy.
Table 2. Laboratory values of patients before the first combined supportive extracorporeal therapy and after the last combined supportive extracorporeal therapy.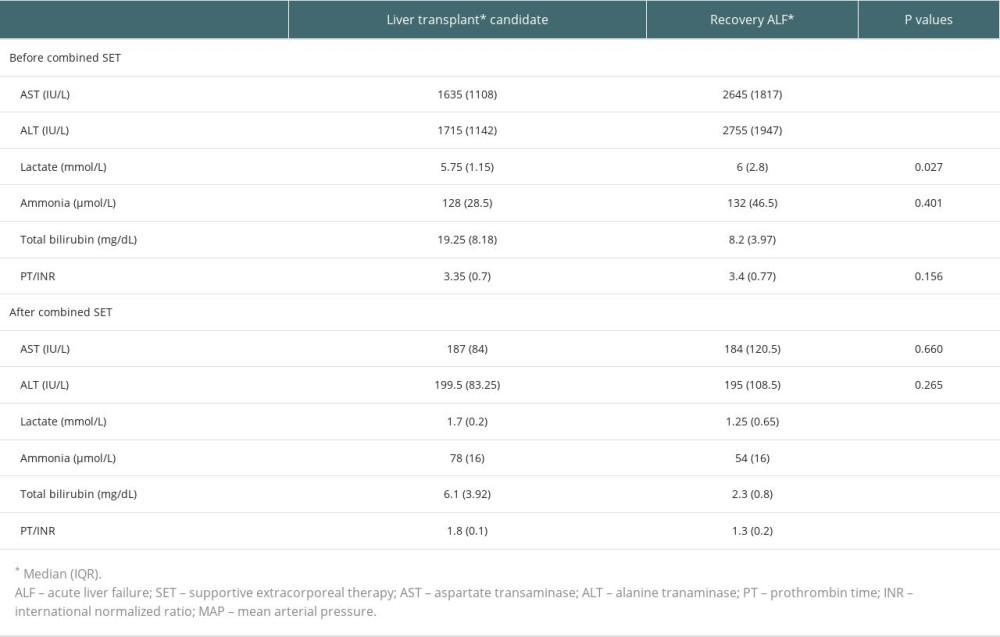 Table 3. Laboratory values of all patients at admission and after treatment.
Table 3. Laboratory values of all patients at admission and after treatment.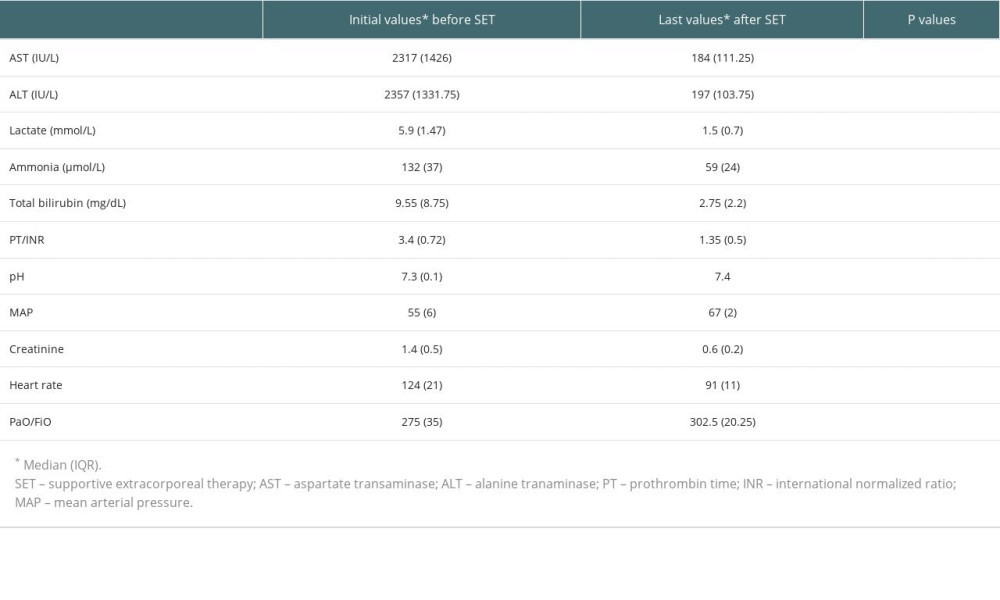 Table 4. Liver transplantation candidates (underwent liver transplantation after last supportive extracorporeal therapy) and non-liver transplant.
Table 4. Liver transplantation candidates (underwent liver transplantation after last supportive extracorporeal therapy) and non-liver transplant.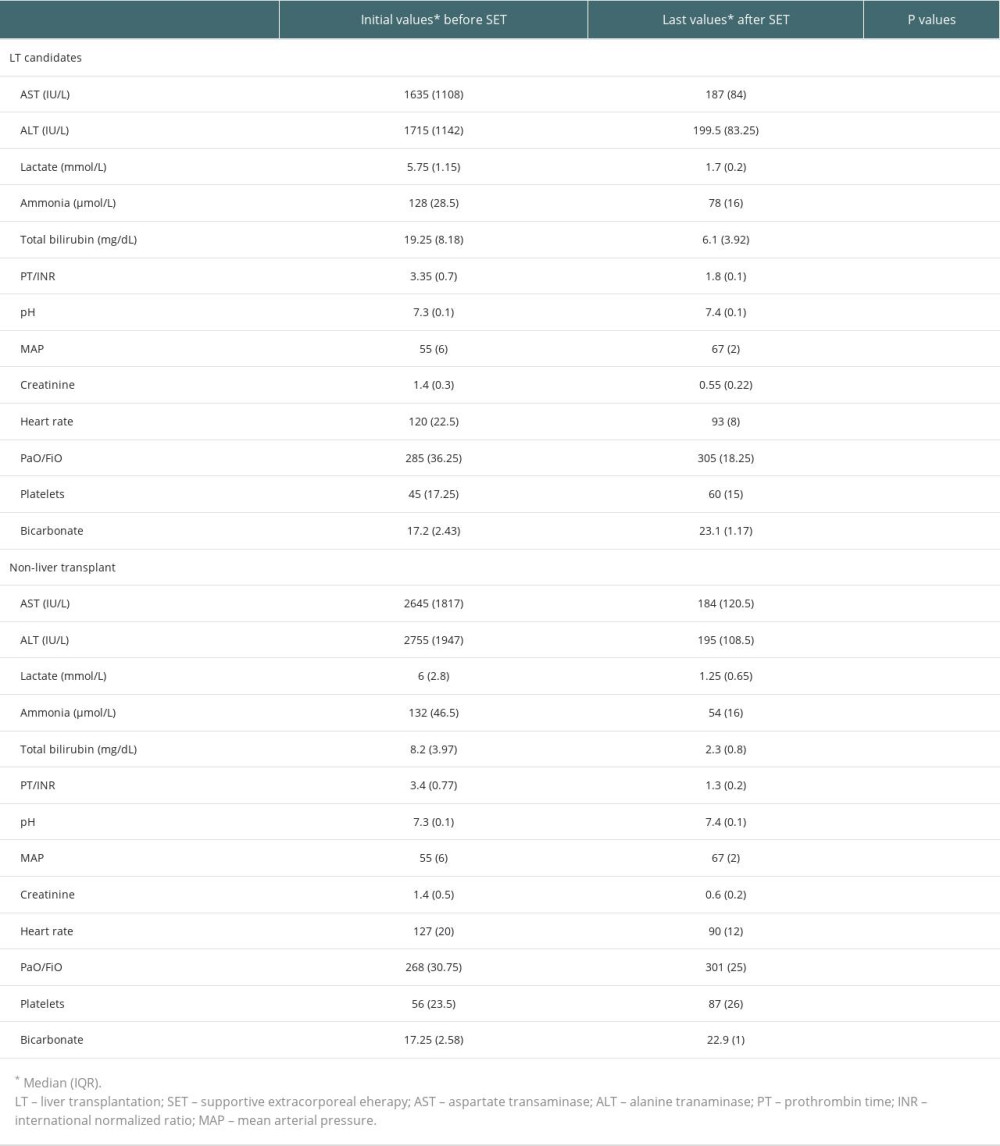 Table 5. Initial values of the survivors and ex-patients (deaths).
Table 5. Initial values of the survivors and ex-patients (deaths).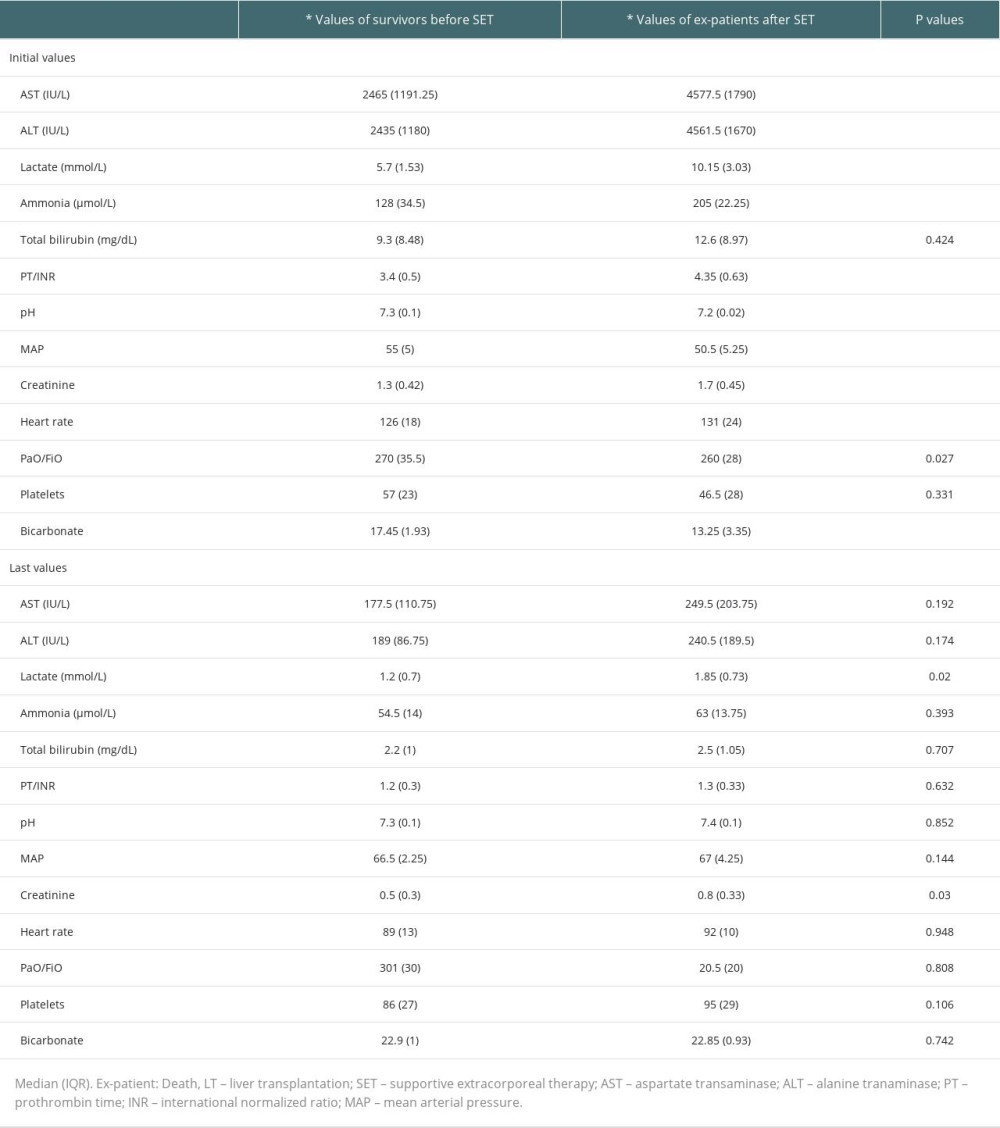
References
1. Matar AJ, Subramanian R, Extracorporeal liver support: A bridge to somewhere: Clin Liver Dis (Hoboken), 2021; 18(6); 274-79
2. Sarin SK, Kedarisetty CK, Abbas Z, Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014: Hepatol Int, 2014; 8(4); 453-71
3. Kurokohchi K, Imataki O, Kubo F, Anti-inflammatory effect of recombinant thrombomodulin for fulminant hepatic failure: World J Gastroenterol, 2015; 21(26); 8203-7
4. Sharma S, Agarwal S, Saraya A, Acute variceal bleeding portends poor outcomes in patients with acute-on-chronic liver failure: A propensity score matched study from the APASL ACLF Research Consortium (AARC): Hepatol Int, 2022; 16(5); 1234-43
5. Xie J, Xiao W, Lin J, Effect of oXiris-CVVH on the clinical outcomes of patients with septic shock: An inverse probability of treatment-weighted analysis: Blood Purif, 2022; 51(12); 972-89
6. Li P, Qu LP, Qi D, High-dose versus low-dose haemofiltration for the treatment of critically ill patients with acute kidney injury: An updated systematic review and meta-analysis: BMJ Open, 2017; 7(10); e014171
7. Wang YH, Zhu X, Feng DY, Wu BQArtificial liver support system for acute-on-chronic liver failure combined with successful liver transplantation in stage III-IV hepatic encephalopathy: An analysis of 14 cases: Zhonghua Gan Zang Bing Za Zhi, 2018; 26(9); 676-79 [In Chinese]
8. Kade G, Lubas A, Rzeszotarska A, Effectiveness of high cut-off hemofilters in the removal of selected cytokines in patients during septic shock accompanied by acute kidney injury-preliminary study: Med Sci Monit, 2016; 22; 4338-44
9. Fujiwara K, Oda S, Abe R, Yokosuka O, On-line hemodiafiltration or high-flow continuous hemodiafiltration is one of the most effective artificial liver support devices for acute liver failure in Japan: J Hepatobiliary Pancreat Sci, 2015; 22(3); 246-47
10. Li M, Sun J, Li J, Clinical observation on the treatment of acute liver failure by combined non-biological artificial liver: Exp Ther Med, 2016; 12(6); 3873-76
11. Yao J, Li S, Zhou L, Therapeutic effect of double plasma molecular adsorption system and sequential half-dose plasma exchange in patients with HBV-related acute-on-chronic liver failure: J Clin Apher, 2019; 34(4); 392-98
12. Li MQ, Ti JX, Zhu YH, Combined use of non-biological artificial liver treatments for patients with acute liver failure complicated by multiple organ dysfunction syndrome: World J Emerg Med, 2014; 5(3); 214-17
13. Gün E, Durak A, Botan E, Extracorporeal therapies in children with acute liver failure: A single-center experience: Turk J Gastroenterol, 2023; 34(1); 73-79
14. Ocak I, Value of extracorporeal artificial liver support in pediatric acute liver failure: A single-center experience of over 10 years: Front Pediatr, 2023; 11; 979619
15. Maiwall R, Moreau R, Plasma exchange for acute on chronic liver failure: Is there a light at the end of the tunnel?: Hepatol Int, 2016; 10(3); 387-89
16. Rotundo L, Pyrsopoulos N, Liver injury induced by paracetamol and challenges associated with intentional and unintentional use: World J Hepatol, 2020; 12(4); 125-36
17. Masior Ł, Grąt M, Primary nonfunction and early allograft dysfunction after liver transplantation: Dig Dis, 2022; 40(6); 766-76
18. Saraiva IE, Ortiz-Soriano VM, Mei X, Continuous renal replacement therapy in critically ill patients with acute on chronic liver failure and acute kidney injury: A retrospective cohort study: Clin Nephrol, 2020; 93(4); 187-94
19. Ide K, Muguruma T, Shinohara M, Continuous veno-venous hemodiafiltration and plasma exchange in infantile acute liver failure: Pediatr Crit Care Med, 2015; 16(8); e268-e74
20. Inoue K, Watanabe T, Maruoka N, Japanese-style intensive medical care improves prognosis for acute liver failure and the perioperative management of liver transplantation: Transplant Proc, 2010; 42(10); 4109-12
21. Lemaire A, Parquet N, Galicier L, Plasma exchange in the Intensive Care Unit: Technical aspects and complications: J Clin Apher, 2017; 32(6); 405-12
22. Mao WL, Chen Y, Chen YM, Li LJ, Changes of serum cytokine levels in patients with acute on chronic liver failure treated by plasma exchange: J Clin Gastroenterol, 2011; 45(6); 551-55
23. Mao WL, Lou YF, Ye B, Changes in peripheral CD4+CD25(high) regulatory T cells in the acute-on-chronic liver failure patients with plasma exchange treatment: Inflammation, 2012; 35(2); 436-44
24. Yokoi T, Oda S, Shiga H, Efficacy of high-flow dialysate continuous hemodiafiltration in the treatment of fulminant hepatic failure: Transfus Apher Sci, 2009; 40(1); 61-70
25. Pu Y, Yang D, Mao Y, Therapeutic effects of blood purification in treatment of fulminant hepatic failure: Braz J Infect Dis, 2013; 17(4); 427-30
26. Ocak İ, Topaloğlu S, Acarli K, Posthepatectomy liver failure: Turk J Med Sci, 2020; 50(6); 1491-503
27. Trepatchayakorn S, Chaijitraruch N, Chongsrisawat V, Therapeutic plasma exchange with continuous renal replacement therapy for pediatric acute liver failure: A case series from Thailand: Indian J Crit Care Med, 2021; 25(7); 812-16
28. Tang WX, Huang ZY, Chen ZJ, Combined blood purification for treating acute fatty liver of pregnancy complicated by acute kidney injury: A case series: J Artif Organs, 2012; 15(2); 176-84
29. Mao W, Ye B, Lin S, Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment: ASAIO J, 2010; 56(5); 475-78
30. Larsen FS, Schmidt LE, Bernsmeier C, High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial: J Hepatol, 2016; 64(1); 69-78
31. Bernal W, Wendon J, Acute liver failure: N Engl J Med, 2013; 369(26); 2525-34
32. Ooishi Y, Ishii T, Takahata T, Efficacy of series double continuous hemodiafiltration using two polymethyl methacrylate membrane hemofilters for patients with hypercytokinemia: Ther Apher Dial, 2014; 18(2); 132-39
33. Nakae H, Igarashi T, Tajimi K, Selective plasma exchange with dialysis in patients with acute liver failure: Ther Apher Dial, 2012; 16(5); 467-71
Figures
 Figure 1. Flow diagram of patients in the study. * 1 year motrality. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation of Figure 1.
Figure 1. Flow diagram of patients in the study. * 1 year motrality. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation of Figure 1. Figure 2. Extracorporeal therapy protocol for acute liver failure or acute-on-chronic liver failure or chronic liver failure. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation ofFigure 2.
Figure 2. Extracorporeal therapy protocol for acute liver failure or acute-on-chronic liver failure or chronic liver failure. PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA) used for creation ofFigure 2. Tables
 Table 1. Data of patients at the time of admission to the Intensive Care Unit.
Table 1. Data of patients at the time of admission to the Intensive Care Unit. Table 2. Laboratory values of patients before the first combined supportive extracorporeal therapy and after the last combined supportive extracorporeal therapy.
Table 2. Laboratory values of patients before the first combined supportive extracorporeal therapy and after the last combined supportive extracorporeal therapy. Table 3. Laboratory values of all patients at admission and after treatment.
Table 3. Laboratory values of all patients at admission and after treatment. Table 4. Liver transplantation candidates (underwent liver transplantation after last supportive extracorporeal therapy) and non-liver transplant.
Table 4. Liver transplantation candidates (underwent liver transplantation after last supportive extracorporeal therapy) and non-liver transplant. Table 5. Initial values of the survivors and ex-patients (deaths).
Table 5. Initial values of the survivors and ex-patients (deaths). Table 1. Data of patients at the time of admission to the Intensive Care Unit.
Table 1. Data of patients at the time of admission to the Intensive Care Unit. Table 2. Laboratory values of patients before the first combined supportive extracorporeal therapy and after the last combined supportive extracorporeal therapy.
Table 2. Laboratory values of patients before the first combined supportive extracorporeal therapy and after the last combined supportive extracorporeal therapy. Table 3. Laboratory values of all patients at admission and after treatment.
Table 3. Laboratory values of all patients at admission and after treatment. Table 4. Liver transplantation candidates (underwent liver transplantation after last supportive extracorporeal therapy) and non-liver transplant.
Table 4. Liver transplantation candidates (underwent liver transplantation after last supportive extracorporeal therapy) and non-liver transplant. Table 5. Initial values of the survivors and ex-patients (deaths).
Table 5. Initial values of the survivors and ex-patients (deaths). In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








