12 January 2022: Original Paper
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of Kidney Transplant Recipients
Camilla Lorant1ABCDEF*, Gabriel WestmanDOI: 10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
Abstract
BACKGROUND: BK virus (BKV) infection after kidney transplantation leads to BKV-associated nephropathy (BKVAN) in up to 10% of recipients, and is associated with an increased risk of allograft dysfunction or loss. The objective of this study was to estimate the incidence of BKVAN and to analyze whether enhanced induction is associated with an increased risk of BKVAN, possibly justifying more intensive surveillance.
MATERIAL AND METHODS: This was a single-center retrospective cohort study. All patients who underwent kidney transplantation or simultaneous pancreas and kidney transplantation at the Uppsala University Hospital in Sweden between 2005 and 2014 were included, a period when BKV screening was not yet implemented. The effect of enhanced induction, defined as treatment with thymoglobulin, rituximab, and/or eculizumab, often in combination with IVIg and glycosorb, immunoadsorption and/or plasmapheresis/apheresis, was analyzed in a multivariable Cox proportional hazards model together with sex, age, cytomegalovirus mismatch (donor+/recipient-) and rejection treatment as co-predictors. Further, the effects of BKVAN on graft survival was analyzed in a univariable Cox proportional hazards model.
RESULTS: In total 44 of 928 (4.7%) patients developed a biopsy-verified BKVAN 4.8 (1.5-34.2) months after transplantation. Male sex was identified as a risk factor (HR 2.02, P=0.04) but not enhanced induction. Patients with BKVAN experienced a significantly higher risk of graft loss (HR 4.37, P<0.001).
CONCLUSIONS: Male sex, but not enhanced induction, was found to be a risk factor for BKVAN development after kidney transplantation. BKVAN is associated with an increased risk of graft loss.
Keywords: BK Virus, Graft Survival, Kidney Transplantation, Risk Factors, Humans, Kidney Diseases, Male, transplant recipients, Tumor Virus Infections
Background
BK virus (BKV) infects up to 90% of the general population and often causes asymptomatic infections during childhood [1]. After primary infection a persistent state is established in renal tubular cells and the uroepithelium with minimal clinical implications [2–4]. When the immune system is suppressed, the virus may reactivate and cause serious complications such as BKV-associated nephropathy (BKVAN). BKVAN affects 1–10% of all kidney transplant recipients (KTR) [5–7], and is associated with a significant risk of allograft dysfunction or loss [5,7,8]. There is no established antiviral therapy available for BKV infection in KTR, but a pre-emptive reduction of immunosuppression when BK viremia is detected has been shown to lower the incidence of BKVAN [9,10]. In 5–15% of all allogenic hematopoietic stem cell transplant patients BKV causes BKV-associated hemorrhagic cystitis [1,11,12]. Since BKVAN and hemorrhagic cystitis are seldom seen in other immunosuppressed patients it is likely that an additional insult is needed which predisposes the graft or the bladder to the damage by BKV [13].
Several risk factors for development of BKVAN have been identified. The single most important factor is thought to be the overall degree of immunosuppression, including general immunosuppression as well as treatment for acute rejection and desensitization [6,14–18]. Other identified risk factors that have been proposed include male sex, age of both donor and recipient [16], obesity [15], and donors and recipients serostatus of both BKV and Cytomegalovirus (CMV) [15]. Also, factors such as long cold ischemia time [15] and delayed graft function [15,17] have been suggested to be of interest. There are only a few prospective studies with multivariable analyses of risk factors and most included less than 500 patients [19].
The aim of this study was to examine the incidence of BKVAN in a Swedish cohort of patients having received a kidney transplant or combined kidney and pancreas transplants, and to evaluate selected risk factors and their association with the development of BKVAN. Further, we aimed to clarify if a sub-group of patients, those having received enhanced induction, is at higher risk of BKVAN and who would therefore possibly benefit from more intense surveillance.
Material and Methods
STUDY DESIGN:
This was a single-center retrospective cohort study based on prospectively collected data. All transplantations in patients aged ≥18 years who underwent kidney transplantation (KTx) or simultaneous pancreas and kidney transplantation (SPK) at the Uppsala University Hospital in Sweden between 1 January 2005 and 31 December 2014 were eligible to be included, a period when BKV screening was not yet implemented at our center. All patients who experienced primary non-function, early allograft loss (<6 months), were lost to follow-up or died within 6 months after transplantation were excluded from the analysis cohort. Of note, none of the excluded patients developed BKVAN.
The primary analysis was the incidence of BKVAN, according to the contemporary local definition including >10% loss of filtration capacity along with histologic findings consistent with BK virus engagement of the graft. Secondary analyses were the association of selected risk factors with BKVAN development, rate of graft loss in patients with and without BKVAN and the proportion of patients who received antiviral treatment with cidofovir. Risk factors were selected on the basis of previous findings and biological rationale rather than univariate analysis, in line with the recommendations by Heinze et al [20]. The selected risk factors were sex, age, CMV mismatch (donor+/recipient−), enhanced induction and rejection treatment. Enhanced induction was defined as treatment with thymoglobulin, rituximab and/or eculizumab, often in combination with IVIg and glycosorb, immunoadsorption and/or plasmapheresis/apheresis. The reasons for administering enhanced induction could be HLA-incompatibility, ABO-incompatibility, SPK and other high-risk immunological scenarios such as previous transplantations. Rejection treatment included methylprednisolone, anti-thymocyte immunoglobulin, rituximab, eculizumab, IVIg or plasmapheresis were analyzed in relation to BKVAN development.
The study was approved by the regional ethics review board in Uppsala (No. 2015/488). All data were collected from electronic medical records and from a local follow-up transplantation registry at the Uppsala University Hospital. At time of transplantation, all patients were informed about the registration of their data and were given a standing option to actively opt-out at any time.
DIAGNOSIS OF BK VIREMIA AND BKVAN:
BK virus was analyzed in serum or plasma samples from the patients using a modified variant of a previously described quantitative TaqMan real time polymerase chain reaction (qPCR) procedure [21,22]. Briefly, BK virus DNA was extracted from 200 μl plasma using the automatic NucliSens easyMAG robot (BioMérieux, Marcy l’Etoile, France). BK virus DNA was then amplified using the Qiagen Rotor-Gene Q thermo cycler (Qiagen, Hilden, Germany).
The study was performed prior to the introduction of a BKV viremia surveillance program. Hence, only patients with deterioration of the kidney function underwent a biopsy to see if they had histological signs of BKVAN. The locally applied indication for a transplant biopsy was an unexplained 10% increase in serum creatinine or more. BKVAN was diagnosed by pathological evaluation of kidney allograft biopsies taken at any time after transplantation and defined as positive immunohistochemical staining for Simian virus 40 (SV40) and a significant level of BK virus copies/mL (>10 000 copies/mL) in serum or plasma.
IMMUNOSUPPRESSIVE REGIMENS:
Several different standard immunosuppressive protocols were used during the study period; most commonly used induction therapy was monoclonal IL-2 receptor blockers (basiliximab or daclizumab) and methylprednisolone or methylprednisolone alone, either followed by tacrolimus, cyclosporine A or other immunosuppressive drugs. In addition, most patients received mycophenolate mofetil. Almost all patients received prednisolone in tapering doses.
ANTIMICROBIAL PROPHYLAXIS:
All KTR received cefuroxime one dose of 1.5 g at transplantation and all SPK recipients received cefotaxime and ampicillin for 2 days and caspofungin for 5–7 days. All CMV mis-matched recipients received valacyclovir or valganciclovir prophylaxis for 3–6 months. All recipients received trimethoprim/sulfamethoxazole one single strength tablet OD for 6 months. In case of non-tolerance, pentamidine inhalations were administered monthly.
STATISTICAL ANALYSIS:
A Cox proportional hazards model was used for analysis of the following risk factors and their association with BKVAN development: recipient sex and age, CMV mismatch (donor+/recipient−), enhanced induction and rejection treatment. For the predictor variable “rejection treatment”, which was not present at baseline but could arise during time of follow-up, the corresponding hazard ratio was assessed dynamically using only time after the administration of such treatment for defining time at risk.
The risk of graft loss in relation to BKVAN was analyzed using a Cox proportional hazards model, using BKVAN as a single-predictor variable. The contribution of time before and after BKVAN was separated on a study subject level. Continuous data were presented as median with ranges. All analyses were conducted in R version 3.5.1 using package
Results
PATIENT CHARACTERISTICS:
In total, 977 transplantations performed in the 963 patients who received a KTx or SPK were assessed for eligibility in the study. Fourteen patients received more than one transplant during the period. Forty-nine transplantations were excluded from the analysis dataset due to age <18 years (n=7), primary non-function (n=9), death within 6 months (n=14), allograft loss within 6 months (n=18) or lost to follow-up within 6 months (n=1) (Figure 1). Hence, the analyzed cohort consisted of 928 transplantations in 919 patients, followed for a median of 83 (range 6–169) months. The patient and transplant characteristics are presented in Table 1, whereas patient survival from first transplantation and graft survival for all transplants is presented in Figure 2.
INDUCTION THERAPY:
In this cohort, 875 patients (94.3%) received methylprednisolone and basiliximab as induction therapy, which was standard of care (SOC) in Uppsala at the time for the study. Forty-seven patients (5.1%) received methylprednisolone alone, 5 patients (0.5%) received methylprednisolone and daclizumab, and 1 patient (0.1%) was given basiliximab only.
Enhanced induction was administered to 138 patients, mostly in addition to SOC. Of these, 93 were ABO-incompatible. The standard treatment for ABO-incompatibility is glycosorb or in some cases immunoadsorption and/or plasmapheresis as well as IVIg and rituximab. The majority of all ABO-incompatible patients received this treatment, except that some did not receive IVIg, and 1 patient received thymoglobulin in addition to standard treatment and 13 patients received eculizumab, 2 of whom did not receive rituximab.
Of the other 45 patients, 23 patients received rituximab. Of these 23 patients, 1 also received thymoglobulin, 4 plasmapheresis, and 5 immunoadsorption. Three of them also received IVIg. Twenty patients out of the 45 received thymoglobulin. Of these, 5 patients also received eculizumab and 4 patients received plasmapheresis and IVIg. Of the other 2 of the 45 patients, 1 received eculizumab only and 1 received plasmapheresis only.
MAINTENANCE THERAPY:
Calcineurin inhibitors were given as maintenance immunosuppression to 881 out of all 928 patients (94.9%), tacrolimus was given to 803/881 patients (91.1%), and cyclosporine A was given to 78/881 patients (8.9%). Other immunosuppressive drugs, for example belatacept and sirolimus, were given to 47 patients (5.1%). In addition, 830 patients (89.4%) received mycophenolate mofetil; in the tacrolimus group it was 732/803 (91.2%), in the cyclosporine A group it was 59/78 (75.6%), and in the group with other immunosuppressive drugs it was 39/47 (83.0%). Almost all patients (903 [97.3%]) received prednisolone in tapering doses.
RISK FACTORS FOR BKVAN:
In the Cox proportional hazards model, male sex was identified as a significant risk factor for developing BKVAN (HR 2.02, P=0.04). No statistically significant differences in risk of BKVAN were found in relation to enhanced induction, age, CMV mismatch (donor+/recipient−), or rejection treatment prior to BKVAN (Table 2).
BKVAN AND GRAFT LOSS:
In total, 44 patients developed biopsy-confirmed BKVAN, resulting in an incidence of 4.7%. Median time to BKVAN was 4.8 months (1.5–34.2 months), and 34 of 44 (77.3%) were diagnosed within 1 year of transplantation. Patients who developed BKVAN experienced a significantly higher risk of graft loss (HR 4.37,
Immunosuppression in BKVAN patients was reduced at the physician’s discretion.
In total, 25 out of 44 patients (56.8%) with BKVAN were treated with adjuvant cidofovir, and treatment was started after a median of 17 days from diagnosis (0–203 days). The dose and duration of the therapy was decided by the treating physician. The majority received a dose of 0.5 mg/kg once a week during a median of 7 weeks (1–20 weeks). The treated group did not differ substantially from the untreated group with respect to demographic data and renal function.
Discussion
In this single-center study, we present data on all patients who received a kidney or a kidney pancreas transplant during a 10-year period between 2005 and 2014 with the aim to estimate the incidence of BKVAN prior to the introduction of a BKV viremia surveillance program. Further, we investigated if patients with enhanced induction are at higher risk of developing BKVAN, which could justify more intense surveillance of this group.
There are several studies on risk factors for BK viremia but only a few large studies with prospectively collected data on risk factors for biopsy-verified BKVAN including multivariable analysis [19]. Of these, 1 study covered the years 1985 to 2005, a period when other immunosuppressive protocols were used [23], while another included recipients with anti-CD52, as induction therapy which is not widely used at other centers. A third comprised, in contrast to our study, children only [23–25].
The incidence of BKVAN of 4.7% in our study is in the range reported by other investigators [5–7] and is even in line with some other studies in which BKV screening was applied [24,26]. Our primary hypothesis was that enhanced induction therapy would lead to more cases of BKVAN. Due to the inherent limits of multivariable modeling in relation to the total number of BKVAN events, we selected 5 risk factors, based on previous findings and biological rationale, to study in the Cox regression analysis.
Enhanced induction was used in 138 (14.9%) of the patients, but this was not associated with a significantly increased risk of developing BKVAN. Most previous studies addressing the association between enhanced induction immunosuppressive therapy and BKV infection have shown a higher incidence of BKV infection in ABO-incompatible recipients [27]. In a large nation-wide study by Ko et al, desensitization therapy both for ABO and HLA-incompability led to more early deaths due to infection, but BKV infection was not described separately in this study [28]. Toyoda et al did not find a higher rate of BK viremia or BKVAN in HLA-desensitized recipients [29]. Radtke et al found that neither induction nor maintenance immunosuppressive therapy influenced BK virus infection [30]. Their conclusion was that when using modern low-dose concepts of immunosuppression in kidney transplantation, the impact of immunosuppression on the incidence of BK viremia is limited.
Other previously reported risk factors such as age or CMV serostatus could not be verified in our cohort. Rejection before the diagnosis of BKVAN was not identified as a significant risk factor, but it was more common that patients were treated for rejection after BKVAN diagnosis, which indicates that this could be a down-stream event related to reduced immunosuppression. Also, in line with previous studies, patients with BKVAN presented with an increased rate of graft loss compared to patients without BKVAN.
The only factor associated with a significant risk increase found in this study was male sex of the recipient. Male sex has been shown to be a risk factor for BK viremia in several studies [19,31,32] and for BKVAN in some [7,14,33], but to the best of our knowledge it is still not known by what mechanism this risk increase is mediated. There are some possible explanatory models.
About half of the patients received treatment with cidofovir, at the discretion of the responsible physician, to initiate treatment. We could not observe any obvious differences between patients with BKVAN receiving cidofovir treatment and those who did not. Since cidofovir treatment was not randomly assigned, it was impossible to evaluate the benefits and risks of this regimen in our study. IVIg can be used as a treatment for BK virus infection, but in the patients in our material (93 patients and mainly AB0 incompatible) who received IVIg as part of their induction, 6.5% developed BK virus nephropathy, which is more than in the cohort.
One limitation of our study is that only 44 BKVAN events were captured, limiting the multivariable analysis. Another limitation is the single-center design of the study, which could affect generalizability of results, as secular trends in methods, therapies, and patient population might differ between transplantation centers. A third limitation is that general screening for BKV DNAemia was not implemented at our center at the time of the study, which is why subclinical BKV DNAemia could have gone undetected in both the BKVAN and non-BKVAN groups. However, we believe our findings are relevant as, to the best of our knowledge, this the largest multivariable analysis of risk factors for biopsy-proven BKVAN. The loss to follow-up was negligible and the primary end-point, BKVAN, is a reliable and clinically relevant outcome. All patients with increased serum creatinine levels and BK viremia were referred for a kidney biopsy. The vast majority of patients had the same basal immunosuppressive therapy consisting of tacrolimus, MMF, and prednisolone. Hence, the tendency of tacrolimus to be more prone to lead to BKVAN than cyclosporine, as previous described, was not expected to affect our results substantially.
Conclusions
BKVAN is a relatively uncommon complication to kidney and kidney/pancreas transplant, but is associated with a considerable risk of graft loss. Male sex, but not enhanced induction, appears to be a significant risk factor for developing BKVAN. Hence, more intense BKV surveillance of patients receiving enhanced induction does not seem justified in this setting.
Figures
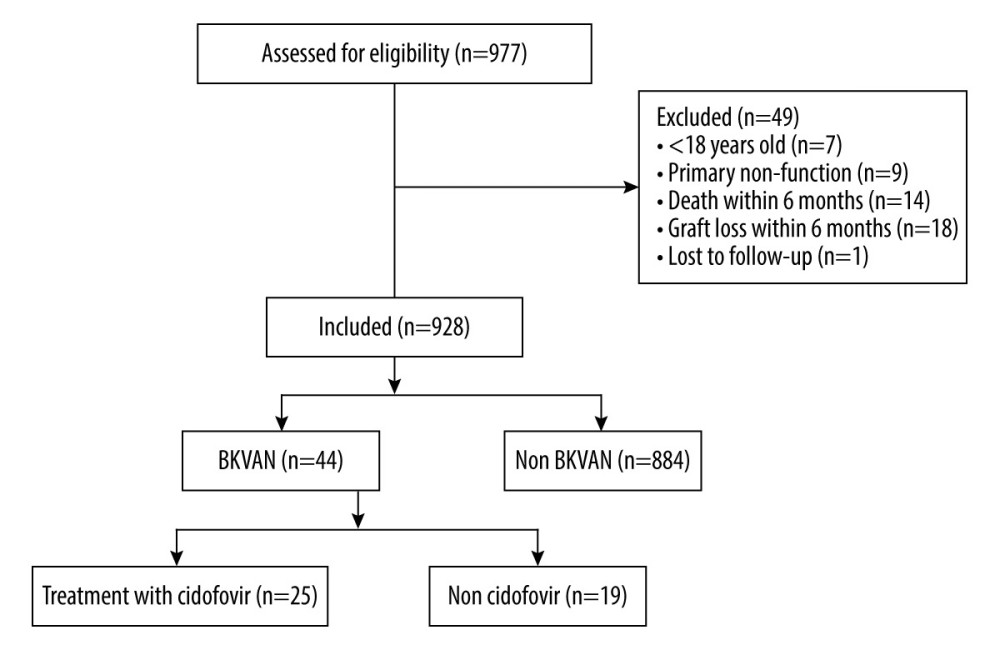 Figure 1. CONSORT flow-chart of patients and grafts in the study. Created in Microsoft Word, version 16.53, 2019.
Figure 1. CONSORT flow-chart of patients and grafts in the study. Created in Microsoft Word, version 16.53, 2019. 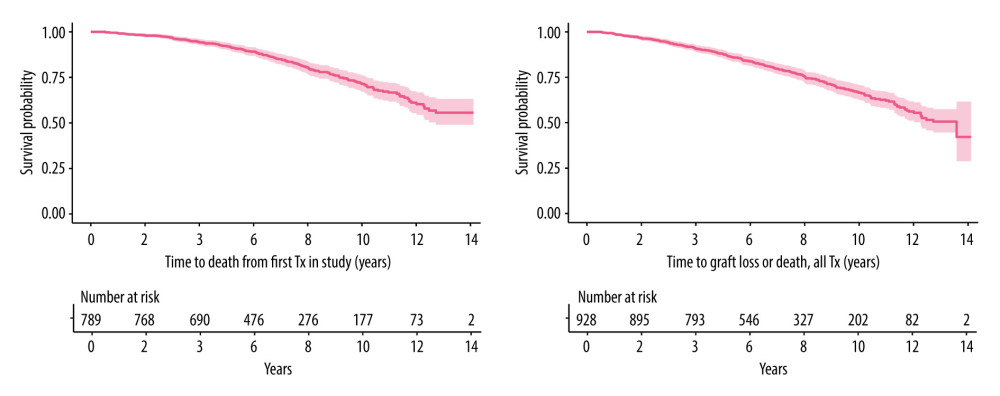 Figure 2. Kaplan-Meier plot of time to death from first tx (upper) and time to death or graft loss for all tx (lower). Created in R version 3.5.1 using package survival version 2.42–3.
Figure 2. Kaplan-Meier plot of time to death from first tx (upper) and time to death or graft loss for all tx (lower). Created in R version 3.5.1 using package survival version 2.42–3. References
1. Hirsch HH, Steiger J, Polyomavirus BK: Lancet Infect Dis, 2003; 3(10); 611-23
2. Chatterjee M, Weyandt TB, Frisque RJ, Identification of archetype and rearranged forms of BK virus in leukocytes from healthy individuals: J Med Virol, 2000; 60(3); 353-62
3. Knowles WA, Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV): Adv Exp Med Biol, 2006; 577; 19-45
4. Chesters PM, Heritage J, McCance DJ, Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues: J Infect Dis, 1983; 147(4); 676-84
5. Binet I, Nickeleit V, Hirsch HH, Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss: Transplantation, 1999; 67(6); 918-22
6. Hirsch HH, Knowles W, Dickenmann M, Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients: N Engl J Med, 2002; 347(7); 488-96
7. Ramos E, Drachenberg CB, Portocarrero M, BK virus nephropathy diagnosis and treatment: Experience at the University of Maryland Renal Transplant Program: Clin Transpl, 2002; 143-53
8. Randhawa PS, Finkelstein S, Scantlebury V, Human polyoma virus-associated interstitial nephritis in the allograft kidney: Transplantation, 1999; 67(1); 103-9
9. Almeras C, Vetromile F, Garrigue V, Monthly screening for BK viremia is an effective strategy to prevent BK virus nephropathy in renal transplant recipients: Transpl Infect Dis, 2011; 13(2); 101-8
10. Hirsch HH, Randhawa PS, BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice: Clin Transplant, 2019; 33(9); e13528
11. Arthur RR, Shah KV, Baust SJ, Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants: N Engl J Med, 1986; 315(4); 230-34
12. Bedi A, Miller CB, Hanson JL, Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation: J Clin Oncol, 1995; 13(5); 1103-9
13. Krejci K, Tichy T, Bednarikova J, BK virus-induced renal allograft nephropathy: Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 2018; 162(3); 165-77
14. Schold JD, Rehman S, Kayle LK, Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States: Transpl Int, 2009; 22(6); 626-34
15. Borni-Duval C, Caillard S, Olagne J, Risk factors for BK virus infection in the era of therapeutic drug monitoring: Transplantation, 2013; 95(12); 1498-505
16. Hirsch HH, Randhawa P, BK polyomavirus in solid organ transplantation: Am J Transplant, 2013; 13(Suppl 4); 179-88
17. Costa JS, Ferreira E, Leal R, Polyomavirus nephropathy: Ten-year experience: Transplant Proc, 2017; 49(4); 803-8
18. Pai D, Mann DM, Malik A, Risk Factors for the development of BK virus nephropathy in renal transplant recipients: Transplant Proc, 2015; 47(8); 2465-69
19. Demey B, Tinez C, Francois C, Risk factors for BK virus viremia and nephropathy after kidney transplantation: A systematic review: J Clin Virol, 2018; 109; 6-12
20. Heinze G, Dunkler D, Five myths about variable selection: Transpl Int, 2017; 30(1); 6-10
21. Hoffman NG, Cook L, Atienza EE, Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays: J Clin Microbiol, 2008; 46(8); 2671-80
22. Hammarin AL, Oqvist B, Wahlgren J, Falk KI, Systematic screening of BK virus by real-time PCR prevents BK virus associated nephropathy in renal transplant recipients: J Med Virol, 2011; 83(11); 1959-65
23. Prince O, Savic S, Dickenmann M, Risk factors for polyoma virus nephropathy: Nephrol Dial Transplant, 2009; 24(3); 1024-33
24. Theodoropoulos N, Wang E, Penugonda S, BK virus replication and nephropathy after alemtuzumab-induced kidney transplantation: Am J Transplant, 2013; 13(1); 197-206
25. Smith JM, Dharnidharka VR, Talley L, BK virus nephropathy in pediatric renal transplant recipients: An analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry: Clin J Am Soc Nephrol, 2007; 2(5); 1037-42
26. Schaub S, Hirsch HH, Dickenmann M, Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy: Am J Transplant, 2010; 10(12); 2615-23
27. de Weerd AE, Betjes MGH, ABO-incompatible kidney transplant outcomes: A meta-analysis: Clin J Am Soc Nephrol, 2018; 13(8); 1234-43
28. Ko EJ, Yu JH, Yang CW, Chung BH, Clinical outcomes of ABO- and HLA-incompatible kidney transplantation: a nationwide cohort study: Transpl Int, 2017; 30(12); 1215-25
29. Toyoda M, Shin BH, Ge S, Impact of desensitization on antiviral immunity in HLA-sensitized kidney transplant recipients: J Immunol Res, 2017; 2017; 5672523
30. Radtke J, Dietze N, Fischer L, Incidence of BK polyomavirus infection after kidney transplantation is independent of type of immunosuppressive therapy: Transpl Infect Dis, 2016; 18(6); 850-55
31. Masutani K, Ninomiya T, Randhawa P, HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia: Nephrol Dial Transplant, 2013; 28(12); 3119-26
32. Hirsch HH, Vincenti F, Friman S, Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: A prospective, randomized, multicenter study: Am J Transplant, 2013; 13(1); 136-45
33. Dharnidharka VR, Cherikh WS, Neff R, Retransplantation after BK virus nephropathy in prior kidney transplant: An OPTN database analysis: Am J Transplant, 2010; 10(5); 1312-15
34. Popik W, Khatua AK, Fabre NF, BK virus replication in the Glomerular Vascular Unit: Implications for BK virus associated nephropathy: Viruses, 2019; 11(7); 583
35. Funk GA, Gosert R, Comoli P, Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants: Am J Transplant, 2008; 8(11); 2368-77
36. Momper JD, Misel ML, McKay DB, Sex differences in transplantation: Transplant Rev (Orlando), 2017; 31(3); 145-50
37. Pivonello R, Auriemma RS, Pivonello C, Sex disparities in COVID-19 severity and outcome: Are men weaker or women stronger?: Neuroendocrinology, 2020; 111(11); 1066-85
38. Torcia MG, Nencioni L, Clemente AM, Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males: PLoS One, 2012; 7(6); e39853
39. Forsyth KS, Anguera MC, Time to get ill: The intersection of viral infections, sex, and the X chromosome: Curr Opin Physiol, 2021; 19; 62-72
Figures
Tables
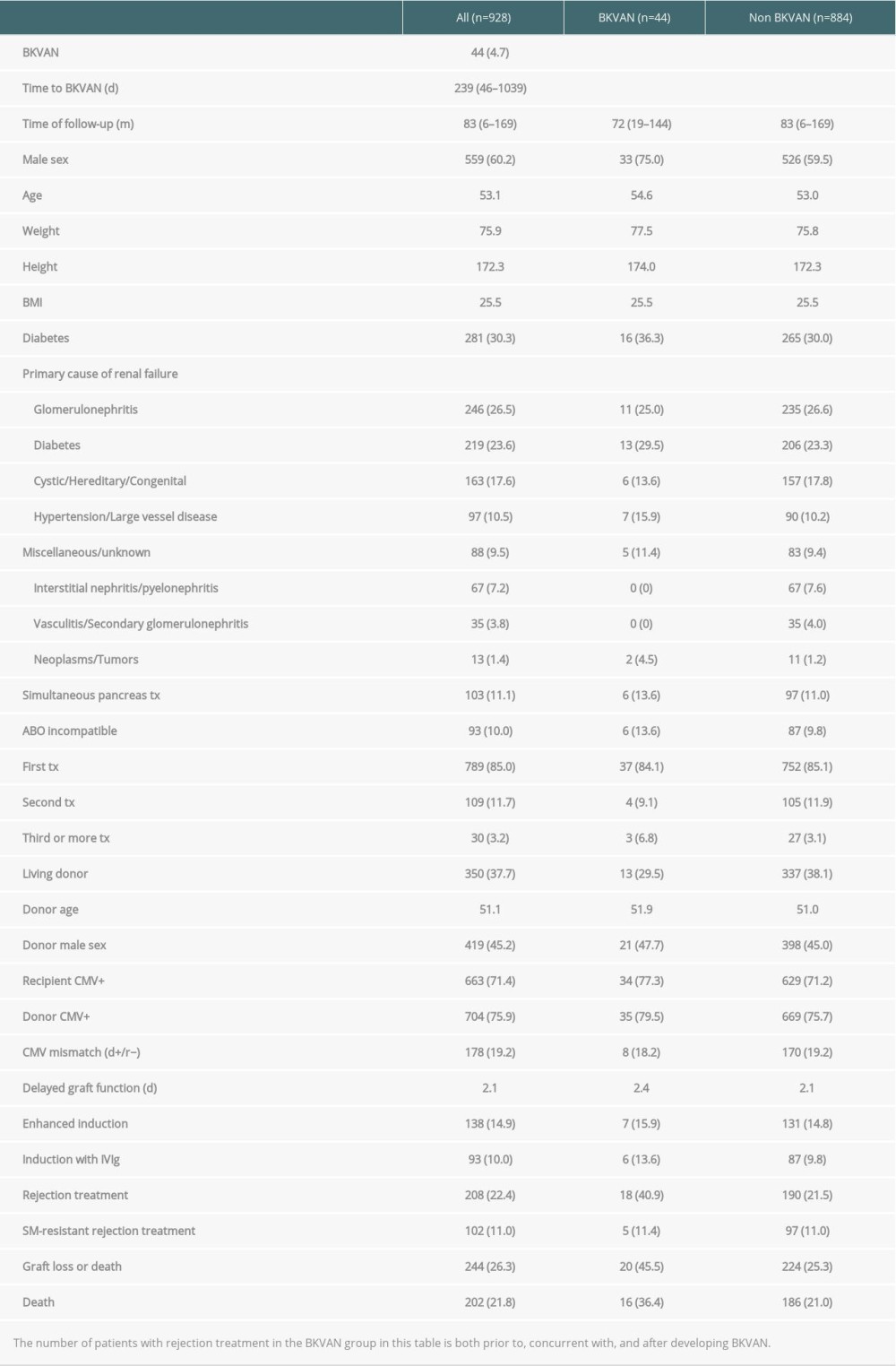 Table 1. Demographics and clinical characteristics of kidney- and kidney/pancreas graft recipients with and without BK virus-associated nephropathy, n (%).
Table 1. Demographics and clinical characteristics of kidney- and kidney/pancreas graft recipients with and without BK virus-associated nephropathy, n (%).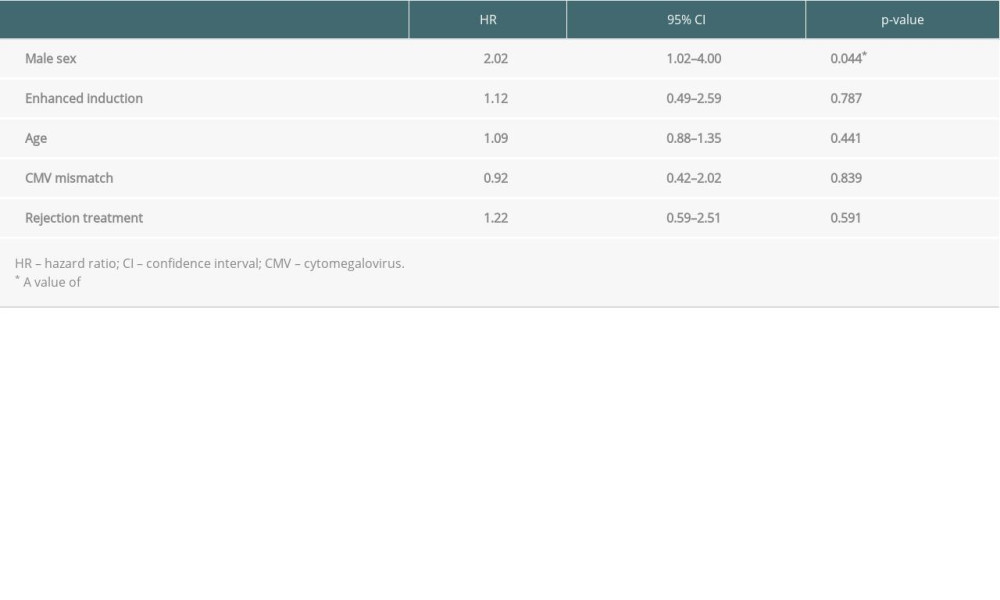 Table 2. Multivariable Cox proportional hazards model of risk factors for BK virus nephropathy after kidney transplantation.
Table 2. Multivariable Cox proportional hazards model of risk factors for BK virus nephropathy after kidney transplantation. Table 1. Demographics and clinical characteristics of kidney- and kidney/pancreas graft recipients with and without BK virus-associated nephropathy, n (%).
Table 1. Demographics and clinical characteristics of kidney- and kidney/pancreas graft recipients with and without BK virus-associated nephropathy, n (%). Table 2. Multivariable Cox proportional hazards model of risk factors for BK virus nephropathy after kidney transplantation.
Table 2. Multivariable Cox proportional hazards model of risk factors for BK virus nephropathy after kidney transplantation. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








