22 March 2022: Original Paper
Pre-Transplant Peripheral Lymphocyte Subsets Predict Pneumonia After Renal Transplantation
Quan Zhuang12ADEG*, Min Yang1BCE, Shu Liu12BCF, Meng Yu1BC, Jie Jiang1BF, Bo PengDOI: 10.12659/AOT.934773
Ann Transplant 2022; 27:e934773
Abstract
BACKGROUND: Kidney transplantation (KTx) has been considered as the most effective therapeutic method for end-stage renal disease. Immune monitoring of peripheral lymphocyte subsets (PLS) reflects the real immune status and has been used for diagnosis of pneumonia after KTx. We aimed to investigate the association between pre-transplant PLS and pneumonia in renal allograft recipients.
MATERIAL AND METHODS: A total of 152 patients receive donation after citizen’s death (DCD) kidney allografts in our center between January 2018 and December 2019. Among them, 114 patients were enrolled in our study based on inclusion and exclusion criteria. During the first-year follow-up after KTx, 32 recipients developed pneumonia, and the other 82 recipients did not (stable group). The pre-clinical information and PLS (including the percentages and absolute numbers (Ab No.) of peripheral T, B, and NK cells, as well as CD4/CD8 ratio) results in these 2 groups were calculated by the Mann-Whitney test and receiver operating curve (ROC) analysis. Univariate and multivariate logistic regression analyses were employed to identify risk factors.
RESULTS: Compared to the stable group, the Ab No. of CD3+, CD8+, and CD4+ T cells, as well as B cells and NK cells, were notably reduced in the pneumonia patients. The area under the curve (AUC) of CD3+ T cell Ab No. was 0.7022. Multivariate analysis demonstrated that pre-transplant B cell Ab No. was the independent risk factor for pneumonia progression after KTx (OR=0.353, P=0.037).
CONCLUSIONS: Pre-transplant Ab No. of PLS were closely related to pneumonia after KTx. Monitoring pre-transplant PLS could provide more timely and effective prevention and therapy for post-operative pneumonia after KTx.
Keywords: Kidney Transplantation, Lymphocyte Subsets, Humans, Kidney Failure, Chronic, Lymphocyte Count
Background
Kidney transplantation (KTx) is the optimal treatment for end-stage renal disease (ESRD) at present, but long-term application of immunosuppressive agents causes many problems, among which infection is considered to be one of the most challenging complications, especially pneumonia, which is the leading cause of death for renal allograft recipients [1,2]. Therefore, the assessment and monitoring of the recipients’ immune status is very important for the preventive and therapeutic approaches of pneumonia after KTx. Although clinical detection techniques have been greatly improved, peripheral blood is still the most common source of samples for immune status detection due to its rich immunological information, minimal invasiveness, and convenience. Since the commonly used immunosuppressants mainly affect the number and function of lymphocytes, monitoring peripheral lymphocytes is considered to be the most direct way to reflect the immune status and function of the body [3]. Thus, previous studies have already focused on the association between peripheral lymphocyte subsets (PLS) and pneumonia after KTx to evaluate its diagnostic value for pneumonia [4–9].
During the ESRD stage (ie, pre-transplant stage), the immune function of renal transplant recipients has already been affected. Under the influence of uremic toxins, metabolic abnormalities, chronic inflammation, dialysis, and water overload, patients tend to have immune impairment or imbalance [10–12]. After renal transplantation, with the recovery of renal function and the reduction of toxins, the immune function of the renal allograft recipients can be gradually rebuilt and repaired [13,14]. Pre-transplant immune function in patients with ESRD can clearly affect post-operative outcome and survival [15]. Therefore, we speculated that there might be links between pre-transplant PLS and post-operative outcomes, especially the incidence and progression of pneumonia. In this study, patients with ESRD who underwent KTx in our center received a PLS test before surgery. During the first-year follow-up after KTx, patients were divided into 2 groups: those who developed pneumonia and those who did not. The association between pre-transplant PLS and the incidence and progression of pneumonia were analyzed. We hope to provide ideas and evidence for the prevention and treatment of post-operative pneumonia through pre-transplant PLS monitoring.
Material and Methods
STUDY DESIGN AND DATA COLLECTION:
A total of 152 patients received donation after citizen’s death (DCD) kidney allografts in our center between January 2018 and December 2019. All transplant operations were approved by the DCD Ethics Committee of the 3rd Xiangya Hospital, Central South University and the grafts were distributed by the China Organ Transplant Response System (COTRS, www.cot.org.cn). For patients requiring immune induction, basiliximab (20 mg on day 0 and day 4) or anti-thymocyte globulin (ATG) (1.00 mg/kg daily for 3 days) was applied. The standard maintenance immunosuppressants consisted of calcineurin inhibitor (CNI), mycophenolate mofetil (MMF), and corticosteroid. The diagnostic criteria for pneumonia were obvious clinical manifestation, positive pathogen culture, and abnormal X-ray or CT scan. Based on inclusion and exclusion criteria (Table 1), 114 patients were enrolled in this study, of which 32 patients had pneumonia during the first-year follow-up period, and the other 82 patients had neither infection nor rejection (stable). Clinical information is provided in Table 2. The study was approved by the Institutional Review Board and the Ethics Committee of the 3rd Xiangya Hospital, Central South University (No. 2018-S347).
BLOOD SAMPLE COLLECTION AND FLOW CYTOMETRY STRATEGY:
The peripheral blood samples from patients were collected several hours before KTx in an EDTA anticoagulant tube. Then, 50 μl of these samples were directly transferred to a BD Trucount tube with BD Multitest 6-color TBNK reagent (10 μl) and incubated in the dark for 15 min. Them, 450 μl red blood cell lysate (MBL Life Science, Nagoya, Japan) was added. The samples were subjected to flow cytometry (BD FACSCanto II) and analyzed using BD FACSCanto clinical software (BD Biosciences, San Jose, CA, USA). The percentage and absolute numbers (Ab No.) of CD3+, CD3+CD4+, CD3+CD8+ T cells, CD19+ B cells, and CD16+CD56+ NK cells, as well as CD4/CD8 ratio, were calculated. Details of the methods used were described in our previous study [16].
STATISTICAL ANALYSIS:
All analyzed data were expressed using mean and standard deviation (SD). At baseline, the sex ratio and the proportion of induction regimen were compared by chi-square (χ2) test. The non-normally distributed data of lymphocyte subsets in the 2 groups were compared by Mann-Whitney U test. The optimal cut-off values of the absolute counts of CD3+, CD8+, and CD4+ T cells, B cells, and NK cells were computed by using receiver operating curve (ROC) analysis. The risk factors of disease progression were screened by applying univariate and multivariate logistic regression analyses. Renal function was assessed via the estimated glomerular filtration rate (eGFR) calculated based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eq. (2009). Statistical calculations were performed using GraphPad Prism version 9.0 (GraphPad Software, Inc., La Jolla, CA, USA) or SPSS version 23.0 (SPSS, Inc., Chicago, IL, USA). Results were considered statistically significant when
Results
CLINICAL INFORMATION:
A total of 114 patients were eligible for this study, of which 32 patients had pneumonia during the first-year follow-up period, and the other 82 patients had neither infection nor rejection (stable). The mean age of all patients was 42.36±10.79 years old. The onset time of pneumonia was 5.47±3.98 months after the operation. Six patients obtained an immunosuppressant withdrawn after a reliable diagnosis of pneumonia. Twenty-one cases of pneumonia were diagnosed by obvious clinical symptoms and CT manifestations, and 11 cases were diagnosed by blood pathogen culture, clinical symptoms, and CT manifestations. The statistical comparison between the 2 groups in all the clinical indices showed no significant difference (P>0.05). The distribution of the pathogens in the pneumonia group were bacteria (n=13, 41%), virus (n=6, 19%), fungus (n=10, 31%), and polyinfection (n=3, 9%) (Table 2).
COMPARISON OF PRE-TRANSPLANT PLS ANALYSIS BETWEEN PNEUMONIA AND STABLE ALLOGRAFT RECIPIENTS:
Compared to stable allograft recipients, the ones with pneumonia presented with markedly lower cell counts of pre-transplant CD3+ T cells (976.10±347.70 vs 725.10±276.00, P=0.0007), CD8+ T cells (411.50±194.30 vs 306.60±157.30, P=0.0051), and CD4+ T cells (539.40±204.60 vs 411.60±145.70, P=0.0037), as well as B cells (139.60±83.39 vs 95.81±61.27, P=0.0056) and NK cells (210.60±186.70 vs 149.50±114.80, P=0.0468). However, there was no significant difference of the frequencies in all the pre-transplant indexes above (P>0.05), as well as CD4/CD8 ratio (Table 3, Figure 1).
ROC CURVE AND CUT-OFF VALUES OF PRE-TRANSPLANT PLS:
We selected statistically significant indexes from the above results to discern patients with infection from stable allograft recipients, including the absolute counts of CD3+, CD8+, and CD4+ T cells, as well as B cells and NK cells. We determined the optimal cut-off values using ROC analysis. Among them, the absolute value of CD3+ T cells had a relatively high AUC (0.7022) (Figure 2). The optimal cut-off values were 822.7/ul, 285/ul, 422.7/ul, 104.5/ul, and 78.50/ul for the absolute counts of CD3+, CD8+, and CD4+ T cells, as well as B cells and NK cells. All AUC and cut-off values are displayed in Table 4.
THE INDEPENDENT RISK FACTOR OF PRE-TRANSPLANT PLS FOR DISEASE PROGRESSION:
We aimed to identify independent predictive factors associated with disease progression based on clinical descriptions, and found none of the clinical indexes were significantly related with disease progression in univariate/multivariate logistic analysis. In Table 5, the univariate analysis demonstrated that the Ab No. of pre-transplant CD3+, CD8+, and CD4+ T cells, as well as B cells, were strongly correlated with disease progression. Multivariate analysis revealed only the Ab No. of pre-transplant B cells (OR=0.353, P=0.037) was an independent risk factor for disease progression.
Discussion
This study successfully detailed the distribution and Ab No. of pre-transplant PLS and demonstrated their significance in predicting pneumonia after KTx. We found that, compared to the stable group, the Ab No. of CD3+, CD8+, and CD4+ T cells, as well as B cells and NK cells, were significantly lower in the pneumonia patients. Among these significant indices above, the AUC of CD3+ T cell Ab No. was 0.7022, and the cut-off value was 822.7/ul. The result of multivariate logistic analysis demonstrated that a low level of pre-transplant B cell Ab No. was an independent risk factor for pneumonia progression after KTx. Therefore, monitoring pre-transplant PLS allow prediction of pneumonia and contribute to prompt prevention and therapy for pneumonia after KTx.
Pneumonia is one of the most common complications in KTx, and morbidity and mortality rates among patients are high. During the early post-transplant period, recipients are at increased risk of infection during hospitalization [1]. In our study, patients in the ATG and non-ATG groups were not analyzed because of data deficiencies in patients who received transplants outside our center. Due to the low routine dose of ATG used in China (1.00 mg/kg for 3 consecutive days), patients typically developed lymphocyte reconstruction 3 months after transplantation (unpublished data). Hence, we only enrolled patients beyond 3 months after transplantation. Some studies showed a strong association between post-operative PLS and pneumonia after kidney transplantation. Luo et al found that decreased Ab No. of T cells and NK cells were related to the infection onset in renal allograft recipients [17]. Fernandez-Ruiz et al also reported that low post-operative Ab No. of PLS could predict post-transplantation opportunistic infections in renal transplant recipients and discovered that CD8+ T cells <100/μl and CD4+ T cells <50/μl at 1 month after transplantation were the optimal predictors discriminating between the non-ATG and ATG groups [5]. In our recent study, we found all the Ab No. of post-operative PLS were closely associated with pneumonia diagnosis in KTx, and among them, we verified that the Ab No. of CD8+ T cells and NK cells showed the best prediction performance, with AUC of 0.940 by machine learning models [16]. We further investigated the diagnostic value of severe pneumonia in KTx and found that the counts of CD4+ T cells and NK cells were significantly decreased in patients with severe pneumonia (unpublished data). Therefore, the diagnostic value of post-operative PLS (mainly the absolute counts) was shown to be very high in pneumonia after KTx. Most transplant experts agree that early prediction and prevention of respiratory infection is even more important than after the onset of infection. However, there are not enough predictable studies of this kind, especially pre-transplant predicts of immune function. Crepin et al performed research similar to the present study and found pre-transplant immune senescence (ie, CD4/CD8 ratio less than 1 and/or CD8+ T cell count exceeding 90th percentile) increased the risk of opportunistic infections and severe bacterial infection after renal transplantation [18]. Fribourg et al monitored peripheral T cell depletion using time-of-flight (CyTOF) mass cytometry and found that there was a correlation between a rise of particular subgroups of CD4+ and CD8+ depleted T cells from before transplantation compared to 6 months after transplantation, and found improved outcomes in kidney transplant recipients, but they also pointed out this would be related to an increased incidence of opportunistic infections after KTx [19]. In our current study, low pre-transplant Ab No. of all the PLS indices showed a strong predictable association with pneumonia after KTx, but there was no significant difference in the distribution of these indices, and they found no significant difference in CD4/CD8 ratio. We also showed post-operative CD4/CD8 ratio might not be a meaningful index for diagnosing the pneumonia after KTx [16]. According to our clinical experience, percentages and CD4/CD8 ratio only had efficacy if the absolute counts were basically normal. Additionally, some special cell markers and cytokines could be also used for predictable indices. CD200 is an immunosuppressive protein expressed on a wide variety of cells such as B cells and activated T cells that inhibits immunological effect through its receptor CD200R1. Oweira et al assessed CD200 and CD200R1 concentrations immediately before KTx and found there was a positive association between pre-transplant CD200R1 concentrations and Cytomegalovirus (CMV) infection [20]. Li et al assessed pre-transplant serum T cell immunoglobulin and mucin domain-3 (Tim-3) and Galectin-9 (Gal-9), but found they were not obviously related to infection after KTx [21].
Renal failure and dialysis alter a patient’s immune profile, and both the innate and adaptive immune systems are seriously impaired because of metabolic disorder, uremic toxin accumulation, chronic inflammatory stimulation, long-term dialysis, and other factors [22]. Although uremia can stimulate the activation of innate immune effectors, their impaired anti-bacterial capacity can lead to a higher risk of extracellular bacterial infections [23]. Premature senescence of CD4+ T cells during ESRD is considered to influence subsequent kidney graft function, and because the senescent T cells cannot be inverted, the peripheral T cell compartments are impaired after KTx using immunosuppressants [24]. Additionally, progressive thymic involution is a predominant characteristic of ESRD-associated immune senescence. Courivaud et al assessed the thymic function before transplantation via recent thymic emigrants (RTE) and found that lower levels of RTE were strongly correlated with poor outcomes in renal allograft recipients [15]. A recent single-cell transcriptome study showed that among ESRD patients there were a marked rise in inflammation reaction, complement cascade, and cell metabolism, and a significant decline in regulating the progression of immune cell cycle in related cell-types [25]. In a word, immune impairment was continuous from ESRD stage to post-transplant period, and the immune function could not be recovered even if the renal dysfunction was restored by transplant surgery. Thus, the infection risk after KTx was tightly associated with a degree of pre-transplant immune status and function. Another interesting issue is that pre-transplant B cell count had a major impact on pneumonia occurrence after KTx by multivariate logistic analysis in our study. Thus, apart from failing to augment immune reactions of efficient T cell, the adaptive humoral reactions also become impaired in uremia patients. As the antigen binds to the surface B cell receptor (BCR), antibodies to the protein antigen are produced in the secondary lymphoid organs. The activated first signal transfers to cogenetic B cells and facilitates the internalized antigen, and is subsequently presented as peptides on cell-surface MHC molecules. The activated second signal is transferred via T follicular helper (Tfh) cells. After recognition of antigen/MHC-complexes on the surface of B cells via the TCR, the final signal reciprocally transfers soluble and membrane-bound effector molecules. B cells are activated in the germinal center reaction, which gives rise to high-affinity antibody-secreting plasma cells and high-affinity memory B cells [26]. Therefore, it is observed that uremia toxins can suppress the immune reactions of helper T cell and result in failing to activate B cells and produce antibody responses [27], perhaps because the uremia toxins have a negative impact on B cells, impairing humoral response among uremia patients. Decline in GFR is related to a reduced number of total B cells, which seemingly influence naïve/memory subsets [28]. The above processes might lead to the non/low response of an anti-infection by B cells after KTx. In addition to the association with pneumonia after KTx, other outcomes, like renal function (creatinine level), are of interest to researchers. Therefore, we performed correlation analysis between post-operative creatinine level and pre-transplant PLS in both patient groups using Pearson correlation analysis. However, we did not find any statistically significance difference (Supplementary Figure 1), which indicated there would be no relationship between post-operative creatinine level and pre-transplant PLS in renal allograft recipients.
There were some limitations to this study. First, our study had a comparatively small number of samples from a single center, which may have contributed to biased data analysis. Due to the imperfect follow-up and the supply of some laboratory reagents, many data such as MFI of CD64 and HLA-DR were missing, which directly led to these indicators being excluded. As a result, our study indicators were reduced. Therefore, future large-scale, multicenter, and multiple-index studies are required to fully identify the mechanisms of pre-transplant PLS in transplant infection and immunity.
Conclusions
This study demonstrated the association between pre-transplant PLS and pneumonia after KTx and found that Ab No. of pre-transplant CD3+, CD8+, and CD4+ T cells, as well as B cells and NK cells, was significantly lower in the pneumonia patients. Among them, CD3+ T cell Ab No. could best identify patients at risk of pneumonia. Furthermore, the low level of pre-transplant B cell Ab No. was an independent risk factor for pneumonia progression. Monitoring pre-transplant PLS can provide important insights for more timely and effective prevention and therapy of post-operative pneumonia in renal allograft recipients.
Figures
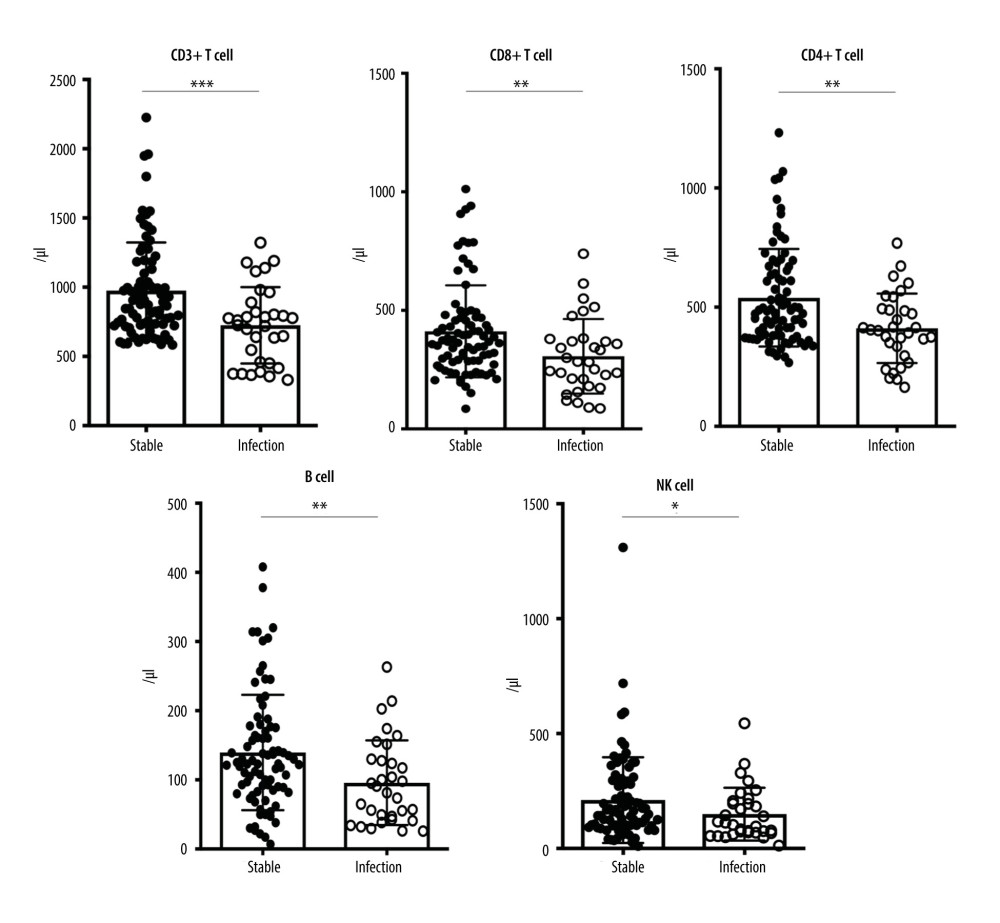 Figure 1. Ab No. of CD3+ T cell, CD8+ T cell, CD4+ T cell, B cell and NK cell between pneumonia and stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA).
Figure 1. Ab No. of CD3+ T cell, CD8+ T cell, CD4+ T cell, B cell and NK cell between pneumonia and stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA). 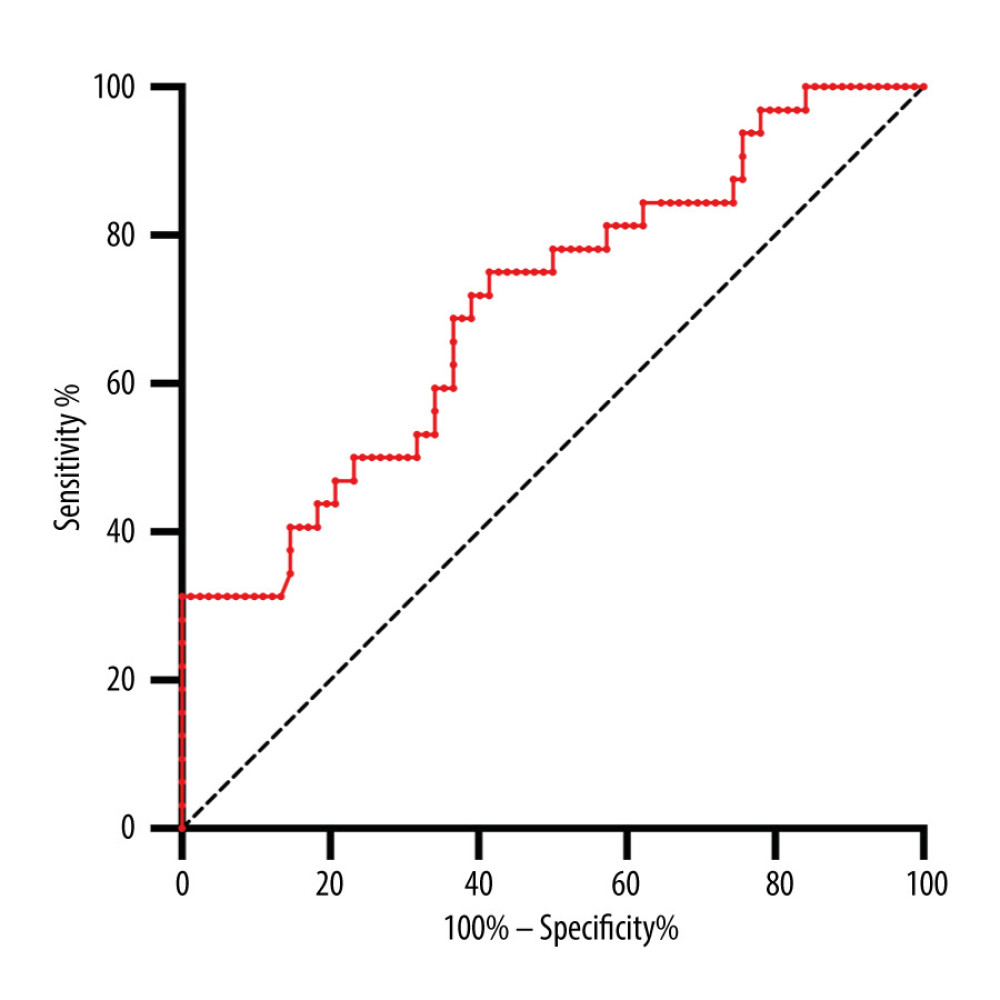 Figure 2. ROC curve of Ab No. of CD3+ T cells used to distinguish pneumonia from stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA).
Figure 2. ROC curve of Ab No. of CD3+ T cells used to distinguish pneumonia from stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA). Tables
Table 1. Inclusion and exclusion criteria for renal allograft recipients.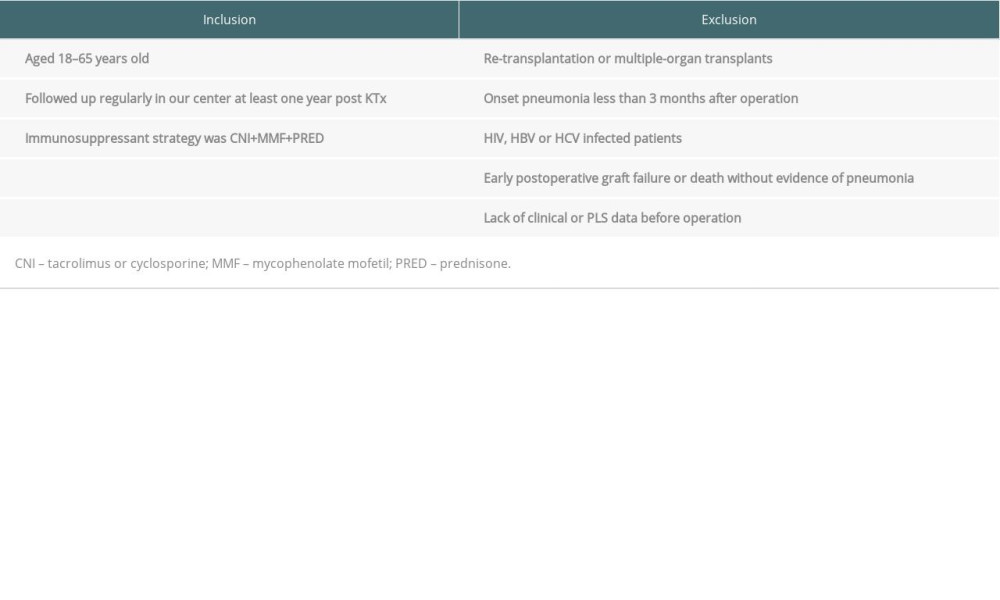 Table 2. Clinical characteristics between pneumonia and stable allograft recipients.
Table 2. Clinical characteristics between pneumonia and stable allograft recipients.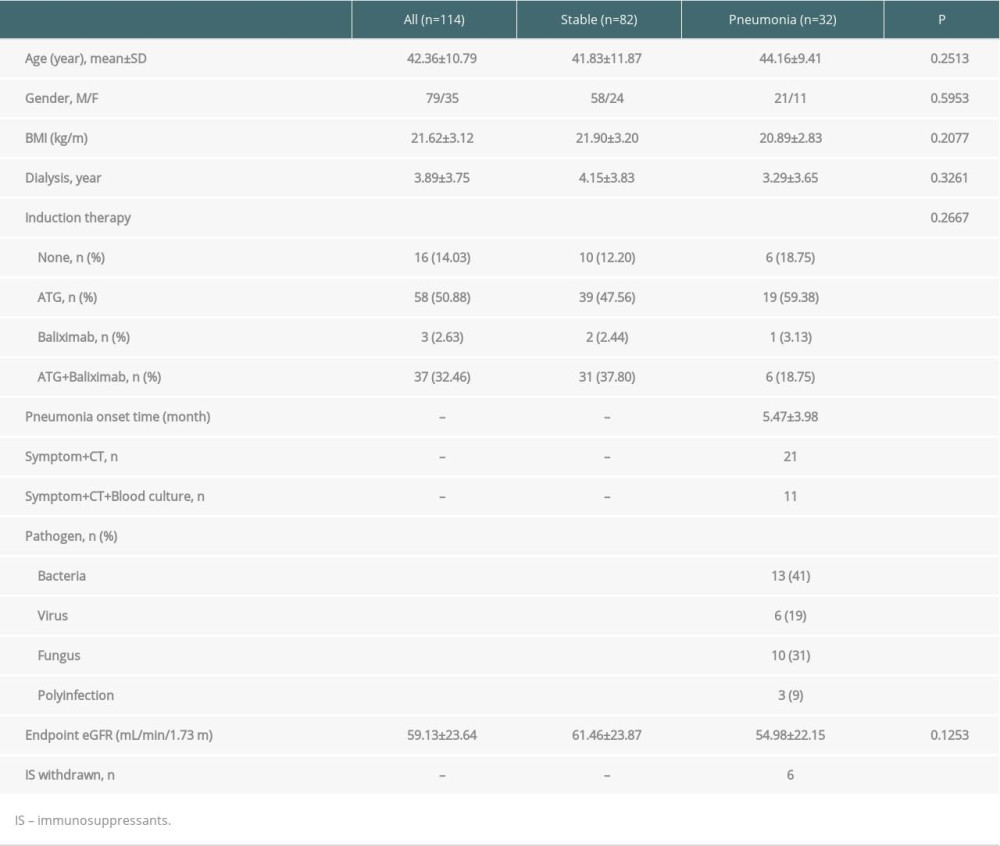 Table 3. Pre-transplant PLS analysis between pneumonia and stable allograft recipients.
Table 3. Pre-transplant PLS analysis between pneumonia and stable allograft recipients.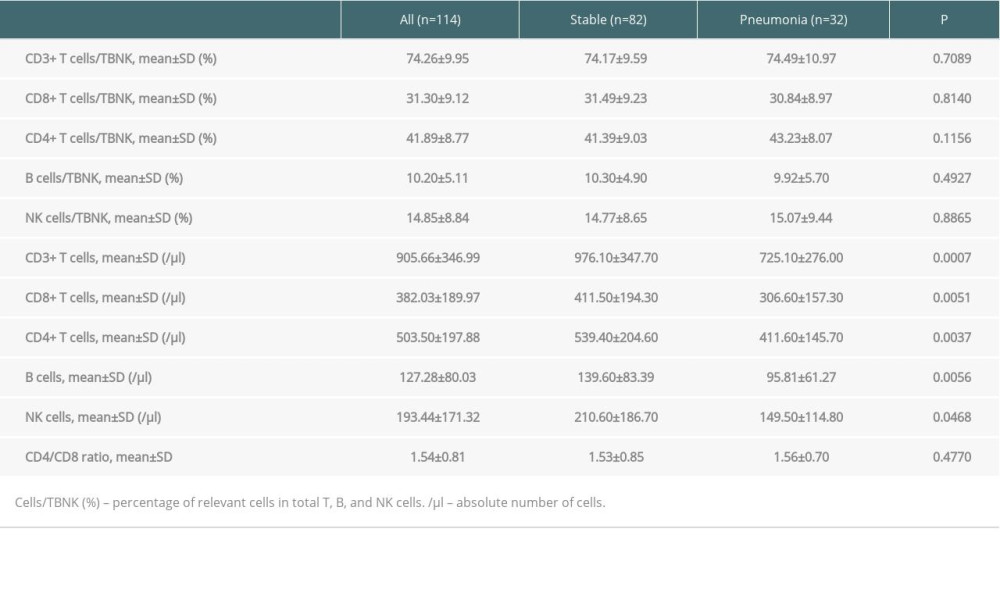 Table 4. Areas under the curve (AUC) and cut-off values of pre-transplant PLS.
Table 4. Areas under the curve (AUC) and cut-off values of pre-transplant PLS.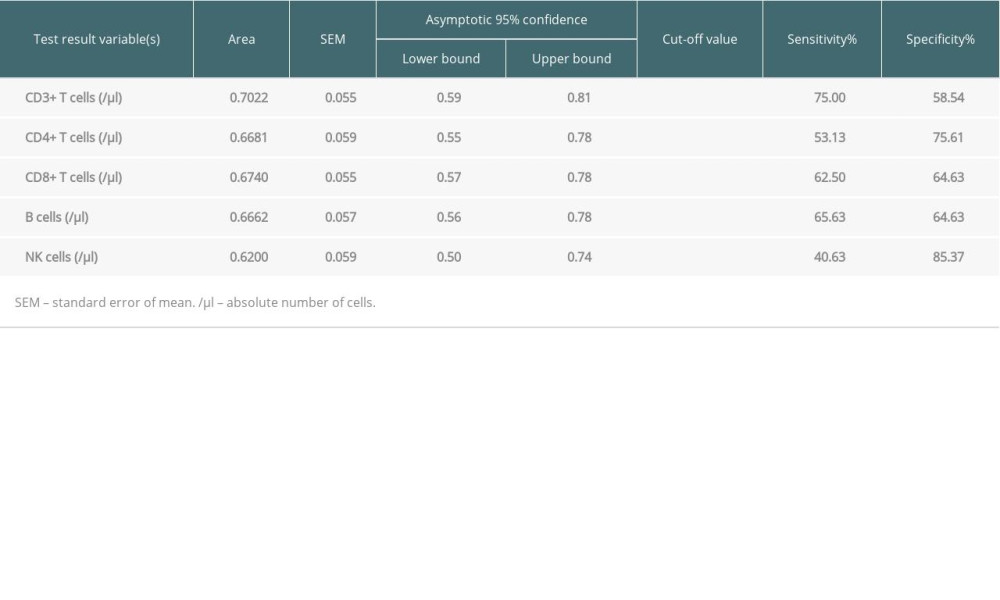 Table 5. Risk factors of pre-transplant PLS for progression by logistic regression.
Table 5. Risk factors of pre-transplant PLS for progression by logistic regression.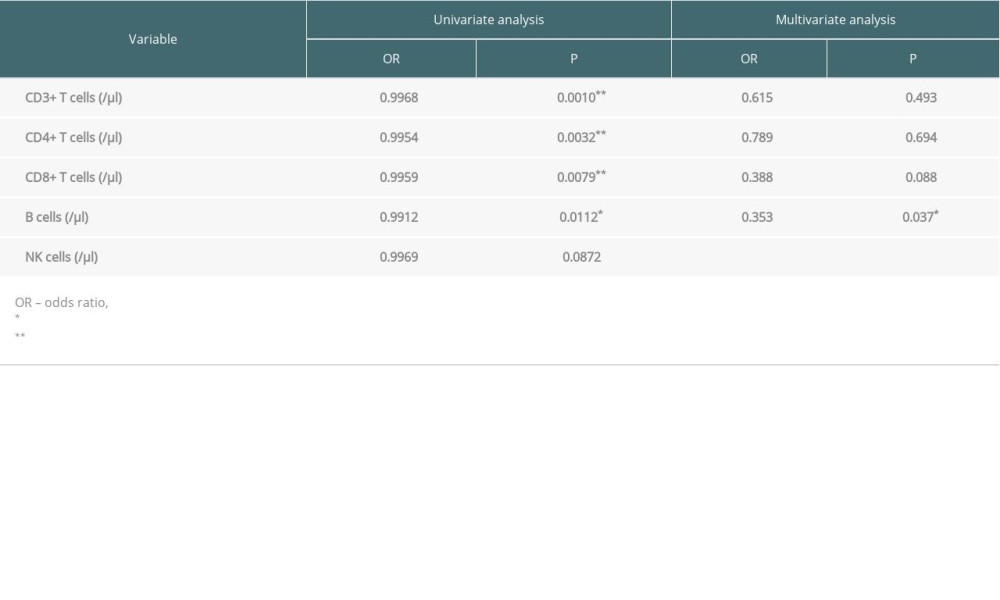
References
1. Wilmes D, Coche E, Rodriguez-Villalobos H, Kanaan N, Bacterial pneumonia in kidney transplant recipients: Respir Med, 2018; 137; 89-94
2. Gudiol C, Sabé N, Carratalà J, Is hospital-acquired pneumonia different in transplant recipients?: Clin Microbiol Infect, 2019; 25(10); 1186-94
3. Fernández-Ruiz M, López-Medrano F, Predictive tools to determine risk of infection in kidney transplant recipients: Expert Rev Anti Infect Ther, 2020; 18(5); 423-41
4. Calarota SA, Zelini P, De Silvestri A, Kinetics of T-lymphocyte subsets and posttransplant opportunistic infections in heart and kidney transplant recipients: Transplantation, 2012; 93(1); 112-19
5. Fernández-Ruiz M, López-Medrano F, Allende LM, Kinetics of peripheral blood lymphocyte subpopulations predicts the occurrence of opportunistic infection after kidney transplantation: Transpl Int, 2014; 27(7); 674-85
6. Bouvy A, Klepper M, Kho M, The impact of induction therapy on the homeostasis and function of regulatory T cells in kidney transplant patients: Nephrol Dial Transplant, 2014; 29(8); 1587-97
7. El Helou G, Lahr B, Razonable R, Absolute lymphocyte count as marker of cytomegalovirus and allograft rejection: Is there a “Safe Corridor” after kidney transplantation?: Transplant Infect Dis, 2021; 23(2); e13489
8. Meesing A, Abraham RS, Razonable RR, Clinical correlation of cytomegalovirus infection with CMV-specific CD8+ T-cell immune competence score and lymphocyte subsets in solid organ transplant recipients: Transplantation, 2019; 103(4); 832-38
9. Werbel WA, Ison MG: Clin Infect Dis, 2018; 20(3); e12876
10. Cohen G, Immune dysfunction in uremia 2020: Toxins (Basel), 2020; 12(7); 439
11. Syed-Ahmed M, Narayanan M, Immune dysfunction and risk of infection in chronic kidney disease: Adv Chronic Kidney Dis, 2019; 26(1); 8-15
12. Betjes MG, Immune cell dysfunction and inflammation in end-stage renal disease: Nat Rev Nephrol, 2013; 9(5); 255-65
13. Zhuang Q, Peng B, Wei W, The detailed distribution of T cell subpopulations in immune-stable renal allograft recipients: A single center study: Peer J, 2019; 7; e6417
14. Zhuang Q, Li H, Yu M, Profiles of B-cell subsets in immunologically stable renal allograft recipients and end-stage renal disease patients: Transpl Immunol, 2020; 58; 101249
15. Courivaud C, Bamoulid J, Crepin T, Pre-transplant thymic function predicts is associated with patient death after kidney transplantation: Front Immunol, 2020; 11; 1653
16. Peng B, Gong H, Tian H, The study of the association between immune monitoring and pneumonia in kidney transplant recipients through machine learning models: J Transl Med, 2020; 18(1); 370
17. Luo Y, Xie Y, Zhang W, Combination of lymphocyte number and function in evaluating host immunity: Aging, 2019; 11(24); 12685-707
18. Crepin T, Gaiffe E, Courivaud C, Pre-transplant end-stage renal disease-related immune risk profile in kidney transplant recipients predicts post-transplant infections: Transpl Infect Dis, 2016; 18(3); 415-22
19. Fribourg M, Anderson L, Fischman C, T-cell exhaustion correlates with improved outcomes in kidney transplant recipients: Kidney Int, 2019; 96(2); 436-49
20. Oweira H, Khajeh E, Mohammadi S, Pre-transplant CD200 and CD200R1 concentrations are associated with post-transplant events in kidney transplant recipients: Medicine, 2019; 98(37); e17006
21. Li YM, Li Y, Yan L, Assessment of serum Tim-3 and Gal-9 levels in predicting the risk of infection after kidney transplantation: Int Immunopharmacol, 2019; 75; 105803
22. Baragetti I, El Essawy B, Fiorina P, Targeting immunity in end-stage renal disease: Am J Nephrol, 2017; 45(4); 310-19
23. Espi M, Koppe L, Fouque D, Thaunat O, Chronic kidney disease-associated immune dysfunctions: Impact of protein-bound uremic retention solutes on immune cells: Toxins (Basel), 2020; 12(5); 300
24. Meijers RW, Litjens NH, de Wit EA, Uremia-associated immunological aging is stably imprinted in the T-cell system and not reversed by kidney transplantation: Transplant Int, 2014; 27(12); 1272-84
25. Luo T, Zheng F, Wang K, A single-cell map for the transcriptomic signatures of peripheral blood mononuclear cells in end-stage renal disease: Nephrol Dial Transplant, 2021; 36(4); 599-608
26. Crotty S, A brief history of T cell help to B cells: Nat Rev Immunol, 2015; 15(3); 185-89
27. Girndt M, Sester M, Sester U, Molecular aspects of T- and B-cell function in uremia: Kidney Int Suppl, 2001; 78; S206-11
28. Fernández-Fresnedo G, Ramos MA, González-Pardo MC, B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2: Nephrol Dial Transplant, 2000; 15(4); 502-10
Figures
 Figure 1. Ab No. of CD3+ T cell, CD8+ T cell, CD4+ T cell, B cell and NK cell between pneumonia and stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA).
Figure 1. Ab No. of CD3+ T cell, CD8+ T cell, CD4+ T cell, B cell and NK cell between pneumonia and stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA). Figure 2. ROC curve of Ab No. of CD3+ T cells used to distinguish pneumonia from stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA).
Figure 2. ROC curve of Ab No. of CD3+ T cells used to distinguish pneumonia from stable allograft recipients. (GraphPad Prism version 9.0, GraphPad Software, Inc., La Jolla, CA, USA). Tables
 Table 1. Inclusion and exclusion criteria for renal allograft recipients.
Table 1. Inclusion and exclusion criteria for renal allograft recipients. Table 2. Clinical characteristics between pneumonia and stable allograft recipients.
Table 2. Clinical characteristics between pneumonia and stable allograft recipients. Table 3. Pre-transplant PLS analysis between pneumonia and stable allograft recipients.
Table 3. Pre-transplant PLS analysis between pneumonia and stable allograft recipients. Table 4. Areas under the curve (AUC) and cut-off values of pre-transplant PLS.
Table 4. Areas under the curve (AUC) and cut-off values of pre-transplant PLS. Table 5. Risk factors of pre-transplant PLS for progression by logistic regression.
Table 5. Risk factors of pre-transplant PLS for progression by logistic regression. Table 1. Inclusion and exclusion criteria for renal allograft recipients.
Table 1. Inclusion and exclusion criteria for renal allograft recipients. Table 2. Clinical characteristics between pneumonia and stable allograft recipients.
Table 2. Clinical characteristics between pneumonia and stable allograft recipients. Table 3. Pre-transplant PLS analysis between pneumonia and stable allograft recipients.
Table 3. Pre-transplant PLS analysis between pneumonia and stable allograft recipients. Table 4. Areas under the curve (AUC) and cut-off values of pre-transplant PLS.
Table 4. Areas under the curve (AUC) and cut-off values of pre-transplant PLS. Table 5. Risk factors of pre-transplant PLS for progression by logistic regression.
Table 5. Risk factors of pre-transplant PLS for progression by logistic regression. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








