22 November 2022: Original Paper
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipients with Hepatocellular Carcinoma
Gonzalo Sapisochin 1ADE** , Wei Chen Lee 2ABCDE , Dong Jin Joo 3ABD , Jae-Won Joh 4ABE , Koichiro Hata 56BCDE , Arvinder Singh Soin 7ACDE , Uday Kiran Veldandi 8ABCDEF , Shuhei Kaneko 9ACDE , Matthias Meier 10ACDEG , Denise Leclair 11DE , Gangadhar Sunkara 11CDEF , Long Bin Jeng 12AB**DOI: 10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
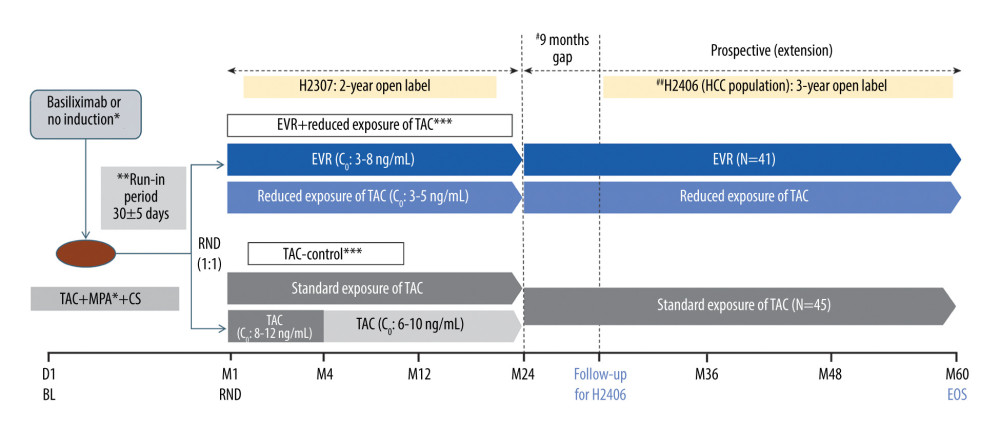
Figure 1 Design of the follow-up study. * Per the center’s choice; ** All patients received TAC (C0: 5–15 ng/mL) during the run-in phase; *** CS in both arms per the local practice; # variable period between the end of study visit in the H2307 study and the start of data collection for study H2406; ## treatment as per the local clinical practice during H2406. C0 – trough level; CS – corticosteroid; EOS – end of study; EVR – everolimus; HCC – hepatocellular carcinoma; LT – liver transplantation; M – month; MPA – mycophenolic acid; RND – randomization; TAC – tacrolimus. Created using Microsoft Office (2016, Microsoft).


