14 September 2021: Original Paper
Impact of Liver Transplantation on Carbon Monoxide Production as Measured by Arterial Carboxyhemoglobin Levels in Cirrhotic Patients with and without Hepatopulmonary Syndrome
Ralph Llewel Sabang1ABCDEF*, Mohammad Abu-Hishmeh2ABC, Roxana Bodin3DEF, Oleg Epelbaum4ABCDEFDOI: 10.12659/AOT.932009
Ann Transplant 2021; 26:e932009
Abstract
BACKGROUND: Hepatic dysfunction is associated with increased production of carbon monoxide. End-stage liver disease patients with hepatopulmonary syndrome (HPS) have been shown to have higher blood carbon monoxide levels than those without HPS. The impact of liver transplantation on blood carbon monoxide levels is currently unknown. We assessed the impact of liver transplantation on blood carbon monoxide and whether this is affected by HPS.
MATERIAL AND METHODS: Eligible liver transplant recipients had room air arterial blood gas testing performed before and after liver transplantation. The carboxyhemoglobin fraction was obtained from arterial co-oximetry and used as a surrogate for carboxyhemoglobin production. Mean arterial carboxyhemoglobin fraction before transplantation was compared to that after transplantation. Mean absolute and median relative pre- to post-transplant within-patient change in carboxyhemoglobin fraction was compared between those with and without HPS.
RESULTS: Thirty-nine transplanted cirrhotic patients were analyzed, of whom 14 (36%) met criteria for hepatopulmonary syndrome. The mean pre-transplant carboxyhemoglobin fraction was higher than the post-transplant fraction (2.6 vs 1.8, difference 0.8 [95% CI 0.4-1.2]; P value 0.0002). Of the 14 patients with HPS, 11 (79%) experienced a decrease in their carboxyhemoglobin fraction after liver transplantation; among the 25 patients without HPS, 16 (64%) experienced such a decrease (P=0.48). Neither the absolute nor relative within-patient pre- to post-transplant change in carboxyhemoglobin fraction was significantly different between patients with and without HPS.
CONCLUSIONS: Blood carbon monoxide levels decreased significantly in cirrhotic patients following liver transplantation, but HPS did not affect the magnitude of this change.
Keywords: Carbon Monoxide, hepatopulmonary syndrome, Liver Cirrhosis, Liver Transplantation, Oximetry, Carboxyhemoglobin, End stage liver disease, Female, Humans, Severity of Illness Index
Background
In liver cirrhosis, arterial vasodilation in the splanchnic circulation as well as in the pulmonary vasculature plays an important role in the development of extrahepatic complications. Among the proposed mediators of decreased vascular tone in hepatic dysfunction, there are 2 gases: nitric oxide (NO) and carbon monoxide (CO) [1,2]. Both NO and CO can be produced endogenously. CO is a byproduct of a pathway catalyzed by heme-oxygenase (HO), an inducible enzyme that degrades heme in the liver. CO can activate soluble guanylate cyclase, leading to generation of cyclic guanosine monophosphate, which in turn mediates various physiological functions such as smooth muscle relaxation (Figure 1). Through this mechanism, CO has been postulated to be a major contributor to the regulation of local and systemic vascular tone [3–5]. Animal studies have shown that levels of CO increase following liver injury as a result of upregulation of the HO pathway [6,7]. Human data likewise point to increased HO activity in cirrhotic patients compared to healthy controls, as indicated indirectly by higher CO and blood carboxyhemoglobin (HbCO) levels in the former group [8].
Hepatopulmonary syndrome (HPS) is a complication of severe liver disease, characterized by abnormal arterial oxygenation due to intrapulmonary vascular dilatation. Overproduction of pulmonary NO has been considered to be the main pathophysiological mechanism behind the abnormal vasodilation [9]. Also found in experimental HPS is markedly increased expression of HO with accompanying elevation of arterial HbCO [10,11]. In a human study, Arguedas et al showed that levels of HbCO were significantly higher in cirrhotic patients with HPS compared to patients without HPS [12]. In contrast, Tran et al found no correlation between HbCO and liver disease severity in cirrhotic outpatients [13].
Liver transplantation can be expected to reduce CO production compared to the pre-transplant level, perhaps especially so in patients with HPS. There is currently no published work examining the effect of liver transplantation on CO production. We performed the present study to assess the impact of liver transplantation on CO production using HbCO levels as a surrogate in patients with chronic liver disease and to look at this interplay differentially between those with and without HPS before transplantation.
Material and Methods
PATIENTS:
Westchester Medical Center (WMC) is an active liver transplantation center located in the state of New York that performs approximately 40 liver transplantation surgeries annually. All adult patients with liver cirrhosis who underwent deceased donor liver transplantation at WMC between January 1, 2014 and February 29, 2020 were eligible for inclusion in the study. Candidate patients were identified through the electronic liver transplantation recipient database belonging to the Section of Transplant Hepatology. For patients thus identified, parameters of interest were then extracted from the hospital electronic medical record and the database maintained by the hospital’s pulmonary function laboratory. The Institutional Review Board of New York Medical College and the Clinical Research Institute of Westchester Medical Center approved the study (protocol #12965). All clinical data used in this study had already been obtained as part of routine patient care, so the requirement for informed consent was waived.
Potentially eligible liver transplant recipients were screened for fulfillment of the following inclusion criteria:
Patients were ineligible for analysis if any of the following exclusion criteria were met:
The presence or absence of chronic pulmonary disease was adjudicated by 2 experienced pulmonologists (OE, MAH) based on review of available history, chest imaging, and pulmonary function testing.
HPS CRITERIA:
In accordance with published guidelines [14], HPS was defined by a positive contrast-enhanced (“bubble”) echocardiogram consistent with intrapulmonary shunting in combination with an alveolar-arterial oxygen gradient of ≥15 mmHg. HPS severity was graded as recommended by the European Respiratory Society’s task force in 2004 [15]:
ARTERIAL BLOOD GAS AND HBCO TESTING:
As part of pre-transplant evaluation, arterial specimens for ABG analysis are customarily obtained in the pulmonary function laboratory from the radial artery with the patient breathing ambient air in a sitting position. The blood is collected into a heparinized syringe and processed routinely for pH and gas tensions as well as typically for co-oximetry using the GEM Premier 4000 system (Instrumentation Laboratory, Bedford, MA, USA). Co-oximetry measurements provide a value for the percentage of HbCO in the sample with the normal range in our institution being 0.5–1.5%. In the event that multiple eligible ABG results were available, the values closest to the date of transplant surgery were chosen for study purposes.
STATISTICAL ANALYSIS:
The primary objective of this study was to compare both the absolute and relative change in HbCO fraction between the pre-transplant and post-transplant values in cirrhotic patients overall as well as separately in those with and without HPS. The absolute within-patient change was defined as the difference between the post-transplant HbCO and pre-transplant HbCO. The relative within-patient change was defined as: (post-transplant HbCO – pre-transplant HbCO)/pre-transplant HbCO. The expected impact of liver transplantation was a decline in HbCO fraction. Patient characteristics were reported as frequency and percentage for categorical variables; mean±SD for normally distributed continuous variables, and median (IQR) for continuous variables that violated normality. Normality of distribution was assessed using the Shapiro-Wilk test. Fisher’s exact test (two-tailed) was used to compare categorical variables. The paired
Results
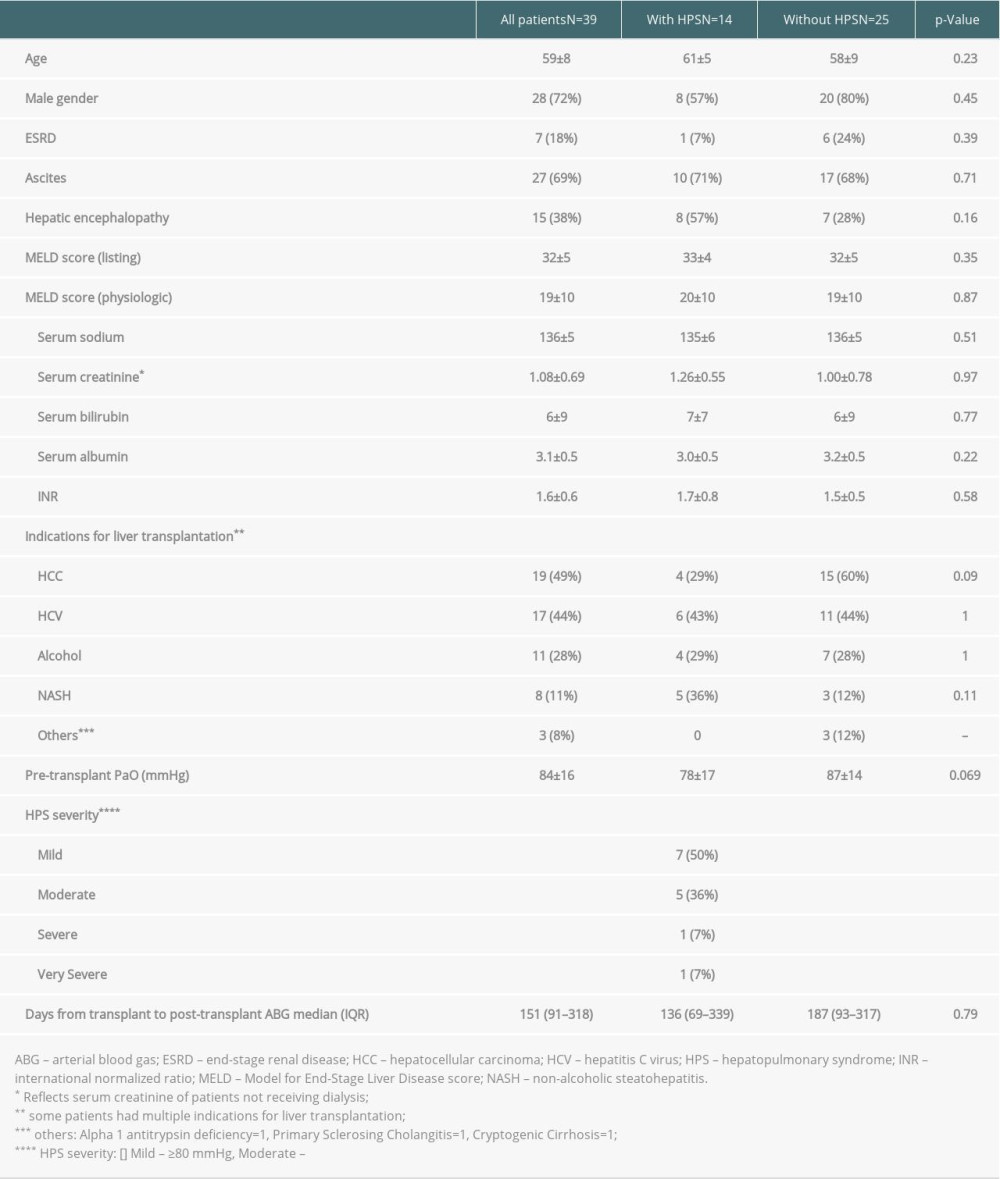
A total of 145 patients underwent liver transplantation at WMC during the study period. Of these, 106 patients (73%) became ineligible for analysis after application of the inclusion and exclusion criteria. Out of the remaining 39 patients, 14 (36%) fulfilled criteria for HPS. The demographic and clinical characteristics of the study population are summarized in Table 1. The mean age of our sample was 59±8 years, and 28 patients were men (72%). The most common indication for liver transplantation in our sample was hepatocellular carcinoma (N=19, 49%) combined with other indications. The second and third most common indications were hepatitis C cirrhosis (N=17, 44%) and alcoholic cirrhosis (N=11, 28%). The mean physiologic Model for End-Stage Liver Disease (MELD) score in the sample was 19±10. With respect to complications of end-stage liver disease, the pre-transplantation course of 27 patients (68%) included ascites, 7 patients (18%) were recipients of hemodialysis, and another 15 (38%) had a history of hepatic encephalopathy. When the baseline characteristics of patients with and without HPS were compared, no significant differences emerged between the 2 groups, including with respect to pre-transplant PaO2 (Table 1). Twelve of the 14 patients (86%) with HPS had mild to moderate disease. The 2 patients with severe to very severe disease were prescribed supplemental oxygen prior to transplantation.
The main results are summarized in Figures 2 and 3. The mean pre-transplant HbCO fraction in the study sample as a whole was 2.6±1 (range 0.3–6.8), whereas the mean post-transplant fraction was 1.8±0.7 (range 0.4–4.1). The absolute difference between the mean pre-transplant value and the mean post-transplant value of 0.8 (95% CI 0.4–1.2) was statistically significant (
Discussion
This study builds on prior findings in experimental acute liver injury [3–6] as well as on human data from chronic liver disease [7] indicating that CO production and therefore HbCO levels may rise in the setting of hepatic dysfunction. The mean pre-transplantation HbCO fraction in our sample (2.6) was almost twice the upper limit of normal (1.5) for our institutional laboratory. The mean post-transplantation HbCO fraction of 1.8 approached the normal range but failed to reach it. Contrary to experimental [10] and clinical [11] reports, however, HPS was not associated with a higher pre-transplant HbCO level nor with a more robust response of HbCO to liver transplantation compared to patients without HPS.
This study is the first to examine the impact of liver transplantation on CO production in cirrhotic patients. As hypothesized, liver transplantation significantly reduced the HbCO fraction measured by arterial co-oximetry in a sample of non-smoking, end-stage liver disease patients without chronic lung disease from a single transplant center. When cases with and without HPS were compared, there was no difference in pre-transplantation or post-transplantation HbCO levels between them nor in the magnitude of absolute or relative within-patient change from before the transplant to after. Most patients in both groups experienced a decrease in HbCO fraction with liver transplantation.
The related questions of whether liver cirrhosis does indeed elevate the HbCO level and whether liver transplantation leads to lowering of CO production have a number of practical implications. One of them is the performance of pulse oximetry, which does not differentiate between types of hemoglobin and thus can overestimate the oxygen saturation by counting HbCO [16]. Abrams et al have shown that in cirrhotic patients, pulse oximetry (SpO2) overestimates oxygen saturation in comparison to co-oximetry (SaO2), which can specifically measure oxyhemoglobin, with a mean absolute bias (SpO2-SaO2) of about 3.5 percentage points [17]. In their sample, there was no significant difference in HbCO fraction between the patients with liver disease and healthy controls, and the HbCO fraction did not correlate well with pulse oximetry bias (Spearman r=0.21). In the current study, the mean HbCO fraction before transplantation was higher than that reported by Abrams et al in their cirrhotic population (2.6% vs 1.7%, respectively), and the figure of 2.6% approximates in magnitude the bias of pulse oximetry in that earlier study. Nonetheless, consistent with the results of Abrams et al, in our patients the pre-transplant HbCO fraction did not correlate significantly with pre-transplant pulse oximetry bias (Spearman r=0.094;
As mentioned earlier, CO has been implicated in the vasodilatory state that accompanies end-stage liver disease and contributes to HPS. The significant drop in HbCO fraction following liver transplantation observed in the present study raises the possibility that reduced CO production could be a contributor to the post-transplant improvement in vascular tone that leads to the higher systemic blood pressure and resolution of HPS commonly seen in transplant recipients [18,19]. If this hypothesis is supported by future investigations, it could refine the current understanding of factors that modulate vascular tone in patients with liver cirrhosis and could potentially offer a pathway for therapeutic targeting [20].
Our study suffers from a number of limitations, primary its retrospective nature and small sample size. Because patients were not studied prospectively, it was impossible to standardize the timing of ABG collections relative to the date of transplantation surgery. Subjects’ cigarette smoking status was determined based strictly on medical record documentation rather than laboratory testing for nicotine or cotinine.
Conclusions
Using arterial HbCO fraction as a marker of endogenous CO production, this study is the first to demonstrate that liver transplantation results in a significant decrease in CO production and consequently in HbCO levels. This effect was observed irrespective of the presence or absence of HPS. The absolute and relative magnitude of the reduction in CO following liver transplantation was similar in those with and without HPS. If confirmed by subsequent studies, this phenomenon could affect the interpretation of pulse oximetry measurements in the pre-transplant period relative to the post-transplant period and could inform the understanding of changes in vascular tone associated with receipt of liver allografts.
Figures
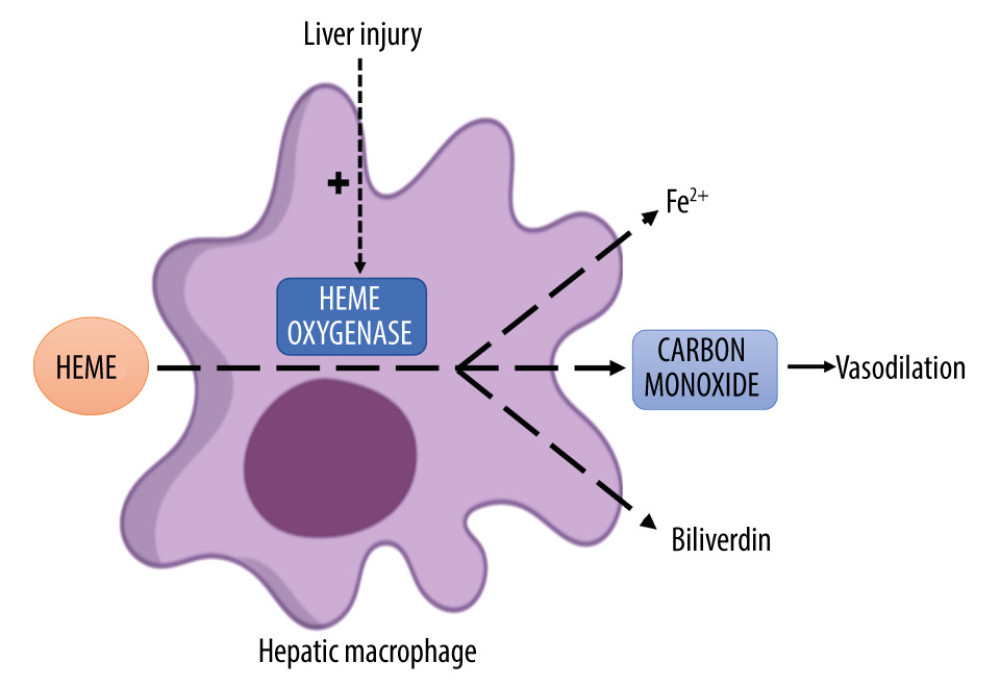 Figure 1. Graphical depiction of the heme-oxygenase (HO) pathway. Heme, a product of hemoglobin degradation, is engulfed by liver macrophages and then broken down to iron (Fe2+), biliverdin, and carbon monoxide in a reaction catalyzed by the inducible enzyme HO-1. This enzyme’s activity can be upregulated by multiple factors, including liver injury. Carbon monoxide, in turn, exerts several downstream effects, including vasodilation.
Figure 1. Graphical depiction of the heme-oxygenase (HO) pathway. Heme, a product of hemoglobin degradation, is engulfed by liver macrophages and then broken down to iron (Fe2+), biliverdin, and carbon monoxide in a reaction catalyzed by the inducible enzyme HO-1. This enzyme’s activity can be upregulated by multiple factors, including liver injury. Carbon monoxide, in turn, exerts several downstream effects, including vasodilation. 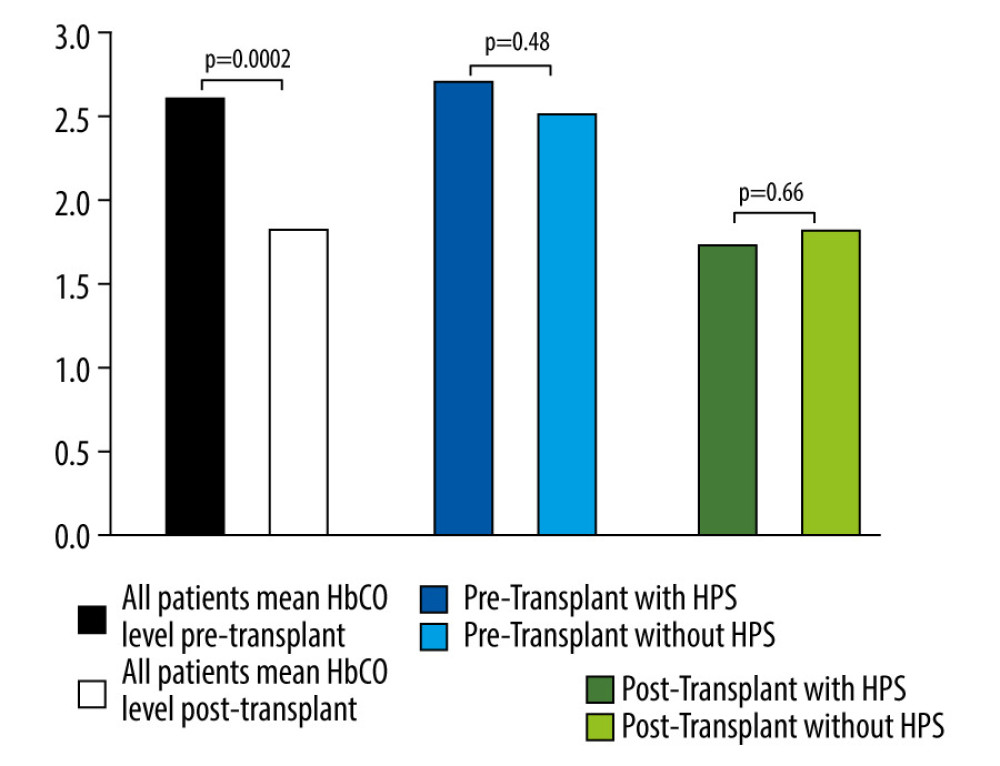 Figure 2. Comparison of mean carboxyhemoglobin (HbCO) levels in all patients before (black bar) and after (white bar) liver transplantation, which were significantly higher prior to transplant. HbCO levels before liver transplantation are also compared separately in those with (solid blue bar) and without (light blue bar) hepatopulmonary syndrome (HPS). The same comparison is likewise shown after transplantation (solid and light geen bars). Differences in the latter 2 comparisons did not reach statistical significance.
Figure 2. Comparison of mean carboxyhemoglobin (HbCO) levels in all patients before (black bar) and after (white bar) liver transplantation, which were significantly higher prior to transplant. HbCO levels before liver transplantation are also compared separately in those with (solid blue bar) and without (light blue bar) hepatopulmonary syndrome (HPS). The same comparison is likewise shown after transplantation (solid and light geen bars). Differences in the latter 2 comparisons did not reach statistical significance. 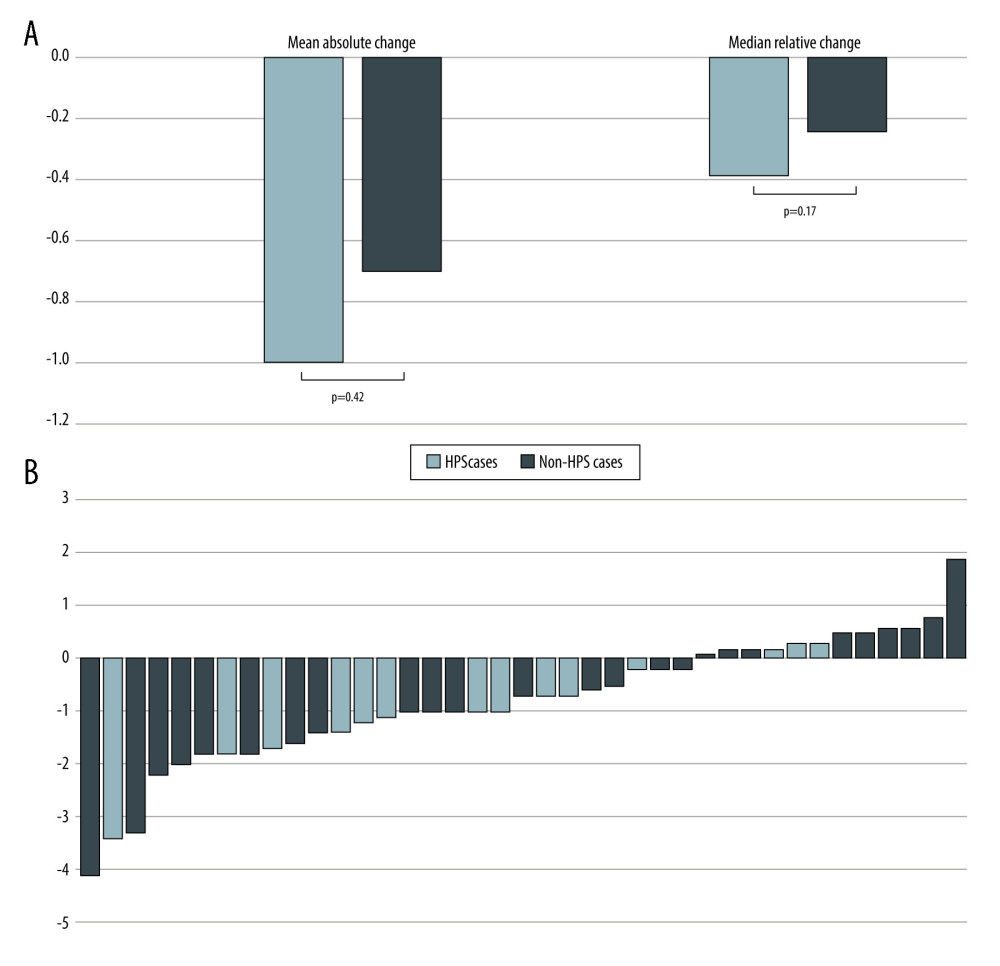 Figure 3. (A) Graph depicting both the mean absolute change in carboxyhemoglobin (HbCO) level from before to after liver transplantation, as well as the median relative change. Light bars represent the group with hepatopulmonary syndrome (HPS), while black bars represent the group without HPS. The differences between the 2 groups were not statistically significant. (B) Individual patient direction and magnitude of absolute change in HbCO level from before liver transplantation to after (N=39). Of the 14 patients with HPS, 11 (79%) experienced a decrease in their HbCO level after liver transplantation; among the 25 patients without HPS, 16 (64%) experienced a decrease (P=0.48).
Figure 3. (A) Graph depicting both the mean absolute change in carboxyhemoglobin (HbCO) level from before to after liver transplantation, as well as the median relative change. Light bars represent the group with hepatopulmonary syndrome (HPS), while black bars represent the group without HPS. The differences between the 2 groups were not statistically significant. (B) Individual patient direction and magnitude of absolute change in HbCO level from before liver transplantation to after (N=39). Of the 14 patients with HPS, 11 (79%) experienced a decrease in their HbCO level after liver transplantation; among the 25 patients without HPS, 16 (64%) experienced a decrease (P=0.48). References
1. Tumgor G, Cirrhosis and hepatopulmonary syndrome: World J Gastroenterol, 2014; 20; 2586-94
2. Soulaidopoulos S, Cholongitas E, Giannakoulas G, Review article: Update on current and emergent data on hepatopulmonary syndrome: World J Gastroenterol, 2018; 24; 1285-98
3. Drummond GS, Baum J, Greenberg M, Lewis D, Abraham NG, HO-1 overexpression and underexpression: Clinical implications: Arch Biochem Biophys, 2019; 673; 108073
4. Fernandez M, Bonkovsky HL, Increased heme oxygenase 1 gene expression in liver cells and splanchnic organs from portal hypertensive rats: Hepatology, 1999; 29; 1672-79
5. Møller S, Bendtsen F, The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis: Liver Int, 2018; 38; 570-80
6. Tang J, Li C, Li L, Wu J, Wang G, Changes in expression and production of heme oxygenase-1 in rats with acute liver injury induced by lipopolysaccharide: J Toxicol Sci, 2016; 41; 469-77
7. Wen T, Guan L, Zhang Y, Zhao J, Dynamic changes of heme oxygenase-1 and carbon monoxide production in acute liver injury induced by carbon tetrachloride in rats: Toxicology, 2006; 228; 51-57
8. De Las Heras D, Fernández J, Ginès P, Increased carbon monoxide production in patients with cirrhosis with and without spontaneous bacterial peritonitis: Hepatology, 2003; 38; 452-59
9. Grace JA, Angus PW, Hepatopulmonary syndrome: Update on recent advances in pathophysiology, investigation, and treatment: J Gastroenterol Hepatol, 2013; 28; 213-19
10. Carter EP, Hartsfield CL, Miyazono M, Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome: Am J Physiol Lung Cell Mol Physiol, 2002; 283; 346-53
11. Zhang J, Ling Y, Luo B, Analysis of pulmonary heme oxygenase-1 and nitric oxide synthase alterations in experimental hepatopulmonary syndrome: Gastroenterology, 2003; 125; 1441-51
12. Arguedas MR, Drake BB, Kapoor A, Fallon MB, Carboxyhemoglobin levels in cirrhotic patients with and without hepatopulmonary syndrome: Gastroenterology, 2005; 128; 328-33
13. Tran TT, Martin P, Ly H, Balfe D, Mosenifa Z, Carboxyhemoglobin and its correlation to disease severity in cirrhotics: J Clin Gastroenterol, 2007; 41; 211-15
14. Krowka MJ, Fallon MB, Kawut SM, International Liver Transplant Society practice guidelines: Diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension: Transplantation, 2016; 100; 1440-52
15. Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MBERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee, Pulmonary-hepatic vascular disorders (PHD): Eur Respir J, 2004; 24; 861-80
16. Ortega R, Hansen CJ, Elterman K, Woo A, Pulse oximetry: N Engl J Med, 2011; 364; e33
17. Abrams GA, Sanders MK, Fallon MB, Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates: Liver Transpl, 2002; 8; 391-96
18. Piscaglia F, Zironi G, Gaiani S, Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: A long-term prospective study: Hepatology, 1999; 30; 58-64
19. Taillé C, Cadranel J, Bellocq A, Liver transplantation for hepatopulmonary syndrome: A ten-year experience in Paris, France: Transplantation, 2003; 75; 1482-89
20. Ryter SW, Choi AM, Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation: Transl Res, 2016; 167; 7-34
Figures
 Figure 1. Graphical depiction of the heme-oxygenase (HO) pathway. Heme, a product of hemoglobin degradation, is engulfed by liver macrophages and then broken down to iron (Fe2+), biliverdin, and carbon monoxide in a reaction catalyzed by the inducible enzyme HO-1. This enzyme’s activity can be upregulated by multiple factors, including liver injury. Carbon monoxide, in turn, exerts several downstream effects, including vasodilation.
Figure 1. Graphical depiction of the heme-oxygenase (HO) pathway. Heme, a product of hemoglobin degradation, is engulfed by liver macrophages and then broken down to iron (Fe2+), biliverdin, and carbon monoxide in a reaction catalyzed by the inducible enzyme HO-1. This enzyme’s activity can be upregulated by multiple factors, including liver injury. Carbon monoxide, in turn, exerts several downstream effects, including vasodilation. Figure 2. Comparison of mean carboxyhemoglobin (HbCO) levels in all patients before (black bar) and after (white bar) liver transplantation, which were significantly higher prior to transplant. HbCO levels before liver transplantation are also compared separately in those with (solid blue bar) and without (light blue bar) hepatopulmonary syndrome (HPS). The same comparison is likewise shown after transplantation (solid and light geen bars). Differences in the latter 2 comparisons did not reach statistical significance.
Figure 2. Comparison of mean carboxyhemoglobin (HbCO) levels in all patients before (black bar) and after (white bar) liver transplantation, which were significantly higher prior to transplant. HbCO levels before liver transplantation are also compared separately in those with (solid blue bar) and without (light blue bar) hepatopulmonary syndrome (HPS). The same comparison is likewise shown after transplantation (solid and light geen bars). Differences in the latter 2 comparisons did not reach statistical significance. Figure 3. (A) Graph depicting both the mean absolute change in carboxyhemoglobin (HbCO) level from before to after liver transplantation, as well as the median relative change. Light bars represent the group with hepatopulmonary syndrome (HPS), while black bars represent the group without HPS. The differences between the 2 groups were not statistically significant. (B) Individual patient direction and magnitude of absolute change in HbCO level from before liver transplantation to after (N=39). Of the 14 patients with HPS, 11 (79%) experienced a decrease in their HbCO level after liver transplantation; among the 25 patients without HPS, 16 (64%) experienced a decrease (P=0.48).
Figure 3. (A) Graph depicting both the mean absolute change in carboxyhemoglobin (HbCO) level from before to after liver transplantation, as well as the median relative change. Light bars represent the group with hepatopulmonary syndrome (HPS), while black bars represent the group without HPS. The differences between the 2 groups were not statistically significant. (B) Individual patient direction and magnitude of absolute change in HbCO level from before liver transplantation to after (N=39). Of the 14 patients with HPS, 11 (79%) experienced a decrease in their HbCO level after liver transplantation; among the 25 patients without HPS, 16 (64%) experienced a decrease (P=0.48). In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860