29 December 2021: Meta-Analysis
Efficacy and Safety of Tacrolimus-Based Maintenance Regimens in De Novo Kidney Transplant Recipients: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
Manjunatha T A1ABCDEF*, Rebecca Chng1ABCDEF, Wai-Ping Yau2ADEDOI: 10.12659/AOT.933588
Ann Transplant 2021; 26:e933588
Abstract
BACKGROUND: Tacrolimus is an established component of immunosuppressive regimens for kidney transplant recipients (KTRs); however, data comparing long-term outcomes between formulations are lacking. We conducted a systematic literature review and network meta-analysis assessing tacrolimus (primarily Advagraf [once-daily] and Prograf [twice-daily])-based maintenance regimens.
MATERIAL AND METHODS: Embase, MEDLINE, and Cochrane databases and congress proceedings were searched to identify studies of adult de novo KTRs who received tacrolimus-based therapy in phase II/III randomized controlled trials. Outcomes were acute rejection, graft/patient survival, and incidence of new-onset diabetes mellitus after transplantation (NODAT) and cytomegalovirus (CMV) infection. Bayesian network meta-analysis was used to analyze treatment effects on graft/patient survival.
RESULTS: Sixty-eight publications (61 primary) were included. Of 21 publications reporting graft rejection following Advagraf or Prograf treatment in ≥1 study arm, 12-month biopsy-proven acute rejection (BPAR) ranged from 3.3% with Prograf to 55.0% with mycophenolic acid (MPA)+corticosteroids (CS); >24 month BPAR ranged from 0% to 58.7% (the latter with bleselumab-based therapy). Fourteen publications reported graft loss following Advagraf (0-9.6%) or Prograf (0-7.5%). Patient mortality ≤24 months after transplantation (14 publications) ranged from 0% to 8.1% with Advagraf or Prograf. Advagraf+MPA+CS and reference treatment, Prograf+MPA+CS, were associated with a similar risk of graft loss (odds ratio 1.19; 95% credible-interval 0.51, 3.06) and mortality (odds ratio 1.21; 95% credible-interval 0.1557, 9.03). Incidence of NODAT and CMV varied by treatment arm.
CONCLUSIONS: Graft loss and patient mortality rates were generally comparable between Advagraf- and Prograf-based regimens. Further prospective studies are needed to evaluate longer-term outcomes.
Keywords: Immunosuppression, Kidney Transplantation, Maintenance, review, Tacrolimus, Adult, Bayes Theorem, Graft Rejection, Humans, Immunosuppressive Agents, Network Meta-Analysis
Background
Chronic kidney disease results in reduced kidney function, with stage 5 chronic kidney disease occurring when the estimated glomerular filtration rate (eGFR) is below 15 mL/min/1.73 m2 [1,2]. Patients with stage 5 chronic kidney disease require either dialysis or kidney transplantation, with transplantation the preferred strategy owing to improved quality of life and life expectancy and superior cost effectiveness compared with dialysis [3]. However, improvements in immunosuppressive regimens are needed to optimize long-term post-transplant outcomes. In particular, there is a need to improve adherence to immunosuppressive regimens. Non-adherence increases the likelihood of graft rejection [4–6], whereas simplifying treatment can help improve adherence among patients taking immunosuppressive regimens [6]. In a meta-analysis of 14 publications, the median proportion of non-adherent patients between kidney transplantation and the time of the study was 22.3%, and in turn, non-adherence was associated with a median of 36.4% of graft losses, ranging from 7.1% to 80.0% [4].
For over 2 decades, tacrolimus (TAC) has been an established component of maintenance immunosuppressive regimens following kidney transplantation. A once-daily (QD), prolonged-release formulation (Advagraf®) is available, which may improve overall adherence compared with twice-daily (BID) dosing, especially in transplant recipients likely to be non-compliant with their treatment regimen [7–9]. While improved adherence to maintenance immunosuppressive regimens could reduce the risk of kidney transplant rejection, there is a need for further analysis of potential differences in long-term outcomes between the QD and BID dosing regimens. We conducted a systematic literature review and network meta-analysis to assess TAC-based maintenance regimens in kidney transplant recipients, with a focus on identifying differences in patient and graft survival between TAC QD and BID dosing regimens.
Material and Methods
SYSTEMATIC LITERATURE REVIEW:
Searches were consistent with recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement, Centre for Reviews and Dissemination guidelines, and Cochrane Collaboration handbook [10–12]. Searches included free-text and comprehensive disease terms, combined with filters to identify randomized controlled trials published by the Scottish Intercollegiate Guidelines Network. The search had neither time nor language restrictions. Searches were performed using Embase (January 1, 1974, to October 3, 2019), Ovid MEDLINE (January 1, 1946, to October 1, 2019) and Evidence-Based Medicine Reviews: Cochrane Central Register of Controlled Trials (August 2019). The search strategies can be found in Supplementary Table 1. The proceedings from 2017 to 2019 for the American Transplant Congress and the Annual Congress of the European Renal Association – European Dialysis and Transplant Association were screened. Abstracts were also searched for the previous 2 International Conferences of the Transplantation Society (2016 and 2019) and the Congresses of the European Society for Organ Transplantation (2017 and 2019).
Eligibility criteria for the efficacy and safety studies followed those proposed in the Population, Intervention, Comparator, Outcomes, and Study design statement. Key eligibility criteria included adult (aged ≥18 years) de novo kidney transplant patients receiving TAC-based monotherapy or combination immunosuppressive regimens. Efficacy outcomes were acute rejection and graft and patient survival. Safety outcomes were cytomegalovirus (CMV) infection and new-onset diabetes after transplant (NODAT). Only phase II/III randomized controlled trials published in English and including regimens used as maintenance therapy were considered. Secondary publications were included if they contained data relevant to this study, beyond those cited in the primary report.
Two reviewers examined each abstract and each full-text paper. Any studies queried at either stage were referred to a third reviewer, and consensus was reached. After the initial search and screening of full text articles, additional criteria were applied. Advagraf was launched in 2007, and outcome data with this formulation was mostly published from 2008 onward. Since combination immunosuppressive therapies after kidney transplantation have generally remained the same clinically since 2008 [13], publications before 2008 were excluded. Publications reporting only efficacy and safety outcomes before 6 months after transplantation were not considered. Treatment modifications after randomization can introduce a source of bias. Therefore, studies for which 1 or more treatments were withdrawn over the course of the trial were excluded.
The following data were extracted independently by 1 reviewer directly into a template: study characteristics (including study design, inclusion/exclusion criteria, interventions, sample size, and primary endpoints), patient baseline characteristics (including recipient and donor age, sex, race, body mass index [BMI], previous transplant history, and living and/or deceased donors), efficacy outcomes (patient survival, graft survival, acute rejection, and/or biopsy-proven acute rejection [BPAR], and timing of rejection), and safety outcomes. A second reviewer validated all data extracted. Discrepancies were resolved by discussion with a third reviewer. The risk of bias was assessed based on the recommendations of the Cochrane guide for systematic reviews [11].
NETWORK META-ANALYSIS:
The network meta-analysis was conducted according to the International Society for Pharmacoeconomics and Outcomes Research Taskforce guidelines [14] using methodologies based on the National Institute for Health and Care Excellence Decision Support Unit Technical Support Document [15]. In line with the focus of the present study, the efficacy endpoints analyzed were graft loss and mortality, examined for the period 6 to 12 months after transplantation. Rejection, NODAT, and CMV infection were not assessed. The studies evaluated a variety of TAC doses, and reporting was heterogenous. Therefore, the number of comparators was consolidated by combining treatments based on whether TAC was given QD or BID. The analysis assumed that maintenance therapies accounted for most of the treatment effects at 6 to 12 months. Data from the upper limit were used when studies reported outcomes for a time range.
Mycophenolate mofetil (MMF) is hydrolyzed to mycophenolic acid (MPA). Therefore, MMF and MPA were combined. All treatment combinations with MMF were labeled as MPA for consistency. The treatment of TAC BID plus MPA plus a corticosteroid (CS) was included in most networks and was therefore chosen as the reference treatment. Pairwise comparisons between all included treatments were used in the base case and meta-regression analyses.
The proportion of patients who experienced graft loss and mortality was modeled over 6 to 12 months [16]. The effect of race (White vs Black and/or Asian) as a covariate was included in the linear predictor model for each endpoint analysis. Imputed values were used for missing data. Estimates of the treatment effect were iteratively sampled using Bayesian methods. For binary endpoints, the mean log odds ratio (OR) was calculated. The credible intervals (CrI) were estimated. A 95% CrI indicates that there is a 95% probability that the true value of the parameter lies within the lower 2.5 and upper 97.5 percentiles. CrIs that included 1 were deemed to signify no significant difference in the effects of 2 treatments. Forest plots were constructed for graft loss and mortality. Meta-regression analyses were conducted to evaluate the effect of the proportion of White race in the patient population on both graft loss and mortality.
Results
Systematic Literature Review
OVERVIEW OF INCLUDED STUDIES: Following removal of duplicates, abstracts for 3574 unique publications were screened. Subsequently, 3288 publications were excluded, and 286 full-text articles were screened (Figure 1). Most conference abstracts were secondary to a primary full-text publication; however, 18 were included. Following full-text review, 78 publications detailing 61 primary articles (55 full text, 6 conference abstracts) and 17 secondary articles (5 full text, 12 conference abstracts) were included. Of these, 10 conference abstracts were secondary publications that did not contain any additional data to the primary publication. Therefore, this review focuses on 68 publications (61 primary and 7 secondary studies) that report efficacy and/or safety data (Table 1 and Supplementary Table 2) [17–79]. Among these publications, the most frequently evaluated regimens were combinations of TAC BID+MPA+CS (41 studies) and TAC QD+MPA+CS (22 studies). In studies in which the authors specified the name of the TAC formulation, Prograf was the most frequently used (21 studies); Advagraf (including Astagraf XL® and Graceptor®) was assessed in 8 studies.
PATIENT DEMOGRAPHICS AND CHARACTERISTICS: Table 2 and Supplementary Table 3 provide a summary of the key baseline characteristics among the 55 primary publications (ie, after excluding secondary publications [to avoid duplication] and conference abstracts [due to limited information]). Of the 20 primary studies in which 1 or more treatment arms used Advagraf or Prograf, mean or median age was reported in all studies except 1 (De Graav et al [25]). Mean age ranged from 29.36 years in the TAC BID+MPA+CS arm of Bakr et al to 53.60 years in the TAC BID+MPA+CS arm of Ferguson et al (Table 2) [22,28]. The proportion of female patients was also reported in all studies except 1 (Asher et al [21]). The percentage of female patients ranged from 17.1% of 35 patients in the TAC BID+MPA+CS arm of Arns et al to 76.2% of 21 patients in the TAC BID+sirolimus (SRL)+CS arm of Chen et al [19,23]. The proportion of female patients was ≤35% of the population in at least 1 study arm in 14 studies.
Six of the 20 primary studies, in which 1 or more treatment arms used Advagraf or Prograf, measured BMI at baseline, with mean values that varied from 21.8 kg/m2 to 29.2 kg/m2 [17,19,24,31,36,80]. Based on 2 studies, the percentage of patients with diabetes in individual treatment arms was between 6.9% of 390 patients and 28.3% of 212 patients [26,37]. People of White race (11 studies) accounted for between 65.8% of 243 and 95.8% of 309 patients in individual treatment arms [17,19,24–26,28,29,32,35,37,80]. People of Black race accounted for between 0.9% of 117 and 26.7% of 243 patients in individual treatment arms [17,25,26,28,29,32,35,37,80]. Ten studies reported the proportions of patients of Asian race, which varied from 0% of 107 to 100% of 20 to 63 patients in individual treatment arms [17,18,23,25,26,32,35–37,80] (Table 2).
ACUTE REJECTION AND BIOPSY-PROVEN ACUTE REJECTION: Overall, 64 studies reported rates of acute rejection and BPAR (Table 3 and Supplementary Table 4). Of the 21 studies that reported on graft rejection following treatment with Advagraf or Prograf (Table 3), the rates of BPAR at 12 months varied from 3.3% (1/30 patients) and 3.8% (1/26 patients) with TAC BID+MPA+CS and SRL+CS, respectively, to 54.2% (of 296 patients) with SRL+MPA+CS and 55.0% (11/20 patients) with MPA+CS [25,26,28]. Liu et al reported BPAR at 24 months, which was 5.6% (2/36 patients) and 8.3% (3/36 patients) with cyclosporin (CsA) BID+MPA+CS and TAC BID+MPA+CS, respectively [36].
Four of the studies that included treatment with Advagraf or Prograf reported outcomes beyond 24 months [20,30,34,80]. Hamdy et al reported no rejection episodes in 132 patients beyond the second year of follow-up [30]. Harland et al reported BPAR rates of 34.1% (15/44 patients), 35.4% (17/48 patients), and 58.7% (27/46 patients) in the TAC BID+bleselumab (BLES)+CS, TAC BID+MPA+CS, and BLES+MPA+CS arms, respectively, at 36 months [80]. Between 6 and 36 months after transplantation in the ATLAS study, the BPAR rates were 2.1% (3/143 patients), 2.2% (3/139 patients), and 2.9% (4/139 patients) in the TAC BID, TAC BID+MPA, and TAC BID+MPA+CS arms, respectively [34]. Arriola et al reported acute rejection rates of 12.7% (of 55 patients) and 11.5% (of 52 patients) in the TAC BID+MPA+SRL+CS and TAC QD+MPA+everolimus (EVR)+CS arms, respectively, at 12 months and 16.6% of 55 patients and 15.3% of 52 patients, respectively, after 6 years [20].
Two studies reported T cell-mediated rejection between 12 and 24 months after transplantation [23,24]. In the study of Chen et al, a single patient in each group (CsA BID+SRL+CS and TAC BID+SRL+CS) experienced Banff IA BPAR (~5% of patients in both groups) [23]. In the study of Cockfield et al, the T cell-mediated rejection rates, including borderline changes after 24 months of follow-up, were 19.8% (14 patients) and 39.6% (27 patients) for low-dose TAC QD arms comprising angiotensin-converting enzyme inhibitor/angiotensin II receptor 1 blocker (ACEi/ARB) and other antihypertensive, respectively, and 24.2% (17 patients) and 16.5% (12 patients) with standard-dose TAC QD regimens comprising ACEi/ARB and other antihypertensive, respectively [24].
GRAFT LOSS: Graft loss was reported in 14 studies of transplant recipients during follow-up of between 6 and 36 months for TAC QD or TAC BID (Advagraf or Prograf) (Table 4) and in a further 28 studies with follow-up of up to 8 years in which the TAC formulation was not reported (Supplementary Table 5).
Of the 14 studies that reported graft rejection at 6 to 12 months following treatment with Advagraf or Prograf, there was variation in the incidence of graft loss, depending on the study population (intention-to-treat, per-protocol, or full analysis set) and the time since transplantation. In studies in which the TAC formulation was known, the QD regimen was associated with graft loss of between 0% [22,31,38] and 9.6% (29/302 patients) [17], while graft loss for the BID regimen ranged from 0% [22,23,25,28,33,38] to 7.5% (8/107 patients) [35]. Across the studies investigating Advagraf or Prograf, the highest rate of graft loss (15.0%; 3/20 patients) was with MPA+CS [25].
MORTALITY: Patient mortality was reported in 19 studies in which the formulation of TAC QD or TAC BID was known (Advagraf or Prograf) (Table 5) and in a further 32 publications in which the TAC formulation was not reported (Supplementary Table 6). Of the Advagraf or Prograf studies, between 6 and 24 months, mortality ranged from 0% (9 studies, various regimens) to 8.1% (5/62 patients) reported with TAC BID_0.08–0.125+MPA+CS (in which the TAC dose was adjusted between 0.08 and 0.125 mg/kg according to CYP3A5 genotype) in the study by Anutrakulchai et al [18]. The highest mortality rates were reported at 6 years in the study of Arriola et al (13.4% and 14.5% with TAC QD+MPA+EVR+CS and TAC BID+MPA+SRL+CS, respectively, although the numbers of deaths and patients treated were not reported) [20].
CMV INFECTION: CMV infection was reported in 14 studies in which the formulation of TAC QD or TAC BID was known (Advagraf or Prograf) (Supplementary Table 7) and in 30 studies in which the TAC formulation was not reported (Supplementary Table 8). Of the Advagraf or Prograf studies, incidence ranged from 1.3% (1/76 patients) with TAC QD+SRL QD+CS [31] to 15.4% (59/384 patients) with CsA BID+MPA+CS [26,27]. In the 13 primary studies in which the formulation of TAC QD or TAC BID was known (Advagraf or Prograf), the assessment period ranged from 6 months to 4 years.
NODAT: The incidence of NODAT was reported in 12 publications in which the formulation of TAC QD or TAC BID was known (Advagraf or Prograf) (Supplementary Table 9) and in 20 studies in which the TAC formulation was not reported (Supplementary Table 10). Of the Advagraf or Prograf studies, the incidence of NODAT ranged from 0% with CsA BID_Std+MPA+CS, SRL QD+MPA+CS, CsA BID_Low+MPA+CS, MPA+CS, and TAC BID+MPA+CS, all reported at 12 months [26,28,38], to 57.6% (19 patients) at 6 months with TAC BID+MPA+CS [80]. The longest assessment period for reporting NODAT was 6 years, with an incidence of 12.7% with TAC BID+MPA+SRL+CS and 11.5% with TAC QD+MPA+EVR+CS (number of patients not reported) [20].
RISK OF BIAS: Across the studies, most domains were at low risk of bias. Risk of bias among the 61 primary studies is shown in Figure 2. Thirty studies were assessed as being at high risk of bias in 1 or more domains. Of these, most studies had a high risk of bias in 1 domain, 4 studies had a high risk of bias in 2 domains, and 1 study had a high risk of bias in 3 domains.
NETWORK META-ANALYSIS:
Data for mortality and graft loss are presented for the random-effects model, which was a better fit based on deviance information criterion (posterior mean deviance plus the number of parameters) and total residual deviance than fixed-effects models. Among the 61 included primary publications, the network analysis was based on 27 studies that evaluated graft loss or mortality. Of these, the formulation of TAC was named as Advagraf or Prograf in 9 studies [17,22,23,25,27,28,32,33,38], and the TAC formulation was not cited in the remaining 18 studies [39,42,44,45,48–50,52–62]. Figures 3 and 4 show the network diagrams for graft loss and mortality rates, respectively. The networks are connected, with no studies disconnected from the larger network of evidence.
GRAFT LOSS: The ORs of graft loss (6–12 months) compared with the reference treatment Prograf+MPA+CS, adjusted for White race, are presented in Supplementary Table 11. Advagraf+MPA+CS and Prograf+MPA+CS were associated with a statistically similar risk of graft loss (median OR 1.19; 95% CrI 0.51, 3.06) since the CrI includes 1. Compared with Prograf+MPA+CS, the risk of graft loss was higher with TAC BID+EVR BID+CS (median OR 3.0; 95% CrI 1.03, 11.36), MPA+CS (median OR 9.49; 95% CrI 2.12, 58.56), and SRL QD+CS (median OR 13.60; 95% CrI 1.02, 221.41). Figure 5 shows the Forest plots for the random-effects model of graft loss. The following treatments were associated with significantly higher odds of graft loss at 6 to 12 months compared with the reference treatment, Prograf+MPA+CS: TAC BID+EVR BID+CS (OR 3.00; 95% CrI 1.03, 11.33), MPA+CS (OR 9.46; 95% CrI 2.11, 58.30), and SRL QD+CS (OR 13.64; 95% CrI 1.02, 221.77). No treatments were associated with significantly lower odds of graft loss than Prograf+MPA+CS. In the meta-regression model adjusted for White race, the effect estimate of 0.04 (95% CrI −0.07, 0.17) indicated that race did not significantly affect graft loss.
MORTALITY: The adjusted ORs of mortality (6–12 months) compared with the reference treatment, Prograf+MPA+CS, are presented in Supplementary Table 11. Advagraf+MPA+CS and Prograf+MPA+CS were associated with a significantly similar risk of mortality (median OR 1.21; 95% CrI 0.1557, 9.03). The risk of mortality associated with each evaluated treatment regimen, including those incorporating TAC QD, TAC BID, and agents other than TAC, was similar to that associated with the Prograf+MPA+CS reference regimen, since the CrI for each comparison includes 1. Figure 6 shows the Forest plots for the random-effects model for mortality. All treatments were associated with a significantly similar risk of mortality at 6 to 12 months compared with Prograf+MPA+CS. For the adjusted model, the covariate for White (%) was −0.031 (95% CrI −0.089, 0.027) and did not indicate that race significantly affected mortality.
Discussion
This systematic literature review and network meta-analysis included randomized controlled trials evaluating TAC immunosuppression in kidney transplant recipients. To the best of our knowledge, this is the most up-to-date systematic review and network meta-analysis of the literature, summarizing the most recent trials relating to TAC. No notable differences were evident from the network meta-analysis between known formulations of TAC QD and TAC BID in relation to graft loss or patient mortality.
Once-daily dosing with TAC is expected to improve overall adherence and potentially improve exposure to immunosuppressive therapy and transplant outcomes, compared with twice-daily dosing [8,9]. However, findings to date have been partially contradictory. A previous systematic literature review compared 12-month post-kidney transplant outcomes between TAC QD and TAC BID in randomized controlled trials and observational studies, with the authors finding no significant differences between the regimens in relation to BPAR, graft survival, or patient survival [81]. By contrast, a more recent systematic literature and meta-analysis of observational studies revealed a 30% lower risk of BPAR at 12 months with TAC QD compared with TAC BID, with no difference in graft or patient survival or kidney function [82]. Herein, none of the treatments analyzed was associated with increased (or decreased) mortality risk compared with the reference regimen, Prograf+MPA+CS. Three regimens, TAC BID+EVR BID+CS, MPA+CS, and SRL+CS, were associated with increased odds of graft loss compared with the reference regimen. The remaining regimens, including those with TAC QD, appeared to offer similar protection against graft loss. The wide CrIs for many of the pairwise comparisons could be accounted for by the small sample sizes for some of the treatment comparisons.
While we did not find evidence for improved transplant outcomes with TAC QD compared with TAC BID, the studies included in the review (and indeed in the previous reviews cited above) were limited regarding the long-term data available for assessment. Data were mainly confined to 6 to 12 months after transplantation, and there were limited published reports of longer-term outcomes, preventing comprehensive analyses beyond this time period. As such, there is need for more prospective, long-term studies to evaluate efficacy outcomes, and it may be useful to stratify patient outcomes by adherence with immunosuppressive regimens and other factors, such as patient characteristics, which could impact post-transplant outcomes.
In the model adjusted for White race, the small, non-significant effect size of the covariate suggested there was limited evidence of a relationship between race (White vs Black and Asian) and graft loss or mortality. However, adequately powered prospective studies are needed to determine any potential effect associated with race or pharmacogenomic variations, as it is known that race can impact TAC dose requirements, for instance [83].
This systematic review and network meta-analysis had limitations. Pooling of results was limited by heterogeneity in the reporting of rejection outcomes, as the terms acute rejection and BPAR were used interchangeably. Furthermore, methods of determining acute rejection were heterogeneous, ranging from clinical judgment and laboratory values to biopsy, and therefore, comparing data across studies was problematic. Several studies did not provide detail regarding TAC formulation, dosing characteristics and trough concentrations. In addition, some studies allowed dose adjustments based on target TAC trough levels at various time points, while other studies did not state whether dose adjustments were allowed. Due to this lack of consistency, it was not always possible to determine whether TAC was administered at a bioequivalent dose across the studies, which in turn could undermine the interpretation of treatment effects.
As some studies were designed to assess the efficacy of induction therapy, they were not necessarily powered to examine the effectiveness of TAC-based maintenance immunosuppression. In addition, for the network meta-analysis, treatments were grouped according to QD or BID dosing of TAC, rather than the dosages of other medications, and so some studies effectively became single-arm trials and were excluded from the analysis because they lacked a comparator. Furthermore, numerous factors influence short- and long-term graft survival, including donor and recipient matching and recipient characteristics, such as age, sex, and history of prior transplantation [84]. These factors were often reported inconsistently across the studies we analyzed. For example, only 2 studies reported the percentage of patients with diabetes [26,37], despite diabetes being a known risk factor for renal transplant outcomes, including lower graft and patient survival [85,86]. However, despite these limitations, to the best of our knowledge, this is the most current systematic review of TAC-based maintenance regimens in kidney transplant recipients.
Conclusions
In this comprehensive review, the treatment effect on graft loss and patient mortality at 6 to 12 months after transplantation was comparable between regimens of QD TAC (Advagraf) and BID TAC (Prograf) when combined with MPA and CS. Race did not significantly impact graft loss and mortality based on the regimen used, although this finding may be limited by the heterogeneity among studies. Conclusions regarding graft rejection were unclear due to lack of consistency in definitions between the studies. Further prospective research may be necessary to evaluate the long-term outcomes of and factors optimizing maintenance immunosuppression in kidney transplant recipients.
Figures
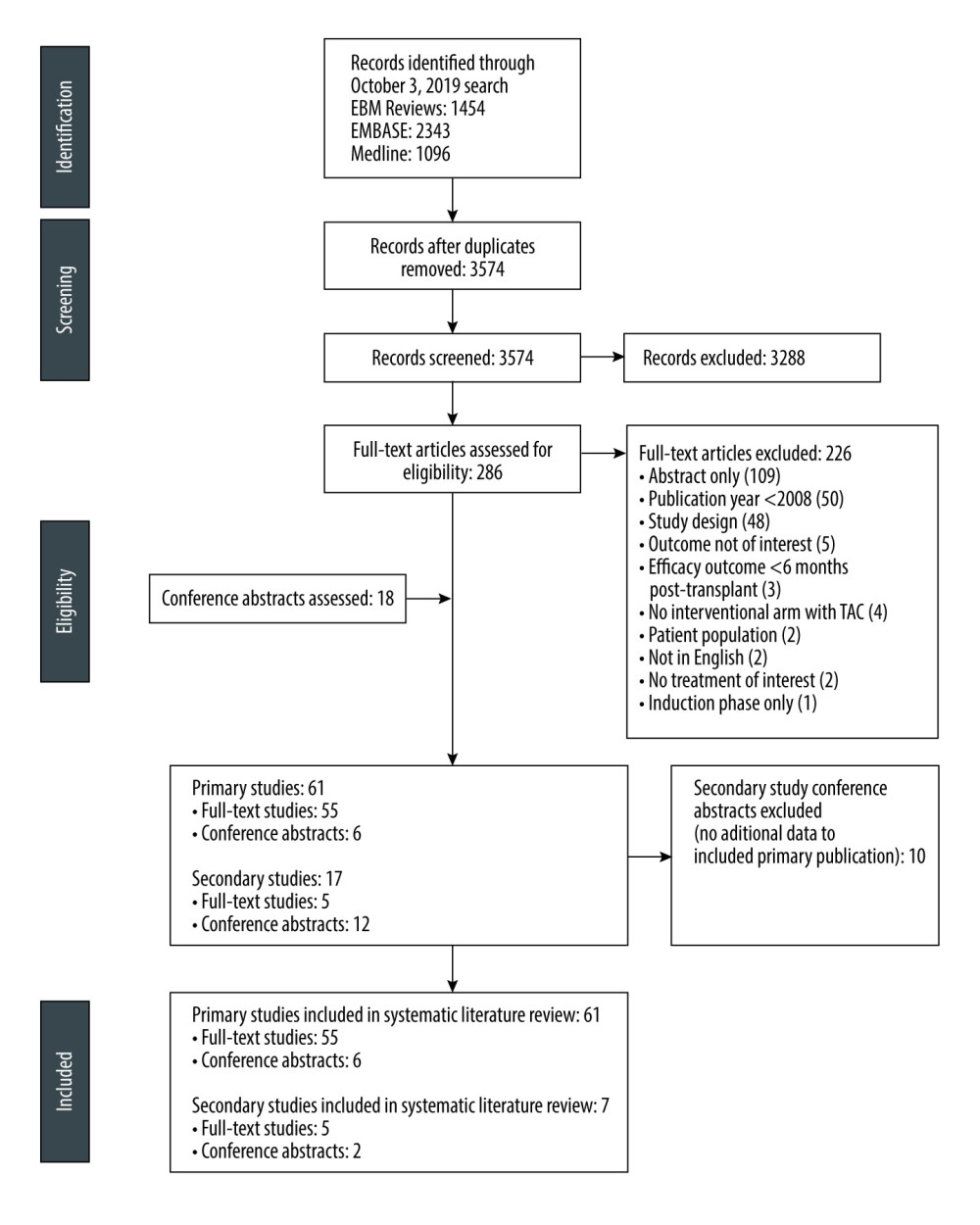 Figure 1. PRISMA flow diagramFigure created using PowerPoint for Microsoft 365, Microsoft. EBM – evidence-based medicine; PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-analyses; TAC – tacrolimus.
Figure 1. PRISMA flow diagramFigure created using PowerPoint for Microsoft 365, Microsoft. EBM – evidence-based medicine; PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-analyses; TAC – tacrolimus. 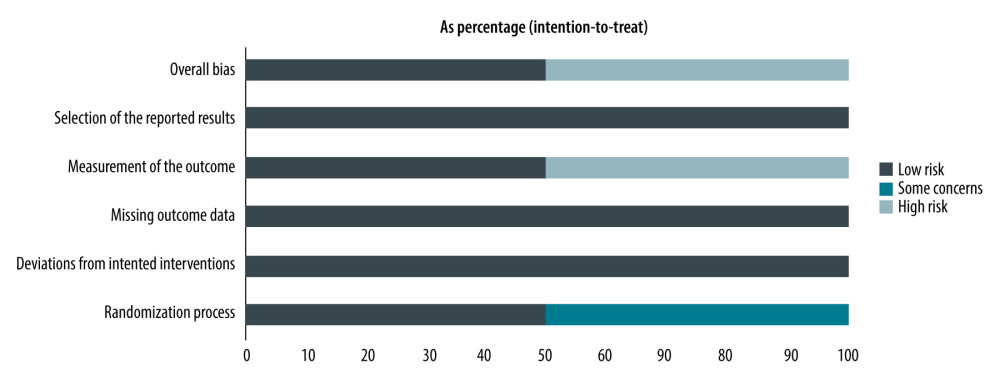 Figure 2. Risk of bias among the 61 primary studies as a percentage of the total included studiesRisk of bias was assessed based on the recommendations of the Cochrane guide for systematic reviews. Figure created using R 3.6.0, The R Foundation.
Figure 2. Risk of bias among the 61 primary studies as a percentage of the total included studiesRisk of bias was assessed based on the recommendations of the Cochrane guide for systematic reviews. Figure created using R 3.6.0, The R Foundation. 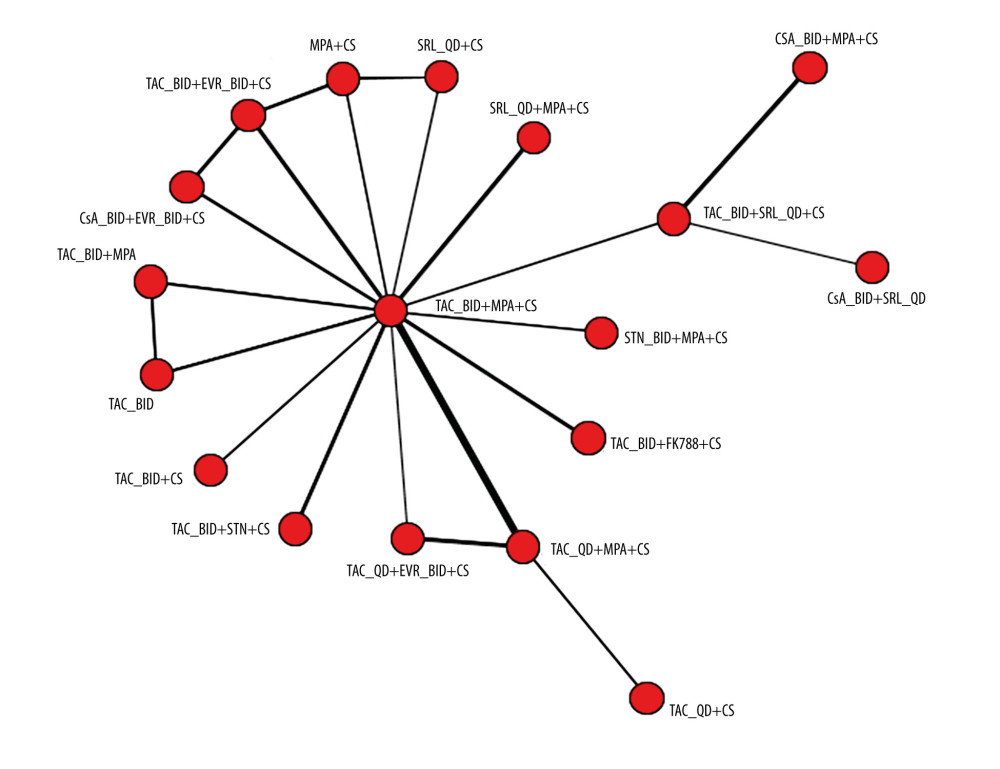 Figure 3. Network plot for graft loss at 6 to 12 monthsThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 3. Network plot for graft loss at 6 to 12 monthsThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. 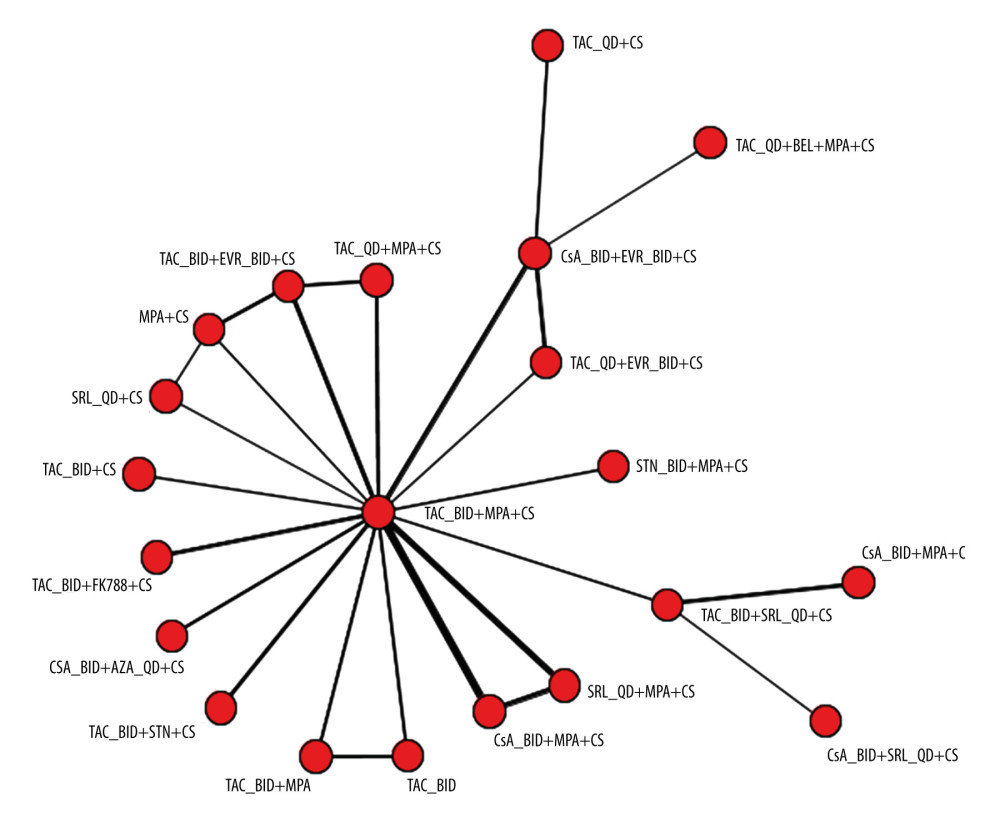 Figure 4. Network plot for mortality rate at 6 to 12 monthsThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 4. Network plot for mortality rate at 6 to 12 monthsThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. 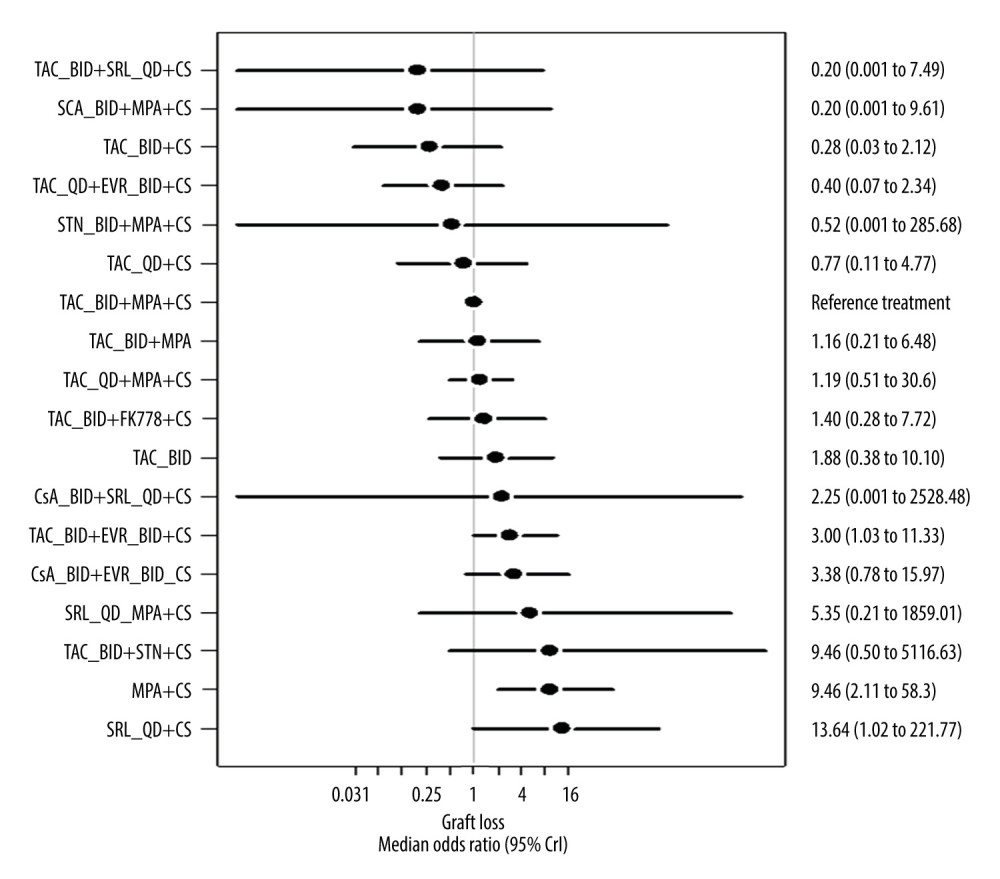 Figure 5. Forest plot for the random-effects model for graft loss (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 5. Forest plot for the random-effects model for graft loss (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. 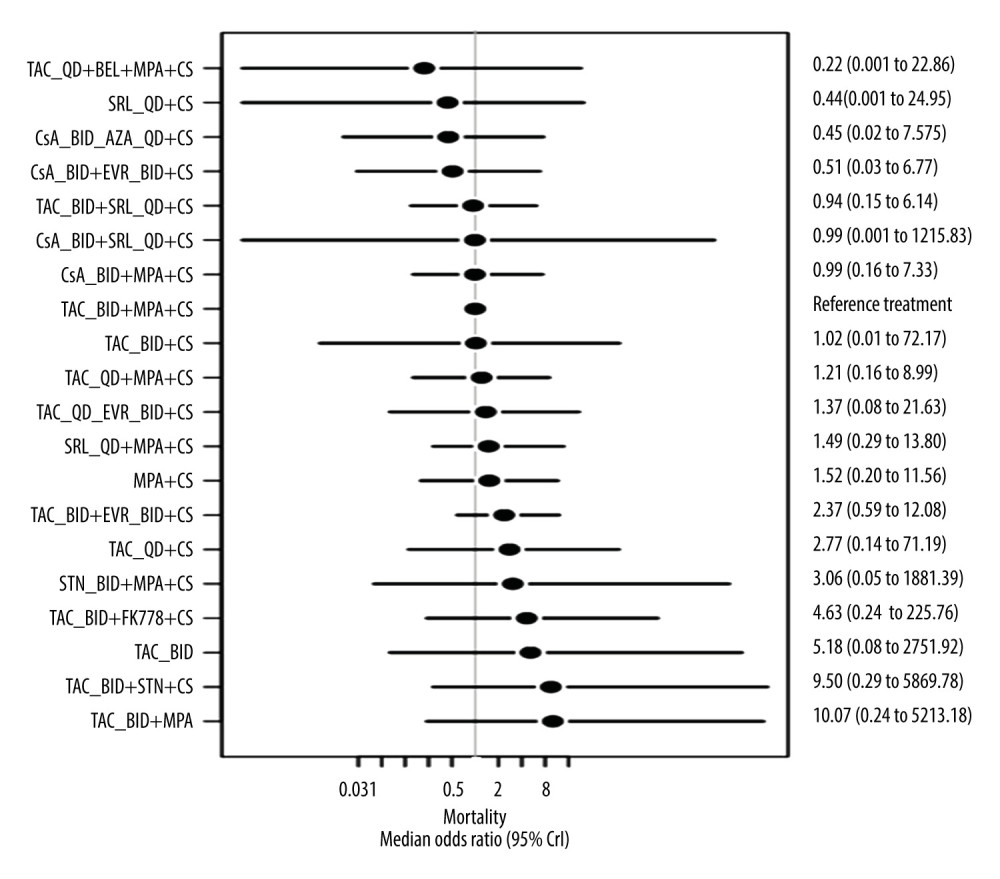 Figure 6. Forest plot for the random-effects model for mortality (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 6. Forest plot for the random-effects model for mortality (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. Tables
Table 1. Overview of included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 2. Key baseline characteristics among primary publications of studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 2. Key baseline characteristics among primary publications of studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 3. Rejection outcomes: acute rejection and biopsy-proven acute rejection among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 3. Rejection outcomes: acute rejection and biopsy-proven acute rejection among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.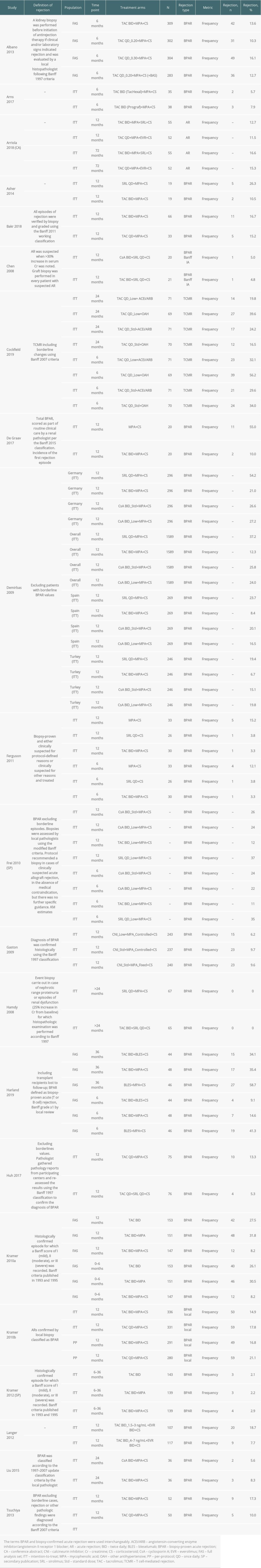 Table 4. Graft loss among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 4. Graft loss among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.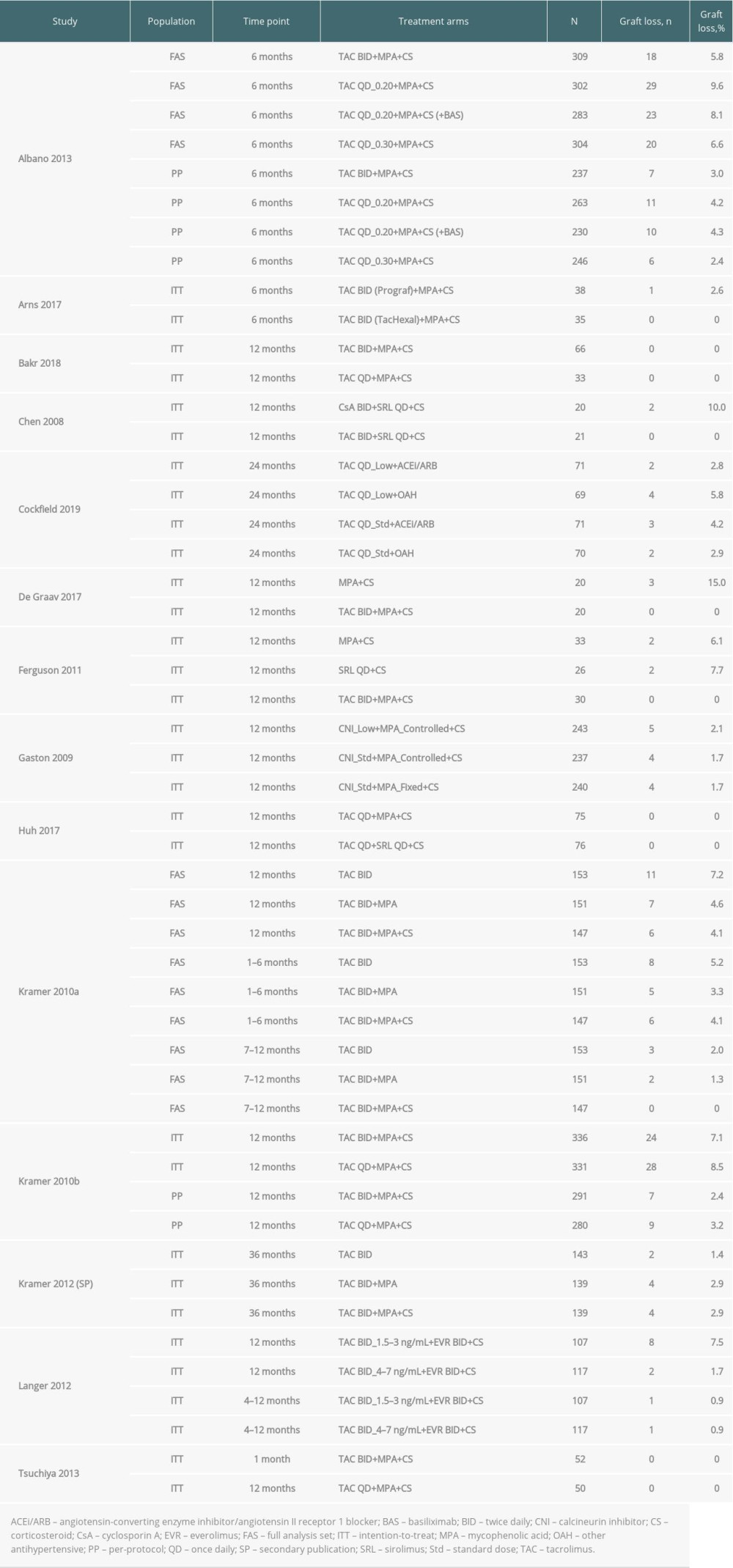 Table 5. Patient death among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 5. Patient death among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.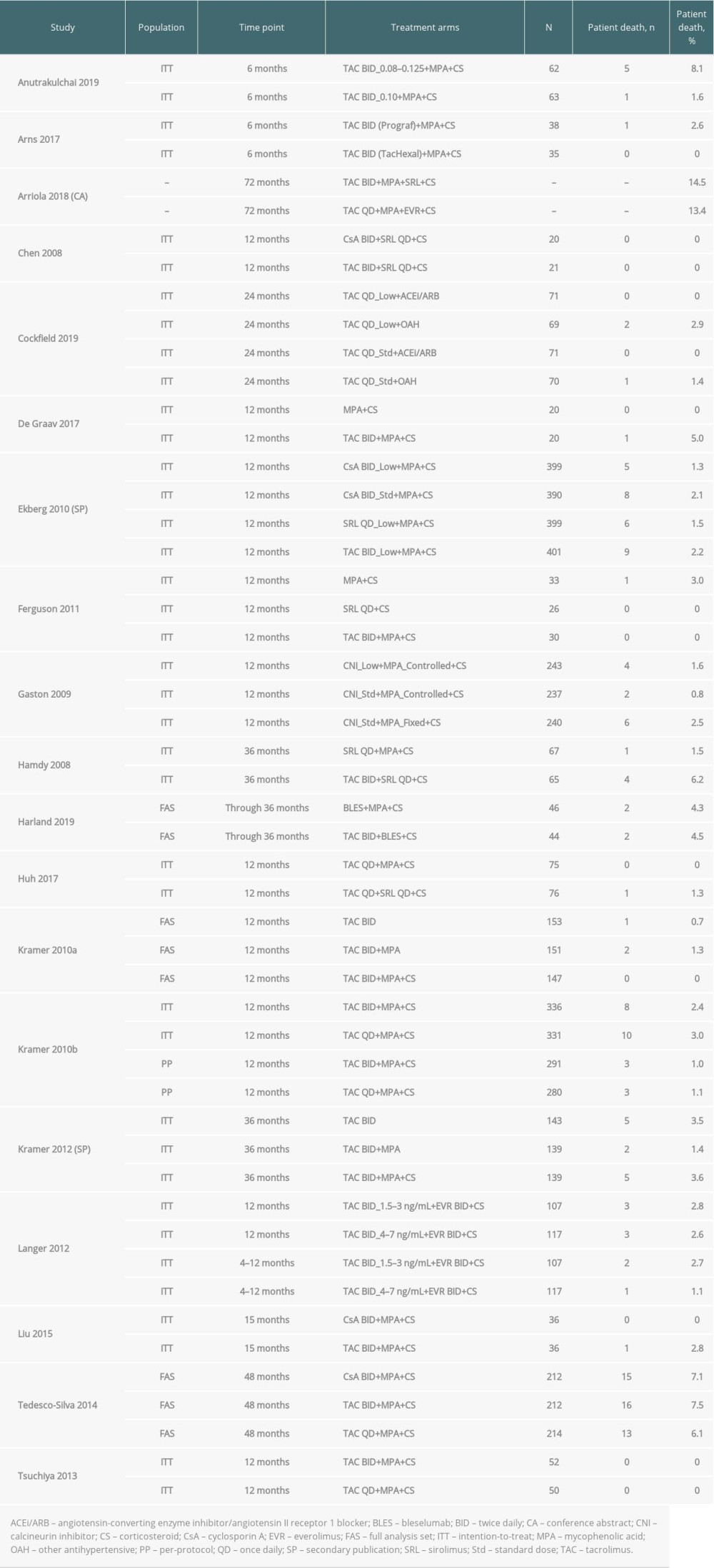
References
1. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group, KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease: Kidney Int Suppl, 2013; 3; 100-50
2. Inker LA, Astor BC, Fox CH, KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD: Am J Kidney Dis, 2014; 63; 713-35
3. Garcia GG, Harden P, Chapman J, The global role of kidney transplantation: Curr Opin Organ Transplant, 2012; 17; 362-67
4. Butler JA, Roderick P, Mullee M, Frequency and impact of nonadherence to immunosuppressants after renal transplantation: A systematic review: Transplantation, 2004; 77; 769-76
5. Banas B, Krämer BK, Krüger B, Long-term kidney transplant outcomes: Role of prolonged-release tacrolimus: Transplant Proc, 2020; 52; 102-10
6. Chisholm-Burns MA, Spivey CA, Sredzinski E, Butler SL, Intervention toolbox to promote immunosuppressant therapy adherence in adult renal transplant recipients: J Am Pharm Assoc (2003), 2012; 52; 816-22
7. Fellström B, Holmdahl J, Sundvall N, Adherence of renal transplant recipients to once-daily, prolonged-release and twice-daily, immediate-release tacrolimus-based regimens in a real-life setting in Sweden: Transplant Proc, 2018; 50; 3275-82
8. Cassuto E, Pageaux GP, Cantarovich D, Adherence to and acceptance of once-daily tacrolimus after kidney and liver transplant: Transplantation, 2016; 100; 2099-106
9. Oh C-K, Bang JB, Kim S-J, Improvement of medication adherence with simplified once-daily immunosuppressive regimen in stable kidney transplant recipients: A prospective cohort study: Asian J Surg, 2020; 43; 660-67
10. Centre for Reviews and Dissemination: Systematic reviews: CRD’s guidance for undertaking reviews in healthcare, 2009 Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf
11. Higgins JPT, Green S: Cochrane Handbook for Systematic Reviews of Interventions, 2011, The Cochrane Collaboration
12. Hutton B, Salanti G, Caldwell DM, The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations: Ann Intern Med, 2015; 162; 777-84
13. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group, KDIGO clinical practice guideline for the care of kidney transplant recipients: Am J Transplant, 2009; 9; S1-155
14. Hoaglin DC, Hawkins N, Jansen JP, Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2: Value Heal, 2011; 14; 429-37
15. Dias S, Welton NJ, Sutton AJ, Ades A: NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/A-general-linear-modelling-framework-for-pair-wise-and-network-meta-analysis-of-randomised-controlled-trials.pdf
16. Dias S, Welton NJ, Sutton AJ: NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD4-Inconsistency.final_.15April2014.pdf
17. Albano L, Banas B, Klempnauer JL, OSAKA trial: A randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation: Transplantation, 2013; 96; 897-903
18. Anutrakulchai S, Pongskul C, Kritmetapak K, Therapeutic concentration achievement and allograft survival comparing usage of conventional tacrolimus doses and CYP3A5 genotype-guided doses in renal transplantation patients: Br J Clin Pharmacol, 2019; 85; 1964-73
19. Arns W, Huppertz A, Rath T, Pharmacokinetics and clinical outcomes of generic tacrolimus (hexal) versus branded tacrolimus in de novo kidney transplant patients: A multicenter, randomized trial: Transplantation, 2017; 101; 2780-88
20. Arriola M, Gaite LE, Favalli C, Advagraf de novo. Use comparative of Advagraf compared with Prograf: Transplantation, 2018; 102; S596
21. Asher J, Vasdev N, Wyrley-Birch H, A prospective randomised paired trial of sirolimus versus tacrolimus as primary immunosuppression following non-heart beating donor kidney transplantation: Curr Urol, 2014; 7; 174-80
22. Bakr MA, Nagib AM, Donia AF, Comparative analysis for optimizing the modified release tacrolimus (Advagraf) after kidney transplantation: A prospective randomized trial: Saudi J kidney Dis Transplant, 2018; 29; 1267-73
23. Chen K-H, Tsai M-K, Lai I-R, Favorable results of concomitant tacrolimus and sirolimus therapy in Taiwanese renal transplant recipients at 12 months: J Formos Med Assoc, 2008; 107; 533-39
24. Cockfield SM, Wilson S, Campbell PM, Comparison of the effects of standard versus low-dose prolonged-release tacrolimus with or without ACEi/ARB on the histology and function of renal allografts: Am J Transplant, 2019; 19; 1730-44
25. De Graav GN, Baan CC, Clahsen-Van Groningen MC, A randomized controlled clinical trial comparing belatacept with tacrolimus after de novo kidney transplantation: Transplantation, 2017; 101; 2571-81
26. Demirbas A, Hugo C, Grinyó J, Low toxicity regimens in renal transplantation: A country subset analysis of the Symphony study: Transpl Int, 2009; 22; 1172-81
27. Ekberg H, Bernasconi C, Nöldeke J, Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study: Nephrol Dial Transplant, 2010; 25; 2004-10
28. Ferguson R, Grinyó J, Vincenti F, Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients: Am J Transplant, 2011; 11; 66-76
29. Gaston RS, Kaplan B, Shah T, Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial: Am J Transpl, 2009; 9; 1607-19
30. Hamdy AF, Bakr MA, Ghoneim MA, Long-term efficacy and safety of a calcineurin inhibitor-free regimen in live-donor renal transplant recipients: J Am Soc Nephrol, 2008; 19; 1225-32
31. Huh KH, Lee JG, Ha J, De novo low-dose sirolimus versus mycophenolate mofetil in combination with extended-release tacrolimus in kidney transplant recipients: A multicentre, open-label, randomized, controlled, non-inferiority trial: Nephrol Dial Transplant, 2017; 32; 1415-24
32. Krämer BK, Charpentier B, Bäckman L, Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized Phase III study: Am J Transplant, 2010; 10; 2632-43
33. Krämer BK, Klinger M, Wlodarczyk Z, Tacrolimus combined with two different corticosteroid-free regimens compared with a standard triple regimen in renal transplantation: one year observational results: Clin Transplant, 2010; 24; E1-9
34. Krämer BK, Klinger M, Vítko Š, Tacrolimus-based, steroid-free regimens in renal transplantation: 3-year follow-up of the ATLAS trial: Transplantation, 2012; 94; 492-98
35. Langer RM, Hené R, Vitko S, Everolimus plus early tacrolimus minimization: A Phase III, randomized, open-label, multicentre trial in renal transplantation: Transpl Int, 2012; 25; 592-602
36. Liu L-S, Li J, Chen X-T, Comparison of tacrolimus and cyclosporin A in CYP3A5 expressing Chinese de novo kidney transplant recipients: a 2-year prospective study: Int J Clin Pract Suppl, 2015; 183; 43-52
37. Tedesco-Silva H, Yang HC, Meier-Kriesche H-U, Long-term follow-up of a Phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients: Transplantation, 2014; 97; 636-41
38. Tsuchiya T, Ishida H, Tanabe T, Comparison of pharmacokinetics and pathology for low-dose tacrolimus once-daily and twice-daily in living kidney transplantation: Prospective trial in once-daily versus twice-daily tacrolimus: Transplantation, 2013; 96; 198-204
39. Banham GD, Flint SM, Torpey N, Belimumab in kidney transplantation: An experimental medicine, randomised, placebo-controlled Phase 2 trial: Lancet, 2018; 391; 2619-30
40. Chan L, Greenstein S, Hardy MA, Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness: Transplantation, 2008; 85; 821-26
41. Cheung CY, Chan HW, Liu YL, Long-term graft function with tacrolimus and cyclosporine in renal transplantation: Paired kidney analysis: Nephrology, 2009; 14; 758-63
42. de Sandes-Freitas TV, Pinheiro PMA, de MBO Sales ML, The impact of everolimus in reducing cytomegalovirus events in kidney transplant recipients on steroid-avoidance strategy: 3-year follow-up of a randomized clinical trial: Transpl Int, 2018; 31; 1345-56
43. Ding C, Xue W, Tian P, Which is more suitable for kidney transplantation at the early post-transplantation phase in China – low dosing or standard dosing of enteric-coated mycophenolate sodium?: Int J Clin Pract Suppl, 2014; 181; 10-16
44. Ferreira AN, Felipe CR, Cristelli M, Prospective randomized study comparing everolimus and mycophenolate sodium in de novo kidney transplant recipients from expanded criteria deceased donor: Transpl Int, 2019; 32; 1127-43
45. Friman S, Arns W, Nashan B, Sotrastaurin, a novel small molecule inhibiting protein-kinase C: Randomized Phase II study in renal transplant recipients: Am J Transplant, 2011; 11; 1444-55
46. Gatault P, Kamar N, Büchler M, Reduction of extended-release tacrolimus dose in low-immunological-risk kidney transplant recipients increases risk of rejection and appearance of donor-specific antibodies: A randomized study: Am J Transplant, 2017; 17; 1370-79
47. Guerra G, Ciancio G, Gaynor JJ, Randomized trial of immunosuppressive regimens in renal transplantation: J Am Soc Nephrol, 2011; 22; 1758-68
48. Hoitsma AJ, Woodle ES, Abramowicz D, FTY720 combined with tacrolimus in de novo renal transplantation: 1-year, multicenter, open-label randomized study: Nephrol Dial Transplant, 2011; 26; 3802-5
49. Kojima CA, Nga HS, Takase HM, Sirolimus associated with tacrolimus at low doses in elderly kidney transplant patients: A prospective randomized controlled trial: Exp Clin Transplant, 2018; 16; 301-6
50. Margreiter R, Klempnauer J, Neuhaus P, Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: Results of a prospective randomized trial: Am J Transplant, 2008; 8; 1480-85
51. Pascual J, Berger SP, Witzke O, Everolimus with reduced calcineurin inhibitor exposure in renal transplantation: J Am Soc Nephrol, 2018; 29; 1979-91
52. Qazi Y, Shaffer D, Kaplan B, Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data: Am J Transplant, 2017; 17; 1358-69
53. Russ GR, Tedesco-Silva H, Kuypers DR, Efficacy of sotrastaurin plus tacrolimus after de novo kidney transplantation: Randomized, Phase II trial results: Am J Transplant, 2013; 13; 1746-56
54. Sampaio EL, Pinheiro-Machado PG, Garcia R, Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus-based immunosuppressive regimen: Clin Transplant, 2008; 22; 141-49
55. Sommerer C, Suwelack B, Dragun D, An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients: Kidney Int, 2019; 96; 231-44
56. Suszynski TM, Gillingham KJ, Rizzari MD, Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation: Am J Transplant, 2013; 13; 961-70
57. Tedesco-Silva H, Felipe C, Ferreira A, Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses: Am J Transplant, 2015; 15; 2655-64
58. Traitanon O, Mathew JM, Shetty A, Mechanistic analyses in kidney transplant recipients prospectively randomized to two steroid free regimen-low dose tacrolimus with everolimus versus standard dose tacrolimus with mycophenolate mofetil: PLoS One, 2019; 14; e0216300
59. Vacher-Coponat H, Moal V, Indreies M, A randomized trial with steroids and antithymocyte globulins comparing cyclosporine/azathioprine versus tacrolimus/mycophenolate mofetil (CATM2) in renal transplantation: Transplantation, 2012; 93; 437-43
60. Weir MR, Mulgaonkar S, Chan L, Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: A randomized, controlled Spare-the-Nephron trial: Kidney Int, 2011; 79; 897-907
61. Welberry Smith MP, Cherukuri A, Newstead CG, Alemtuzumab induction in renal transplantation permits safe steroid avoidance with tacrolimus monotherapy: A randomized controlled trial: Transplantation, 2013; 96; 1082-88
62. Wlodarczyk Z, Vanrenterghem Y, Krämer BK, A multicenter, randomized, double-blind study comparing different FK778 doses (manitimus) with tacrolimus and steroids vs. MMF with tacrolimus and steroids in renal transplantation: BMC Nephrol, 2012; 13; 68
63. Busque S, Cantarovich M, Mulgaonkar S, The PROMISE study: A Phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation: Am J Transplant, 2011; 11; 2675-84
64. Ciancio G, Burke GW, Gaynor JJ, A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow-up: Clin Transplant, 2008; 22; 200-10
65. Ciancio G, Burke GW, Gaynor JJ, Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplant recipients given tacrolimus and daclizumab/thymoglobulin: One year follow-up: Transplantation, 2008; 86; 67-74
66. Feng JJ, Zhang LW, Zhao P, Enteric-coated mycophenolate sodium given in combination with tacrolimus has a lower incidence of serious infections in Asian renal-transplant recipients compared with mycophenolate mofetil: Int J Clin Pract, 2015; 69; 1-7
67. Garcia VD, Carvalho DBM, Gonçalves RT, Randomized trial of early corticosteroid reduction vs. regular-dose corticosteroid maintenance in combination with tacrolimus and mycophenolate mofetil in living donor kidney transplant recipients: The Brazilian CORRETA trial: Clin Transplant, 2010; 24; E109-15
68. Nematalla AH, Bakr MA, Gheith OA, Steroid-avoidance immunosuppression regimen in live-donor renal allotransplant recipients: A prospective, randomized, controlled study: Exp Clin Transpl, 2007; 5; 673-79
69. Hidaka Y, Yamanaga S, Toyoda M, One year outcomes of low-dose vs very low-dose extended-release tacrolimus/mycophenolate mofetil in de novo kidney transplantation: A multi-center randomized controlled trial: Am J Transpl, 2017; 17; 739 [Abstract D87]
70. Ishida H, Takahara S, Amada N, A prospective randomized, comparative trial of high-dose mizoribine versus mycophenolate mofetil in combination with tacrolimus and basiliximab for living donor renal transplant: A multicenter trial: Exp Clin Transpl, 2016; 14; 518-25
71. Kim Y, Chiang Y, Kim S, Prolonged-release tacrolimus dosing in de novo kidney transplantation: Randomized, open-label, pilot study: Am J Transpl, 2018; 18; 808 [Abstract C118]
72. Narumi S, Watarai Y, Goto N, Comparison of low dose and very low dose extended-release tacrolimus/MMF in de novo kidney transplant recipients-3 years follow-up: Am J Transpl, 2018; 18; 802 [Abstract C100]
73. Nashan B, Tedesco H, van den Hoogen M, CFZ533, a new anti-CD40 mAB demonstrates comparable efficacy and better renal function versus tacrolimus in de-novo CNI-free kidney transplantation: Am J Transpl, 2018; 18; 400 [Abstract 400]
74. Okumi M, Unagami K, Furusawa M, Once-daily vs twice-daily tacrolimus for de novo living kidney transplantation patients including ABO/HLA compatible and incompatible: A randomized trial: Clin Transplant, 2018; 32; e13423
75. Shihab F, Qazi Y, Mulgaonkar S, Association of clinical events with everolimus exposure in kidney transplant patients receiving low doses of tacrolimus: Am J Transplant, 2017; 17; 2363-71
76. Spagnoletti G, Salerno M, De Gennaro F, Combination of extended-release tacrolimus plus everolimus once daily in de novo kidney transplant recipients: ER-Tac vs LCPT: Am J Transpl, 2019; 19; 690 [Abstract A251]
77. Takahara S, Takahashi K, Akiyama T, Randomized comparative trial of mizoribine versus mycophenolate mofetil in combination with tacrolimus for living donor renal transplantation: Clin Exp Nephrol, 2013; 17; 899-904
78. Watarai Y, Narumi S, Okada M, Optimization of extended-release tacrolimus dose with mycophenolate mofetile based immunosuppression in de novo kidney transplant recipients: 2 years outcome: Am J Transpl, 2017; 17; 739 [Abstract D86]
79. Yan T, Wu X, Rong L, A four-drug combination therapy consisting of low-dose tacrolimus, low-dose mycophenolate mofetil, corticosteroids, and mizoribine in living donor renal transplantation: A randomized study: SAGE Open Med, 2016; 4; 2050312116643672
80. Harland RC, Klintmalm G, Jensik S, Efficacy and safety of bleselumab in kidney transplant recipients: A Phase 2, randomized, open-label, noninferiority study: Am J Transplant, 2020; 20; 159-71
81. Ho ETL, Wong G, Craig JC, Chapman JR, Once-daily extended-release versus twice-daily standard-release tacrolimus in kidney transplant recipients: A systematic review: Transplantation, 2013; 95; 1120-28
82. Vadcharavivad S, Saengram W, Phupradit A, Once-daily versus twice-daily tacrolimus in kidney transplantation: A systematic review and meta-analysis of observational studies: Drugs, 2019; 79; 1947-62
83. Coto E, Tavira B, Suárez-Álvarez B, Pharmacogenetics of tacrolimus: Ready for clinical translation?: Kidney Int Suppl, 2011; 1; 58-62
84. Guerra AA, Cesar CC, Cherchiglia ML, Cyclosporine versus tacrolimus in immunosuppressive maintenance regimens in renal transplants in Brazil: Survival analysis from 2000 to 2004: Ann Pharmacother, 2010; 44; 192-201
85. Shivaswamy V, Boerner B, Larsen J, Post-transplant diabetes mellitus: Causes, treatment, and impact on outcomes: Endocr Rev, 2016; 37; 37-61
86. Eide IA, Halden TAS, Hartmann A, Associations between posttransplantation diabetes mellitus and renal graft survival: Transplantation, 2017; 101; 1282-89
Figures
 Figure 1. PRISMA flow diagramFigure created using PowerPoint for Microsoft 365, Microsoft. EBM – evidence-based medicine; PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-analyses; TAC – tacrolimus.
Figure 1. PRISMA flow diagramFigure created using PowerPoint for Microsoft 365, Microsoft. EBM – evidence-based medicine; PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-analyses; TAC – tacrolimus. Figure 2. Risk of bias among the 61 primary studies as a percentage of the total included studiesRisk of bias was assessed based on the recommendations of the Cochrane guide for systematic reviews. Figure created using R 3.6.0, The R Foundation.
Figure 2. Risk of bias among the 61 primary studies as a percentage of the total included studiesRisk of bias was assessed based on the recommendations of the Cochrane guide for systematic reviews. Figure created using R 3.6.0, The R Foundation. Figure 3. Network plot for graft loss at 6 to 12 monthsThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 3. Network plot for graft loss at 6 to 12 monthsThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. Figure 4. Network plot for mortality rate at 6 to 12 monthsThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 4. Network plot for mortality rate at 6 to 12 monthsThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. Figure 5. Forest plot for the random-effects model for graft loss (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 5. Forest plot for the random-effects model for graft loss (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 23 studies (8 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 15 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. Figure 6. Forest plot for the random-effects model for mortality (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus.
Figure 6. Forest plot for the random-effects model for mortality (6–12 months) relative to reference treatment with Prograf plus mycophenolic acid plus a corticosteroidThe plot is based on 25 studies (7 studies in which 1 or more of the treatment arms used Advagraf or Prograf, and 18 studies in which the tacrolimus formulation was unknown). Figure created using R 3.6.0, The R Foundation. AZA – azathioprine; BEL – belimumab; BID – twice daily; CrI – credible interval; CS – corticosteroids; CsA – cyclosporin; EVR – everolimus; FK778 – manitimus; MPA – mycophenolic acid; QD – once daily; SRL – sirolimus; STN – sotrastaurin; TAC – tacrolimus. Tables
 Table 1. Overview of included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 1. Overview of included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 2. Key baseline characteristics among primary publications of studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 2. Key baseline characteristics among primary publications of studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 3. Rejection outcomes: acute rejection and biopsy-proven acute rejection among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 3. Rejection outcomes: acute rejection and biopsy-proven acute rejection among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 4. Graft loss among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 4. Graft loss among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 5. Patient death among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 5. Patient death among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 1. Overview of included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 1. Overview of included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 2. Key baseline characteristics among primary publications of studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 2. Key baseline characteristics among primary publications of studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 3. Rejection outcomes: acute rejection and biopsy-proven acute rejection among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 3. Rejection outcomes: acute rejection and biopsy-proven acute rejection among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 4. Graft loss among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 4. Graft loss among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf. Table 5. Patient death among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf.
Table 5. Patient death among all included studies in which 1 or more of the treatment arms used Advagraf or Prograf. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








