26 October 2021: Original Paper
Safety of Antithymocyte Globulin Use in Kidney Graft Recipients During the COVID-19 Pandemic
Aureliusz Kolonko1ABCDEF*, Andrzej Więcek1AEDOI: 10.12659/AOT.933001
Ann Transplant 2021; 26:e933001
Abstract
BACKGROUND: There are many safety concerns regarding the use of antithymocyte globulin (ATG) in kidney transplant recipients (KTRs) during the ongoing COVID-19 pandemic. Hereby, we present our recent experience with ATG administration both as induction therapy and as an anti-rejection treatment.
MATERIAL AND METHODS: We retrospectively analyzed all patients transplanted during the first 12 months of the COVID-19 pandemic who were treated with thymoglobulin. The ATG dosing, lymphocyte number and percentage in blood smear, adverse effects (thrombocytopenia and infectious complications), and kidney graft function up to 12 months and patients’ outcomes were analyzed and compared to KTRs who received basiliximab induction.
RESULTS: During pandemic, a total of 31 patients were treated with ATG and 59 received basiliximab. The median cumulative ATG doses were 275 (175-325) mg in the induction subgroup and 263 (200-275) mg in the anti-rejection treatment subgroup. Mild thrombocytopenia was noted in 7 (22.6%) and 13 (29.5%) patients, respectively. There were more infectious complications among patients treated with ATG as compared with the basiliximab subgroup (32.3 vs 10.2%, P<0.01), but there were similar incidence rates of thrombocytopenia. Kidney graft function up to 12 months after transplant was comparable (1.1 [1.0-1.9] vs 1.1 [1.0-1.4] mg/dl, respectively).
CONCLUSIONS: 1. ATG use in the induction protocol or as the anti-rejection treatment during the COVID-19 pandemic appears to be safe and the risk of adverse events is acceptable. 2. During the COVID-19 pandemic the necessary use of ATG should not be postponed, especially in KTRs with increased immunologic risk.
Keywords: COVID-19, Graft Rejection, Thymoglobulin, Antilymphocyte Serum, basiliximab, COVID-19, Graft Survival, Humans, Immunosuppressive Agents, Kidney, Kidney Transplantation, Pandemics
Background
The ongoing pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) profoundly changed daily practice in kidney transplantation. During the spring and autumn outbreaks of this novel infection, both the donor reporting rate and the number of performed kidney transplantations were markedly reduced worldwide [1–3]. This was mainly the result of compromised hospital resources, especially in intensive care units, and serious doubts concerning the safety of potential organ recipients [4]. Advanced chronic kidney disease itself, as well as high co-morbidity (including hypertension, diabetes and obesity), put this population at very high risk of complications in case of accidental COVID-19 infection during or after the kidney transplantation procedure [5–7].
As any immunosuppressed patient seemed to be at greater risk of potentially deleterious viral infection, even more concerns arose about the safety of new kidney transplant patients, taking into account the need for relatively intensified immunosuppression within the first few months after transplantation. The major issue is induction therapy, with the growing need for antithymocyte globulin (ATG) use, especially in kidney transplant candidates with increased immunological risk [8]. These concerns were mirrored by a substantial drop in the use of lymphocyte-depleting induction agents during first “pandemic” months, which was noted in the USA [9] and also in Poland (unpublished data). On the other hand, avoiding ATG could lead to increased incidence of rejection episodes, while the full anti-rejection protocol accumulates a much larger dose of immunosuppression [10]. Another consequence is the possible transplantation delay in case of high-immunologic risk patients, as recent evidence clearly suggested the advance of ATG over basiliximab induction in this population [11,12]. Until now, except for few case reports [13,14], there is no publication reporting the safety of routine ATG use during the COVID-19 pandemic. Thus, in our present single-center study, we report the details of restored ATG use in the center and the short-term outcomes of patients who were transplanted during the first 12 months of the COVID-19 era.
Material and Methods
This single-center analysis included all consecutive kidney recipients transplanted during the COVID-19 pandemic who received antithymocyte globulin (ATG, Thymoglobulin, Sanofi) as an induction therapy or anti-rejection treatment in the early post-transplant period from February 24, 2020 to February 23, 2021. The control group consisted of kidney recipients transplanted during the same period who received basiliximab induction. The study was performed in accordance with the Declaration of Helsinki. The Bioethics Committee of the Medical University of Silesia granted permission to keep the prospective transplant database and also to perform this analysis based on anonymized data (No PCN/CBN/0022/KB/142/21). Informed consent was not necessary, as the data analysis does not meet the criteria of a medical experiment.
The overall prevalence of COVID-19 infection in the whole country and in the local Silesia region was similar (49 960 and 53 690 cases per million, respectively), with 1245 and 1205 deaths per million, respectively, as of March 24, 2021.
According to our immunosuppressive protocol, an induction with ATG was prescribed in patients with an increased immunologic risk. Notably, all wait-listed kidney transplant candidates were screened for the presence of anti-HLA antibodies (class I and II), and single-bead antigen identification was carried out in patients that showed positive results. Based on last pre-transplant Luminex results, donor-specific antibodies (DSA) were identified during a virtual crossmatch procedure. Immediately before transplantation, patients were stratified to the increased immunologic risk group if they showed presence of DSA (mean fluorescence index, MFI >2000), a substantial (>50%) percentage of positive historic cross-matches during wait-listing, or had an established immunological cause of failure during the previous transplantation. Notably, ATG induction therapy was also prescribed to patients with a low DSA titer but with an anti-HLA MFI >2000.
The rest of the transplanted patients routinely received basiliximab induction. All analyzed kidney transplantations were AB0-compatible transplantations and were performed after the negative traditional cross-match. All organs were procured from deceased donors.
All acute rejection episodes, when ATG treatment was prescribed, were diagnosed based on protocol or indication biopsy. Protocol biopsies were performed routinely during the first post-transplantation hospital stay, usually between 8 and 10 days after surgery. At transplantation, all those patients received induction therapy with basiliximab (but they are not included in the characteristics of the basiliximab subgroup).
The administration of ATG induction was initiated immediately prior to the transplant procedure, after routine premedication, and usually was 50 or 75 mg, depending on patient weight. In our center, we used the protocol of ATG intermittent dosing based on the lymphocyte count, the effectiveness of which has been previously proven [15–17]. Starting from the first post-operative day, ATG doses were prescribed based on the current diuresis and serum creatinine levels, and were adjusted for leukopenia, lymphopenia, and thrombocytopenia, as described previously [18]. There were neither fixed daily doses nor fixed individual cumulative doses in our program. ATG was usually given until an acceptable diuresis and a decrease in serum creatinine down to approximately 2 mg/dl were achieved. Importantly, anti-CMV prophylaxis with ganciclovir/valganciclovir for 1 month after the end of ATG administration was given to all patients. Trimethoprim/sulfamethoxazole anti-pneumocystis prophylaxis was prescribed for 3 months. Basiliximab (20 mg) was given intravenously twice: immediately before transplantation and on the 4th post-operative day.
The standard maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil, and steroids, with both calcineurin inhibitor and antimetabolite drug started before transplantation, and their dosing based on drug therapeutic monitoring. The steroid dose was usually tapered to 7.5–10 mg/day at 3 months after transplant.
In addition to the reasons for ATG therapy and its dosing, the available 1-month, 3-month, and 12-month absolute lymphocyte count in 1 μl and the blood smear lymphocyte percentage were reported, as well as thrombocytopenia episodes during and after the ATG treatment. The time-points mentioned above were counted from the first day of ATG treatment for any cause. We also analyzed the occurrence of infectious complications and death. Additionally, kidney graft function up to 12 months after transplant was also analyzed. Data were collected from the center-operated prospective database and medical records.
Statistical analysis was carried out using STATISTICA 13.3 PL for Windows software package (Tibco, Inc., Palo Alto, CA, USA). Values are presented as means with 95% confidence interval and medians with interquartile ranges or frequencies. The comparison between main study subgroups (patients treated with ATG and patients who did not receive ATG, but had induction therapy using basiliximab) was performed using the
Results
STUDY GROUP:
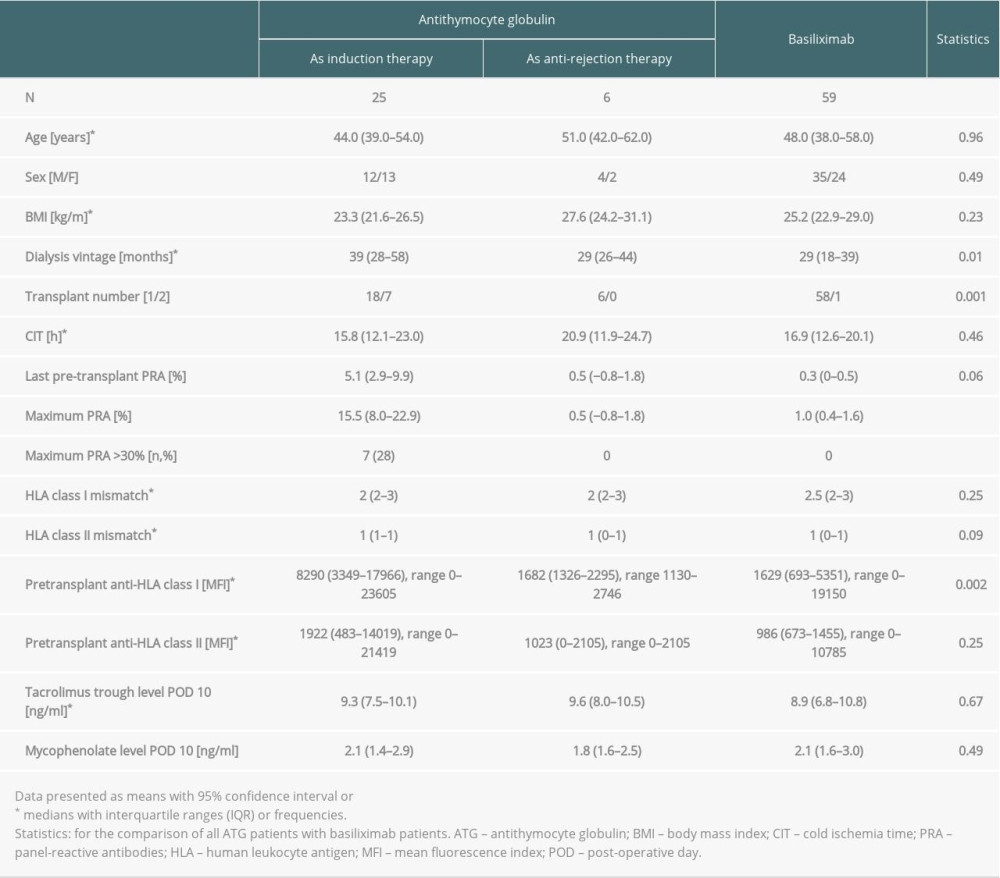
During the analyzed COVID-19 pandemic period, a total of 90 kidney transplantations were performed in our center. Twenty-five patients received ATG as an induction therapy and 6 as an anti-rejection treatment in the early post-transplant period, whereas 59 patients during the study period received the basiliximab induction. The clinical characteristics of those 3 subgroups are given in Table 1. There was no difference in the median post-transplant observation time: 11 (7–13) months in the ATG vs 12 (11–13) in the basiliximab subgroup; P=0.12.
The whole subgroup of patients who were treated with ATG was characterized by a significantly longer time of pre-transplant dialysis therapy (median 36 [26–57] vs 29 [18–39] months; P=0.01), greater anti-HLA class I MFI (median 6550 [2171–16899] vs 1629 [693–5351]; P<0.01), as well as maximum PRA titer (mean 12.6 [6.3–18.9] vs 1.0 [0.4–1.6]; P<0.001) and borderline greater last pre-transplant PRA titer (4.2 [0.3–8.1] vs 0.3 [0–0.5]; P=0.06) and HLA class II mismatch (mean 0.77 [0.59–0.96] vs 0.54 [0.41–0.67]; P=0.09) in comparison to the basiliximab subgroup. There were also more re-transplanted patients in this subgroup (22.6% vs 1.7%; P=0.001) and more patients with maximum PRA titer >30% (Table 1).
In the ATG induction subgroup, 18 patients (72%) had >50% positive historic cross-matches, 3 (12%) had pre-transplant DSA, and 1 patient lost her previous graft in the course of acute humoral rejection. Additionally, 20 patients (80%) had class I or class II anti-HLA antibodies detected in the Luminex screening, with MFI >2000 (Table 1).
THE CHARACTERISTICS OF ACUTE REJECTION EPISODES TREATED WITH ATG:
In the rejection subgroup, 4 cases of acute rejection were diagnosed based on protocol biopsy performed during the first hospitalization (approximately at post-transplant days 8–9) and 2 other cases were diagnosed based on indication biopsies performed at post-transplant days 35 and 65. All biopsies were analyzed by 1 experienced pathologist. Of importance, prior to the diagnosis of rejection which required the ATG treatment, both those patients had developed COVID-19 during the first post-transplant hospitalization (their clinical course was described previously [19] and their mycophenolate doses were substantially reduced. Then, they were diagnosed with T-cell-mediated acute rejection and treated with methylprednisolone pulses, but the ATG treatment was introduced after the second worsening of serum creatinine, based on the results of indication biopsies (vascular rejection V2 and mixed T-cell/antibody-mediated rejection with C4d-positive staining and the presence of DSA). Among the patients diagnosed immediately after transplantation, there was 1 case of mixed T-cell/antibody-mediated rejection with intimal arteritis and microvascular injury, and 3 cases of vascular rejection (with the presence of intimal arteritis, PTC-itis and the obliteration of arterial lumen) with negative C4d staining.
ATG DOSING:
During the COVID-19 pandemic, the median cumulative ATG dose was 275 (175–325) mg in the induction subgroup and 263 (200–275) mg in the anti-rejection treatment subgroup. The corresponding median doses per kg of body weight were 3.8 (3.2–5.1) and 3.3 (2.4–4.2) mg/kg, respectively.
LABORATORY PARAMETERS DURING AND AFTER THE ATG TREATMENT:
As expected, the median absolute lymphocyte counts in 1μl during the whole 12-month observation were significantly lower in patients who received ATG as compared to the basiliximab subgroup: 1-month: 0.53 (0.38–1.16)×103/μl vs 1.92 (1.31–2.62)×103/μl;
In the ATG subgroup, there were 8 patients (25.8%) with mild thrombocytopenia during and after the ATG treatment, with a median nadir of 90 (75–98)×103/μl. In the basiliximab subgroup, there were 10 patients (16.9%) with mild thrombocytopenia with median nadir of 110 (100–113)×103/μl. This difference was not statistically significant (
MAIN TRANSPLANT OUTCOMES AND INFECTIOUS COMPLICATIONS:
Of the whole study group, 2 patients died soon after transplantation after cardiac arrest (1 after venous thrombosis and subsequent graftectomy and 1 due to the sepsis during COVID-19 infection), and they were treated with basiliximab. Another patient from the basiliximab subgroup lost his graft due to the venous thrombosis in the course of post-operative hypotonia and 1 patient returned to hemodialysis therapy after an ineffective treatment of acute rejection with ATG.
Of patients treated with ATG during the COVID-19 pandemic, 7 patients (22.6%) were hospitalized due to infection (4 had urinary tract infection [UTI], 1 developed orchitis, SARS-Cov-2 infection was diagnosed in 2 others), and 3 others (9.7%) had UTI treated at home. One patient died within 1 month after hospital discharge due to acute pulmonary and peripheral embolism, but she had no clinical or laboratory signs of sepsis. No other post-transplant complications were reported in this cohort. In the basiliximab subgroup, 6 patients (10.2%) developed infections (4 UTI, 1 kidney cyst inflammation, and 1 of unknown origin), and 3 (5.1%) of them were hospitalized. As expected, the percentage of patients with infection was significantly greater in the ATG subgroup (
Importantly, there were no significant differences in ATG doses, both median cumulative (250 [150–300] vs 275 [213–338] mg, respectively;
KIDNEY GRAFT FUNCTION:
There was no difference in median serum creatinine concentration between both main study subgroups during the first post-transplant year (3-month: 1.2 [0.9–1.4] in the ATG subgroup vs 1.2 [1.0–1.4] mg/dl in the control group; 6-month [n=70]: 1.3 [1.1–1.7] vs 1.2 [1.0–1.4] mg/dl, respectively; 12-month [n=40]: 1.1 [1.0–1.9] vs 1.1 [1.0–1.4] mg/dl, respectively).
Discussion
In our present study, we report our experience with the use of polyclonal antithymocyte globulin as both an induction therapy and anti-rejection treatment during the COVID-19 pandemic. In contrast to our previous clinical dilemmas, this powerful immunosuppressive agent was found to be relatively safe, enabling return to the full program of kidney transplantations, and also resuming kidney transplantations in patients with high immunologic risk.
Since the early spring of 2020, ATG use in kidney transplantation was significantly reduced, based mostly on previous reports about the increased risk of infectious complications, including death, in ATG-treated patients [20–22]. Importantly, a recent study confirmed that a higher ATG dosage in high-risk patients is associated with an increased risk of cytomegalovirus disease and death [23]. In our center, we previously demonstrated that age >50 years and an absolute lymphocyte count <200/1 μl at post-transplant day 7 were significant independent risk factors for death due to infections [18]. Consequently, when restoring the use of ATG in our center as the first wave of the COVID-19 pandemic subsided, the cumulative ATG doses were reduced to approximately 3–5 mg/kg. This may partially explain the relatively low and acceptable number of infectious complications in our present study, as the 3-month absolute lymphocyte number in the present analysis was significantly higher than was observed during the previous observation (median 0.58 [0.44–0.82]×103/μl). Noticeably, the percentage of infections was higher than in basiliximab subgroup, as was reported previously [24], but there were no significant differences in thrombocytopenia occurrence or in absolute lymphocyte count and lymphocyte percentage between patients with and without infections. Importantly, despite the decreased total ATG dose, the kidney graft function up to 12 months after transplantation was excellent. In agreement with our experience, the authors of the first report of COVID-19 infection within 1 month of living kidney transplant suggested that, among other factors, a lower dosage of ATG possibly contributed to the positive outcomes of those 2 recipients [13]. However, they used much smaller doses of ATG (1 mg/kg on the day of surgery), which probably would be insufficient in recipients with increased immunologic risk. On the other hand, much higher cumulative doses of ATG (7.5 and 5 mg/kg) were used in 2 simultaneous pancreas-kidney transplant recipients, who developed acute rejection shortly after mild COVID-19 infection [14]. Despite the intensive anti-rejection treatment, they did not present any signs or symptoms of recurrent COVID-19.
The major limitation of the present analysis is the low number of patients. It is worth noting that due to the COVID-19 pandemic, according to the medical literature and guidelines issued in March 2020 for Polish transplantation centers, we temporarily (for 4 months) stopped performing transplantation in patients who needed polyclonal antibody induction. In addition, the donor reporting rate diminished significantly, resulting in a relatively low (34.4%) percentage of patients with ATG induction who were eligible for the analysis. However, this is the first systematized report concerning the safety and effectiveness of clinical use of ATG during the ongoing COVID-19 pandemic, also including data regarding drug doses, subsequent kidney graft function, and lymphocyte count. As the current literature on this specific topic is scarce, our real-life experience, including cautious ATG dosing policy, may help other clinicians to make the right decision when choosing the induction therapy in kidney transplant recipients.
Conclusions
In conclusion, reasonable ATG use in the induction protocol or as the anti-rejection treatment during the COVID-19 pandemic appears to be safe and the risk of adverse events is acceptable. During COVID-19 pandemic, the necessary use of ATG should not be postponed in kidney transplant recipients with increased immunologic risk.
References
1. Dominguez-Gil B, Coll E, Fernandez-Ruiz M, COVID-19 in Spain: Transplantation in the midst of the pandemic: Am J Transplant, 2020; 20; 2593-98
2. Loupy A, Aubert O, Reese PP, Organ procurement and transplantation during the COVID-19 pandemic: Lancet, 2020; 395(10237); e95-96
3. Qu Z, Oedingen C, Bartling T, Schrem H, Krauth C, Organ procurement and transplantation in Germany during the COVID-19 pandemic: Lancet, 2020; 396(10260); 1395
4. Martino F, Plebani M, Ronco C, Kidney transplant programmes during the COVID-19 pandemic: Lancet Respir Med, 2020; 8; e39
5. Alberici F, Delbarba E, Manenti C, A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-Cov2 pneumonia: Kidney Int, 2020; 97; 1083-88
6. Banerjee D, Popoola J, Shah S, COVID-19 infection in kidney transplant recipients: Kidney Int, 2020; 97; 1076-82
7. Dorjee K, Kim H, Bonomo E, Dolma R, Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients: PLoS One, 2020; 15; e0243191
8. Pascual J, Zuckermann A, Djamali A, Rabbit antithymocyte globulin and donor-specific antibodies in kidney transplantation – A review: Transplant Rev, 2016; 30; 85-91
9. Bae S, McAdams-DeMarco MA, Massie AB, Early changes in kidney transplant immunosuppression regimens during the COVID-19 pandemic: Transplantation, 2021; 105; 170-76
10. Imam A, Tzukert K, Merhav H, Practical recommendations for kidney transplantation in the COVID-19 pandemic: World J Transplant, 2020; 10; 223-29
11. Goumard A, Sautenet B, Bailly E, Increased risk of rejection after basiliximab induction in sensitized kidney transplant recipients without pre-existing donor-specific antibodies – a retrospective study: Transplant Int, 2019; 32; 820-30
12. Michielsen LA, Wisse BW, Kamburowa EG, A paired kidney analysis on the impact of pre-transplant anti-HLA antibodies on graft survival: Nephrol Dial Transplant, 2019; 34; 1056-63
13. Shingare A, Bahadur MM, Raina S, COVID-19 in recent kidney transplant recipients: Am J Transplant, 2020; 20; 3206-9
14. Barros N, Sharfuddin AA, Powelson J, Rabbit anti-thymocyte globulin administration to treat rejection in simultaneous pancreas and kidney transplant recipients with recent COVID-19 infection: Clin Transplant, 2020; 35(2); e14149
15. Marfo K, Akalin E, Wang C, Lu A, Clinical and economic analysis of short-course versus standard-course antithymocyte globulin (rabbit) induction therapy in deceased-donor renal transplant recipients: Am J Health-Syst Pharm, 2011; 68; 2276-82
16. Peddi VR, Bryant M, Roy-Chaudhury P, Safety, efficacy, and cost analysis of thymoglobulin induction therapy with intermittent dosing based on CD3+ lymphocyte counts in kidney and kidney-pancreas transplant recipients: Transplantation, 2002; 73; 1514-18
17. Abouna GM, al-Abdullah IH, Kelly-Sullivan D, Randomized clinical trial of antithymocyte globulin induction in renal transplantation comparing a fixed daily dose with dose adjustment according to T cell monitoring: Transplantation, 1995; 59; 1564-68
18. Styrc B, Sobolewski M, Chudek J, Effectiveness and safety of two different antithymocyte globulins used in induction therapy in kidney transplant recipients: A single center experience: Clin Transplant, 2019; 33; e13680
19. Kolonko A, Dudzicz S, Więcek A, Król R, COVID-19 infection in solid organ transplant recipients: A single-center experience with patients immediately after transplantation: Transpl Infect Dis, 2021; 23(1); e13381
20. Hill P, Cross NB, Barnett ANRCochrane Database Kidney and Transplant Group, Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients: Cochrane Database Syst Rev, 2017; 2017(1); CD004759
21. Ducloux D, Kazory A, Challier B, Long-term toxicity of antithymocyte globulin induction may vary with choice of agent: A single center retrospective study: Transplantation, 2004; 77; 1029-33
22. Pham C, Kuten SA, Knight RJ, Assessment of infectious complications in elderly kidney transplant recipients receiving induction with anti-thymocyte globulin vs basiliximab: Transpl Infect Dis, 2020; 22; e13257
23. Bayraktar A, Catma Y, Akyildiz A, Infectious complications of induction therapies in kidney transplantation: Ann Transplant, 2019; 24; 412-17
24. Brennan DC, Daller JA, Lake KDThymoglobulin Induction Study Group, Rabbit antithymocyte globulin versus basiliximab in renal transplantation: N Engl J Med, 2006; 355; 1967-77
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860