23 August 2022: Original Paper
Management of Spontaneous Portosystemic Shunts in 231 Patients Who Underwent Living Donor Liver Transplantation: A Retrospective Study from a Single Center in Nagasaki, Japan
Takashi Hamada1ABCDEF, Masaaki Hidaka1ACDEF*, Akihiko SoyamaDOI: 10.12659/AOT.936371
Ann Transplant 2022; 27:e936371
Abstract
BACKGROUND: We have ligated spontaneous portosystemic shunts (SPSS) in living donor liver transplantation (LDLT) when a postoperative interventional radiology (IVR) approach was impossible or the intraoperative hepatopetal flow was insufficient. This retrospective study from a single center in Nagasaki, Japan aimed to investigate the management of SPSS in 231 patients who underwent LDLT between January 2006 and December 2019.
MATERIAL AND METHODS: SPSS were identified in 63 patients (27.3%). Perioperative factors and survival rates were compared in the study population with SPSS divided into 2 groups: the ligation group and the non-ligation group. The post-transplant course was examined in greater detail in the non-ligation group.
RESULTS: SPSS were ligated in 20 patients (31.7%). The indication for shunt ligation was an impossible postoperative approach (10 patients; 50%) or poor intraoperative hepatopetal flow (10 patients; 50%). There was no significant difference in the 1- and 5-year overall survival rates between the ligation and non-ligation group (80%, 80% vs 76%, 55%, respectively, P=0.17). Of the 34 patients in the non-ligation group who could be observed for 6 months, 14 patients (48.3%) had a spontaneous regression of SSPS. Additionally, 5 patients who required postoperative IVR had a good clinical course. There was no graft failure or adverse events in the non-ligation group.
CONCLUSIONS: Unnecessary ligation could be avoided by using our criteria. When postoperative IVR is possible with sufficient intraoperative hepatopetal flow, SPSS do not always need to be ligated in LDLT.
Keywords: Ligation, Liver Transplantation, Patent Ductus Venosus, Radiology, Interventional, Humans, Japan, Living Donors, Portasystemic Shunt, Transjugular Intrahepatic
Background
In patients with liver cirrhosis, portal hypertension leads to development of spontaneous portosystemic shunts (SPSS), which are potentially hemodynamically significant [1,2]. SPSS are channels that bypasses the high vascular resistance of the portal system, flowing down a pressure gradient to a low-pressure systemic venous system [3]. In approximately 20–60% of cirrhotic patients, some types of SPSS are detected by imaging [4,5].
Despite the relatively high frequency of SPSS due to portal hypertension in liver transplantation (LT) patients, there are no guidelines on their management [1]. SPSS are rarely ligated in deceased donor liver transplantation (DDLT) because whole-liver grafts have a larger capacitance for portal flow and can effectively decompress the portal system, thereby closing down the SPSS in a shorter time [6]. However, in living donor liver transplantation (LDLT), SPSS leads to the portal flow steal phenomenon, and subsequent decreased portal inflow to the graft; thus, they are associated with an increased risk of postoperative graft failure, portal vein thrombosis, and hepatic encephalopathy [7,8]. Although ligation of SPSS prevents these complications [9–11], access to SPSS may be a technically difficult procedure that is often associated with massive bleeding due to remarkably high portal pressure in patients with liver cirrhosis [12]. Additionally, ligation of SPSS sometimes leads to inferior vena cava (IVC) thrombosis [13] and can cause severe portal hypertension, resulting in small-for-size graft dysfunction [14].
A study by Gómez-Gavara et al that included 66 patients with splenorenal shunts of >1 cm reported that approximately half of them underwent shunt ligation during liver transplantation (LT) [12], which supports routine ligation of large SPSS during LT whenever feasible. A recent review by Vidal-González et al reported that management of SPSS in LT is still controversial. Additionally, when there is persistence of symptomatic large SPSS during long-time follow-up, interventional radiology (IVR) could be considered, although experience in IVR after LT is extremely limited [15].
This retrospective study from a single center in Nagasaki, Japan aimed to investigate the management of SPSS in 231 patients who underwent LDLT between January 2006 and December 2019. Regarding the management, if the hepatopetal flow was not stolen and IVR was available for SPSS, there was no need for ligation. Additionally, the present study aimed to clarify the conditions under which SPSS should be ligated in LDLT and how SPSS affect the outcome after LDLT.
Material and Methods
PATIENTS:
The present study, which involved retrospective review of the patients’ medical and imaging records, was approved by our research ethics committee (IRB: 20012022). This study was a retrospective analysis of all consecutive adult patients who underwent LDLT at Nagasaki University Hospital in Japan from January 2006 to December 2019. Standard indications for LDLT and the surgical techniques for both the donor and recipient operations have been described in detail elsewhere [16]. The study population included all consecutive patients who underwent LDLT with or without SPSS ligation. Patients with SPSS were divided into 2 groups: the ligation group and the non-ligation group. Additionally, the non-ligation group was divided into 2 groups: the survivor group and the non-survivor group.
DEFINITION OF SPONTANEOUS PORTOSYSTEMIC SHUNTS (SPSS):
A shunt was defined as the presence of collateral veins on pretransplant diagnostic imaging, including a 3-dimensional computed tomography (CT) scan. SPSS was defined as a venous communication between the portal and venous systems of ≥10 mm in largest diameter [9,10,12]. SPSS was identified and classified as the following types of shunts: spleno-renal, gastrorenal, left gastric-azygous, and mesenteric-iliac. In this study, no patients with SPSS underwent pretransplant embolization.
STRATEGY FOR SPSS LIGATION:
The strategy for SPSS ligation in our department is shown in Figure 1. First, we considered - using preoperative CT – whether postoperative IVR for SPSS were possible. If postoperative IVR was impossible, SPSS were ligated during the operation. For example, when no SPSS (such as a renal-splenic shunt) was observed, there was no pathway to approach the left gastric-azygous shunt, and the left gastric vein was ligated. If a postoperative approach was possible or the hepatopetal flow was sufficient, we did not occlude the SPSS during LT. If hepatopetal flow was poor, we ligated the SPSS. Specific values on Doppler ultrasound (DUS) were not determined because the hepatopetal flow differs in individual cases. The portal vein was opened before the anastomosis to remove the thrombus and to actually assess the momentum of the hepatopetal flow. After LT, DUS was routinely performed twice a day for 1 week and once a day for 1 month. Postoperative portal steal syndrome was defined as a stagnant hepatopetal flow on postoperative DUS examinations without any evidence of anastomotic stenosis or thrombosis.
If the hepatopetal flow was poor, IVR was performed to occlude the SPSS after LDLT. Poor hepatopetal flow was assumed to be a clear decrease compared to the time of LT. Specific values were not determined because the hepatopetal flow immediately after LT differs in individual cases. Additionally, the blood test (elevated hepatobiliary enzymes and PT-INR) was also considered as indicating hepatic function. IVR was performed as soon as possible if the hepatopetal flow was poor due to SPSS and there were no other obvious causes. In the case of hepatic encephalopathy, IVR was also performed, even if the hepatopetal flow was sufficient. The detail indication of IVR for hepatic encephalopathy due to SPSS is symptoms and persistent high ammonia levels despite amino acid therapy. IVR was performed when the patient’s condition was not improved by conservative treatment and no cause other than SPSS was evident.
FOLLOW-UP AND DATA ANALYSIS:
Routine blood tests, including ammonia (NH3), were performed in the perioperative period. Evaluations were performed preoperatively, on postoperative day 1, and on postoperative day 7. Amino acid preparations were not used for hepatic insufficiency unless the NH3 level was unusually high (>200 μg/dl) or hepatic encephalopathy was observed.
SPSS were evaluated by CT. Routine CT was also performed on postoperative day 7 and at 1, 3, and 6 months, then once a year after discharge. Additional CT was performed in the event of DUS abnormalities and/or unexplained rebounding of transaminase levels. SPSS regression was defined as a >50% decrease in the maximum variceal diameter.
STATISTICAL ANALYSIS:
All data were statistically analyzed using BellCurve for Excel version 2.21 for Windows (Social Survey Research Information Co., Ltd., Tokyo, Japan). All quantitative data are expressed as the median (range). Univariate analyses were performed using the Mann-Whitney U test or paired
Results
PATIENTS CHARACTERISTICS:
A total of 231 patients, excluding those undergoing re-transplantation, underwent LDLT at our institution during the study period. In the present study population, SPSS were identified in 63 patients (27.3%). The SPSS were classified as splenorenal (n=23; 36.5%), gastrorenal (n=7; 11.1%), left gastric-azygous (n=25; 39.7%), and mesenteric-iliac (n=8; 12.7%). Twenty patients underwent SPSS ligation (31.7%). Intraoperatively, both groups obtained satisfactory portal flow. The indication for SPSS ligation was an impossible postoperative approach (n=10; 50%) or poor intraoperative hepatopetal flow (n=10; 50%). In the non-ligation group, 26 patients survived, 16 patients died, and 1 patient underwent re-transplantation 5 years after transplantation. No patients were lost to follow-up. The patient characteristics are shown in Table 1. The coexistence of hepatocellular carcinoma was detected in 29 patients. The 2 groups were comparable with regard to age, sex, Child-Pugh score, model for end-stage liver disease (MELD) score, graft, operative time, blood loss, splenectomy, and postoperative hospital stay. However, the graft weight (GW)/standard liver volume (SLV) was significantly lower in the non-ligation group (ligation group, 45.2%; non-ligation group, 38.1%; P=0.04).
COMPARISON OF SPSS, SURVIVAL BETWEEN THE LIGATION AND NON-LIGATION GROUPS:
The features of the SPSS and perioperative NH3 levels are shown in Table 2. Left gastric-azygous shunt accounted for half of the shunts in the ligation group, while splenorenal shunt accounted for over 40% of shunts in the non-ligation group. The maximum shunt diameter was significantly greater in the ligation group (18 mm vs 14 mm; P=0.01). The NH3 level on POD1 was significantly higher in the non-ligation group (52 vs 154 μg/dl; P=0.01).
The median follow-up periods of the ligated and non-ligation groups were 39.5 months (range, 1.0–146.3) and 36.5 months (range, 0.5–154.6), respectively. No patients were lost to follow-up or excluded from the survival analysis. The overall survival rates at 1, 3, and 5 years were 80%, 80%, and 80%, respectively, in the ligation group; and 76%, 68%, and 55%, respectively, in the non-ligation group (P=0.17, Figure 2). There was no significant difference in overall survival between the ligation and non-ligation groups and a type 2 error appeared to be unlikely. In the non-ligation group, 1 patient who had received transplantation 4 years previously required re-transplantation due to graft failure. The cause of death of 4 patients in the ligation group was bacterial infection, while the causes of death in the non-ligation group were bacterial infection (n=7), recurrent hepatocellular carcinoma (n=2), cytomegalovirus (n=1), cholestasis (n=1), portal thrombosis (n=1), fungal infection (n=1), cardiac failure (n=1), arrhythmia (n=1), and cerebral hemorrhage (n=1). No deaths were related to SPSS in either group.
COMPARISON OF OUTCOMES OF THE SURVIVOR GROUP AND THE NON-SURVIVORS/RE-TRANSPLANTATION GROUP AMONG PATIENTS MANAGED WITHOUT LIGATION:
No patients had liver dysfunction or primary nonfunction after LDLT. The characteristics of the non-ligation group are shown in Table 3. All features of the 2 groups were comparable. The features of SPSS, the perioperative level of NH3, and postoperative IVR are shown in Table 4. Splenorenal shunt accounted for >50% of the non-survivor/re-transplantation group. The maximum diameter of the SPSS was significantly greater in the survivor group (survivor group, 15.4 mm; non-survivor/re-transplantation group, 12 mm; P=0.02), but the perioperative NH3 values of the 2 groups did not differ to a statistically significant extent.
Three patients in the survivor group and 2 patients in the non-survivor/re-transplantation group received IVR after LT. The remaining 58 patients (92.1%) did not receive IVR or surgical ligation. In the survivor group, 1 patient underwent surgical ligation of the SSPS because the hepatopetal flow was not improved by IVR. In the non-survivor/re-transplantation group, 1 patient who received IVR due to hepatic encephalopathy had no problems for 4 years, and then underwent re-transplantation. The indication for re-transplantation was cholestasis and was not considered to be related to SPSS. The other patient who received IVR due to poor hepatopetal flow died due to brain bleeding 3 years after LT. The other 2 cases in which IVR was performed had improved hepatopetal flow or encephalopathy without complications.
Of the 34 patients who were observed for 6 months in the non-ligation group, 5 required IVR, while approximately half of the patients (14/29, 48.3%) had spontaneous regression of the SSPS. Two representative cases are shown in Figure 3. The case of gastrorenal shunt showed remarkable shrinkage at 2 years after LT. In another case, the left gastric-azygous shunt was too thin to detect at 6 months after LT. In the other 15 patients, the SPSS was not enlarged or significantly changed after LT.
Discussion
In the present study, SPSS were identified in 63 of 231 patients, and approximately 30% of them were ligated. Intraoperatively, either ligation or non-ligation had good hepatopetal flow. There was no significant difference in survival between the ligation and non-ligation groups, and there were no cases of liver failure or shunt-related death. Detailed examinations of the non-ligation group revealed that patients who required postoperative IVR had a good clinical course. Additionally, regression was observed in half of the cases in the non-ligation group. Although Gómez-Gavara et al reported that routine ligation of large SPSS during LT should be performed whenever feasible [12], the present single-center study using our criteria showed that SPSS should not be always ligated.
SPSS has been associated with hepatic encephalopathy [17,18]. Simón-Talero et al showed that hepatic encephalopathy was more frequent in SPSS (≥8 mm), indicating that the diameter of the shunt plays a role in this complication [19]. Regarding the management of SPSS, Gomez et al suggested that SPSS ligation should be routinely performed during liver transplantation whenever feasible. Some studies reported post-liver transplant encephalopathy caused by persistent portosystemic shunts despite good graft function, and was treated with IVR [20,21]. In the present study, SPSS tended to be ligated. Consequently, NH3 levels were successfully lowered and hepatic encephalopathy was prevented. However, even if postoperative hepatic encephalopathy occurs without SPSS ligation, it can be treated with IVR. Therefore, in our study population, it was possible to cope with perioperative hepatic encephalopathy by performing selective SPSS ligation, regardless of the diameter.
IVR treatment for vascular and biliary complications after liver transplantation has become a common procedure [22,23]. IVR after liver transplantation typically involves stent insertion and balloon angioplasty for portal vein stenosis [24,25]; however, there are a few reports of IVR for SPSS occlusion in the treatment of hepatic encephalopathy or poor hepatopetal flow after LT [26,27]. Balloon-occluded retrograde transvenous obliteration (BRTO) is an effective and less invasive IVR method for the occlusion of gastric varices [28]. Furthermore, in SPSS occlusion, the BRTO technique can even be applied in the treatment splenorenal [29], gastrorenal, and mesenteric-iliac shunts. In the present study, 5 patients received IVR including BRTO for the treatment of hepatic encephalopathy or poor hepatopetal flow. There were no complications and all patients recovered from hepatic encephalopathy or increased portal flow. Thus, IVR for the SPSS occlusion after LT was a safe and effective method for treating hepatic encephalopathy.
Many authors recommend routine ligation of SPSS during LDLT to avoid the portal steal phenomenon and graft hypoperfusion [12,30,31]. However, in cases involving a small-for-size graft, SPSS ligation leads to high portal pressure, which can cause graft injury in the immediate post-transplant period due to sinusoidal hypertension and a reciprocal reduction in hepatic flow. Reddy et al proposed that SPSS ligation should be considered in 2 situations during LDLT [32]. The first is when the SPSS steals blood flow from the graft after reperfusion. The second is when the portal pressures are acceptable but are associated with poor hepatopetal flow. In our criteria, both situations could be overcome in LDLT or during the post-transplantation period. During transplantation, we can check the hepatopetal flow by DUS and help determine whether ligation of the SPSS is required. After transplantation, we could also check the hepatopetal flow by DUS and decide when to perform IVR. The present study showed that, when our criteria were applied, the survival rate did not differ between the ligation and non-ligation groups. Therefore, it is not necessary to perform ligation in every case.
Few studies have investigated the hepatopetal flow dynamics in DDLT. SPSS has been rarely acknowledged as an important problem in DDLT because the grafts are large enough to accept hepatopetal flow. Additionally, DDLT grafts can decompress the portal system; thus, the regression of SPSS occurs faster [6]. Although small-volume grafts in LDLT cannot decompress the portal system as effectively, the SPSS may close with time. In some of the cases in the present study that were managed without ligation, the SPSS shrank within a few months after LDLT (Figure 3). This delayed shrinkage occurs because of the time taken to regenerate the liver volume. By following our criteria, shrinkage of the SPSS was possible, even in LDLT.
This study has some limitations. First, we were unable to measure the portal flow precisely. However, the hepatopetal flow was estimated by DUS during LDLT and in the post-transplant period. The information was sufficient to judge whether the post-transplant hepatopetal flow was significantly lower than during transplantation. Second, it was a retrospective study and depended on the completeness of medical records. Third, the outcomes were obtained from a single LDLT center. Therefore, the conclusions may not be directly applicable to others center.
Conclusions
In the present single-center study, unnecessary ligation could be avoided in LDLT when our criteria were applied. Therefore, in cases where the hepatopetal flow is maintained and postoperative IVR is possible, SPSS may not always require ligation in patients undergoing LDLT.
Figures
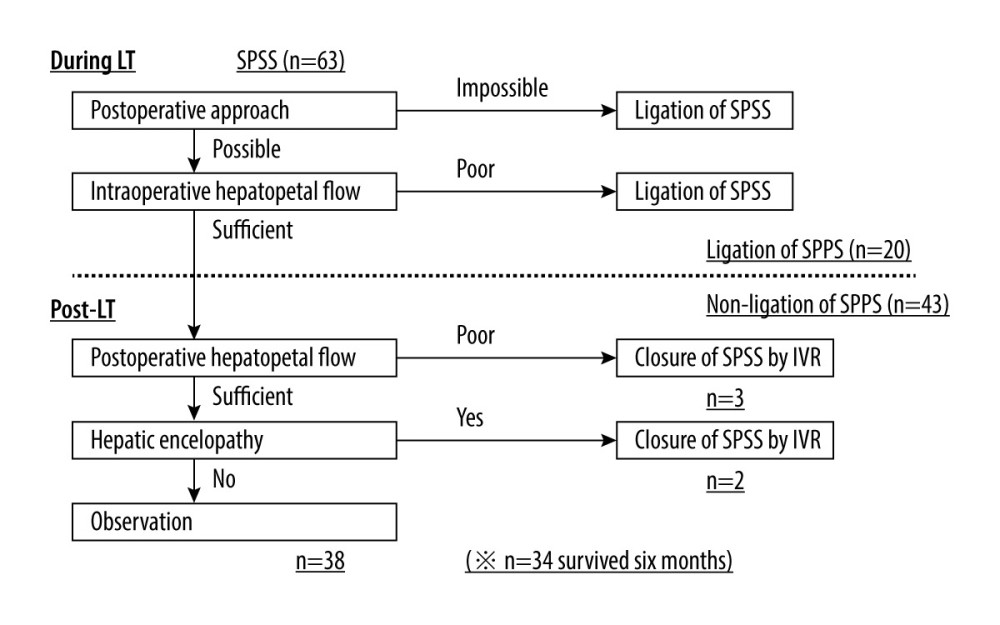 Figure 1. Criteria for spontaneous portosystemic shunts (SPSS) ligation in our departmentFirst, we considered whether postoperative interventional radiology (IVR) for SPSS was possible. If postoperative IVR was impossible, SPSS were ligated during the operation. Next, we observed the hepatopetal flow during liver transplantation (LT). If the hepatopetal flow was poor, we ligated the SPSS. After LT, if the hepatopetal flow was poor, IVR was performed to occlude the SPSS. In case of hepatic encephalopathy, IVR was also performed, even if the hepatopetal flow was sufficient. SPSS was defined as venous communication between the portal and venous systems with a largest diameter of ≥10 mm.
Figure 1. Criteria for spontaneous portosystemic shunts (SPSS) ligation in our departmentFirst, we considered whether postoperative interventional radiology (IVR) for SPSS was possible. If postoperative IVR was impossible, SPSS were ligated during the operation. Next, we observed the hepatopetal flow during liver transplantation (LT). If the hepatopetal flow was poor, we ligated the SPSS. After LT, if the hepatopetal flow was poor, IVR was performed to occlude the SPSS. In case of hepatic encephalopathy, IVR was also performed, even if the hepatopetal flow was sufficient. SPSS was defined as venous communication between the portal and venous systems with a largest diameter of ≥10 mm. 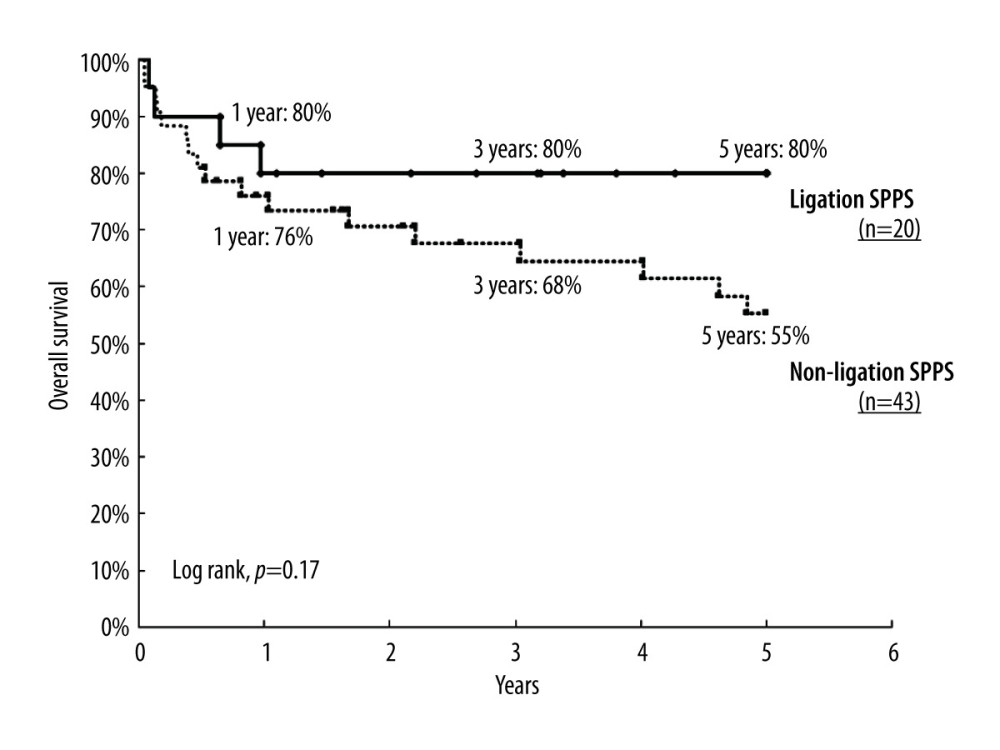 Figure 2. Overall survival of patients with spontaneous portosystemic shunts (SPSS) in ligation and non-ligation groupsThe overall survival rate at 1, 3, and 5 years were 80%, 80%, and 80%, respectively, in the ligation group, and 76%, 68%, and 55%, respectively, in the non-ligation group. This difference was not statistically significant (P=0.17).
Figure 2. Overall survival of patients with spontaneous portosystemic shunts (SPSS) in ligation and non-ligation groupsThe overall survival rate at 1, 3, and 5 years were 80%, 80%, and 80%, respectively, in the ligation group, and 76%, 68%, and 55%, respectively, in the non-ligation group. This difference was not statistically significant (P=0.17). 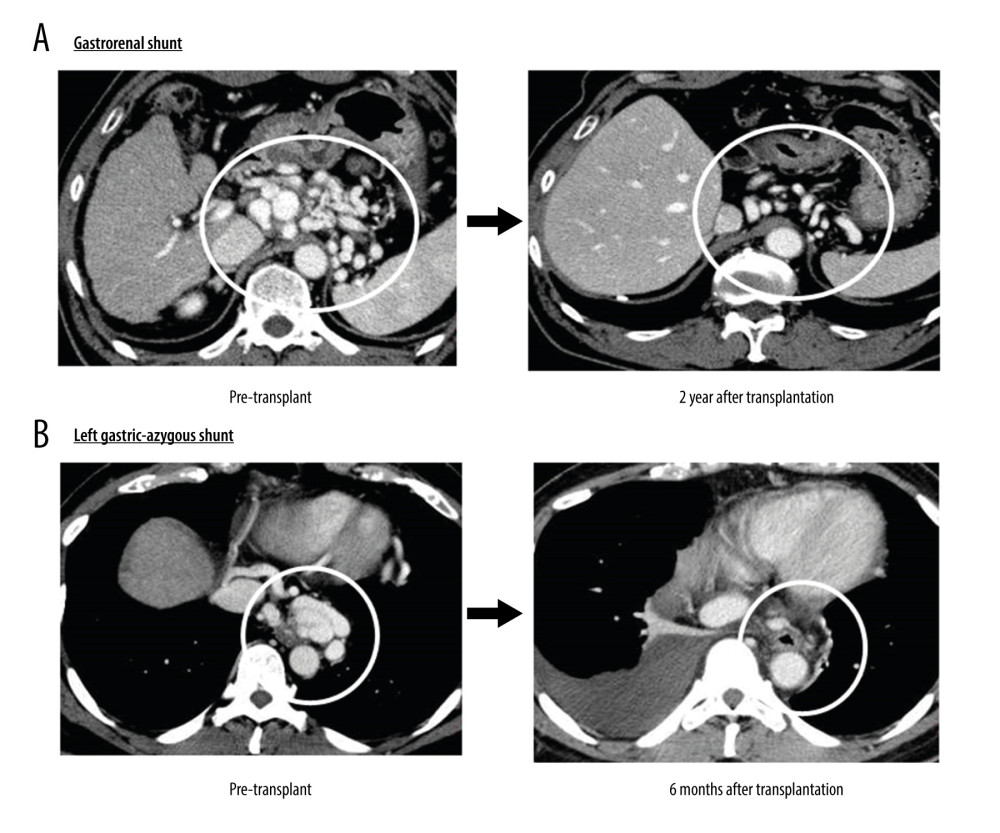 Figure 3. Regression of spontaneous portosystemic shunts (SPSS)(A) Gastrorenal shunt: The gastrorenal shunt had shrunk remarkably at 2 years after liver transplantation (LT). (B) Left gastric-azygous shunt: The left gastric shunt was too thin to detect 6 months after LT.
Figure 3. Regression of spontaneous portosystemic shunts (SPSS)(A) Gastrorenal shunt: The gastrorenal shunt had shrunk remarkably at 2 years after liver transplantation (LT). (B) Left gastric-azygous shunt: The left gastric shunt was too thin to detect 6 months after LT. Tables
Table 1. Patient characteristics (ligation group vs non-ligation group).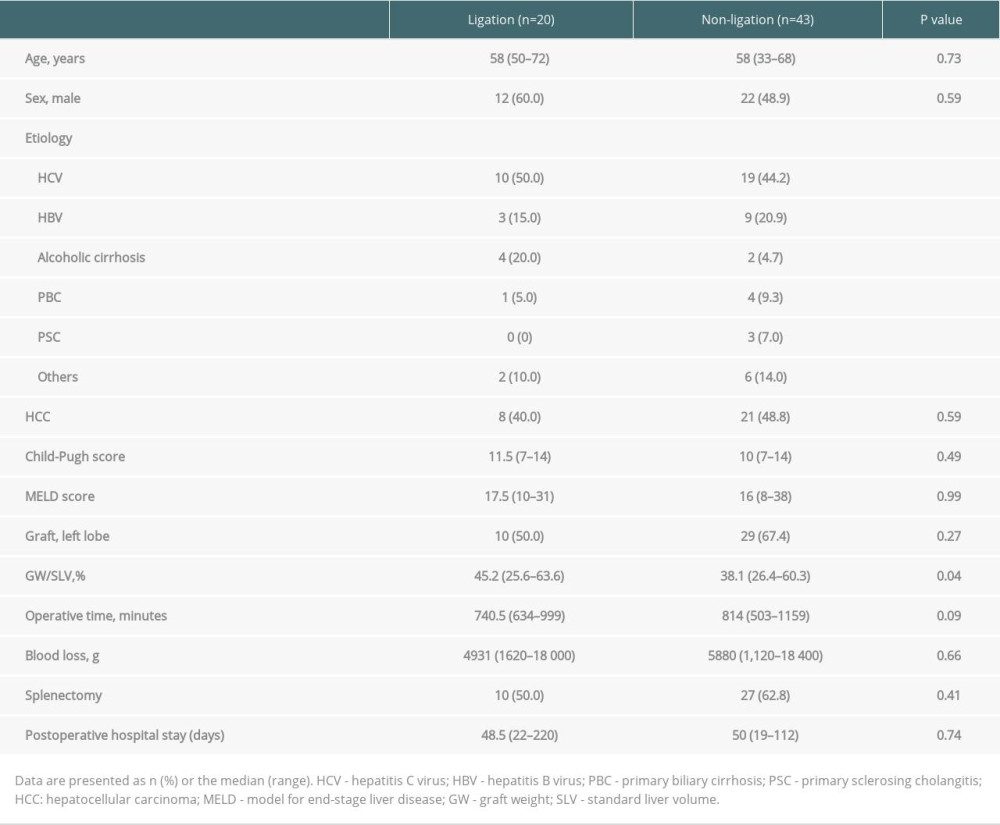 Table 2. The feature of spontaneous portosystemic shunts (SPSS) and the perioperative level of NH3.
Table 2. The feature of spontaneous portosystemic shunts (SPSS) and the perioperative level of NH3.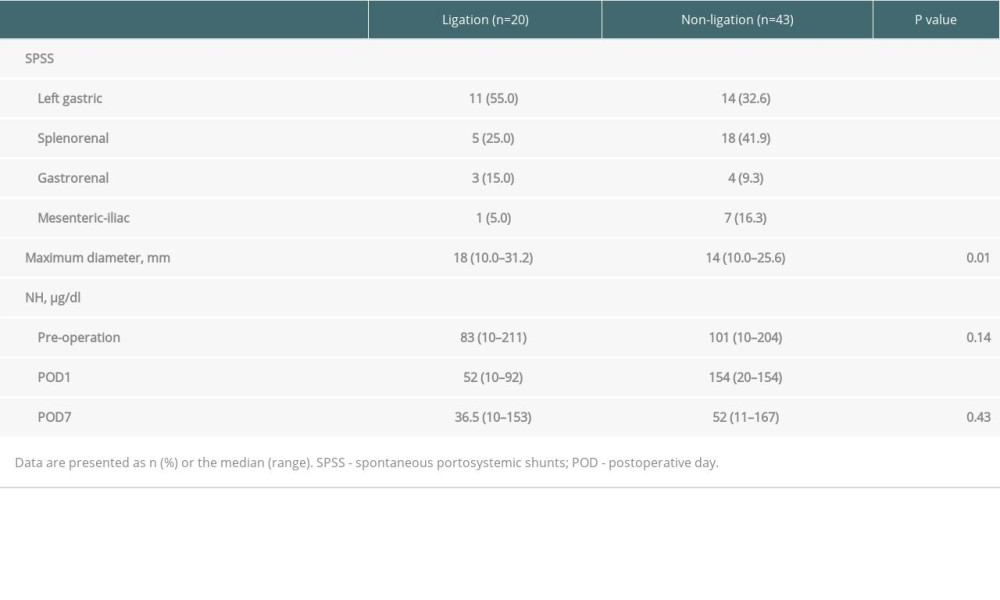 Table 3. Characteristics of the non-ligation group (survivors vs non-survivors/re-transplantation).
Table 3. Characteristics of the non-ligation group (survivors vs non-survivors/re-transplantation).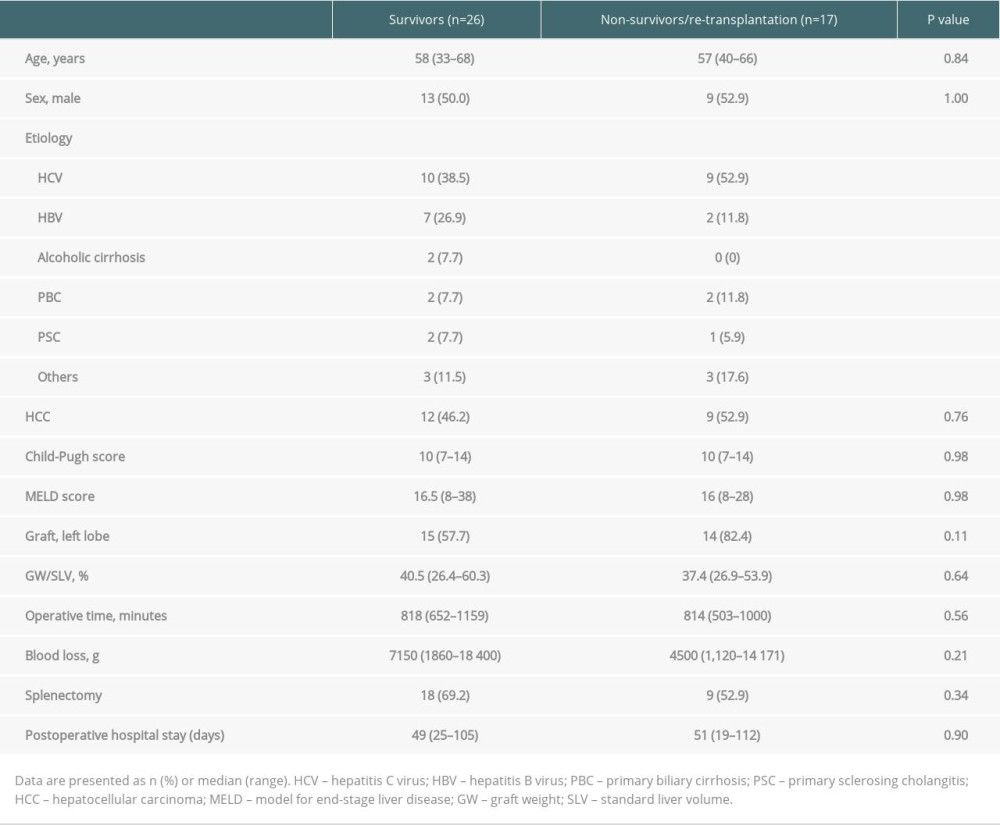 Table 4. Features of spontaneous portosystemic shunts (SPSS), perioperative level of NH3, and postoperative interventional radiology (IVR) in the non-ligation group.
Table 4. Features of spontaneous portosystemic shunts (SPSS), perioperative level of NH3, and postoperative interventional radiology (IVR) in the non-ligation group.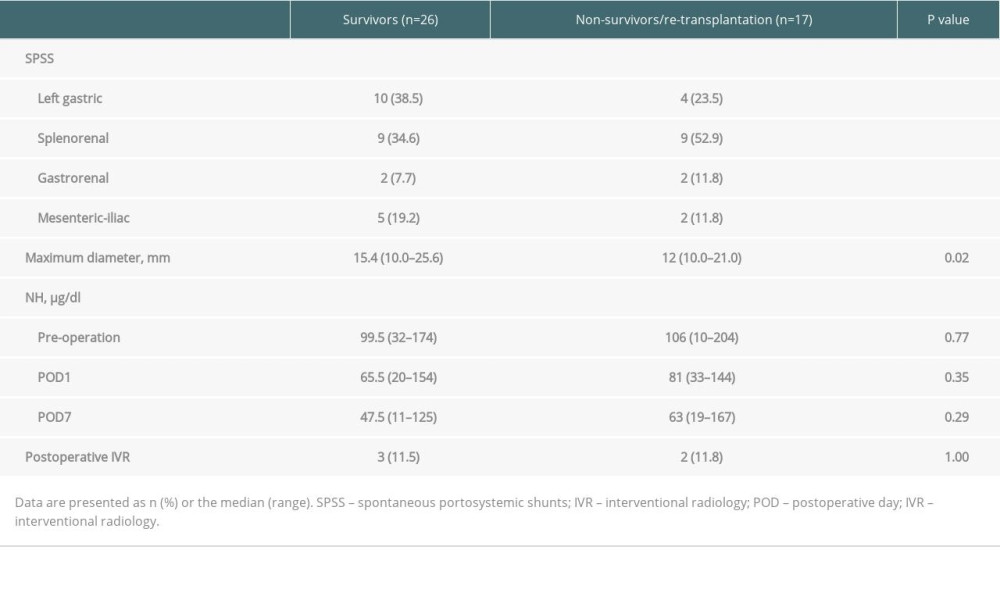
References
1. Miranda PB, Artacho GS, Bellido CB, Management of large, spontaneous portosystemic shunts in liver transplantation: Case report and review of literature: Transplant Proc, 2020; 52(2); 566-68
2. Praktiknjo M, Simon-Talero M, Romer J, Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis: J Hepatol, 2020; 72(6); 1140-50
3. Rodriguez EA, Perez R, Zhang N, Clinical outcomes of portosystemic shunts on the outcome of liver transplantation: Liver Transpl, 2020; 26(5); 693-701
4. Zardi EM, Uwechie V, Caccavo D, Portosystemic shunts in a large cohort of patients with liver cirrhosis: Detection rate and clinical relevance: J Gastroenterol, 2009; 44(1); 76-83
5. Chikamori F, Nishida S, Selvaggi G, Effect of liver transplantation on spleen size, collateral veins, and platelet counts: World J Surg, 2010; 34(2); 320-26
6. Jiang SM, Zhang QS, Zhou GW, Differences in portal hemodynamics between whole liver transplantation and living donor liver transplantation: Liver Transpl, 2010; 16(11); 1236-41
7. Awad N, Horrow MM, Parsikia A, Perioperative management of spontaneous splenorenal shunts in orthotopic liver transplant patients: Exp Clin Transplant, 2012; 10(5); 475-81
8. Pawlicki J, Kurek A, Oczkowicz G, Król R, Ligation of the left renal vein in liver transplant recipients diagnosed with a spontaneous splenorenal shunt - case report: Transplantation Reports, 2020; 5(3); 100053
9. Golse N, Mohkam K, Rode A, Surgical management of large spontaneous portosystemic splenorenal shunts during liver transplantation: splenectomy or left renal vein ligation?: Transplant Proc, 2015; 47(6); 1866-76
10. Kim H, Yoon KC, Lee KW, Tips and pitfalls in direct ligation of large spontaneous splenorenal shunt during liver transplantation: Liver Transpl, 2017; 23(7); 899-906
11. Elshobary M, Shehta A, Salah T, Ligation of huge spontaneous porto-systemic collaterals to avoid portal inflow steal in adult living donor liver transplantation: A case-report: Int J Surg Case Rep, 2017; 31; 214-17
12. Gomez Gavara C, Bhangui P, Salloum C, Ligation versus no ligation of spontaneous portosystemic shunts during liver transplantation: Audit of a prospective series of 66 consecutive patients: Liver Transpl, 2018; 24(4); 505-15
13. Kamei H, Onishi Y, Ishigami M, Development of extensive inferior vena cava thrombosis due to the ligation of a large mesenteric-caval shunt during liver transplantation: A case report: Int J Surg Case Rep, 2016; 29; 211-14
14. Hessheimer AJ, Fondevila C, Taurá P, Decompression of the portal bed and twice-baseline portal inflow are necessary for the functional recovery of a “small-for-size” graft: Ann Surg, 2011; 253(6); 1201-10
15. Vidal-Gonzalez J, Quiroga S, Simon-Talero M, Genesca J, Spontaneous portosystemic shunts in liver cirrhosis: New approaches to an old problem: Therap Adv Gastroenterol, 2020; 13; 1756284820961287
16. Hara T, Soyama A, Hidaka M, Analysis of early relaparotomy following living donor liver transplantation: Liver Transpl, 2016; 22(11); 1519-25
17. Vilstrup H, Amodio P, Bajaj J, Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver: Hepatology, 2014; 60(2); 715-35
18. Wijdicks EF, Hepatic encephalopathy: N Engl J Med, 2016; 375(17); 1660-70
19. Simon-Talero M, Roccarina D, Martinez J, Association between portosystemic shunts and increased complications and mortality in patients with cirrhosis: Gastroenterology, 2018; 154(6); 1694-705e4
20. St Barritt A, Fried MW, Hayashi PH, Persistent portosystemic shunts after liver transplantation causing episodic hepatic encephalopathy: Dig Dis Sci, 2010; 55(6); 1794-98
21. Herrero JI, Bilbao JI, Diaz ML, Hepatic encephalopathy after liver transplantation in a patient with a normally functioning graft: Treatment with embolization of portosystemic collaterals: Liver Transpl, 2009; 15(1); 111-14
22. Saad WE, Portal interventions in liver transplant recipients: Semin Intervent Radiol, 2012; 29(2); 99-104
23. Sanada Y, Katano T, Hirata Y, Interventional radiology treatment for vascular and biliary complications following pediatric living donor liver transplantation - a retrospective study: Transpl Int, 2018; 31(11); 1216-22
24. Mehrzad H, Mangat K, The role of interventional radiology in treating complications following liver transplantation: ISRN Hepatol, 2013; 2013; 696794
25. Kim KS, Kim JM, Lee JS, Stent insertion and balloon angioplasty for portal vein stenosis after liver transplantation: long-term follow-up results: Diagn Interv Radiol, 2019; 25(3); 231-37
26. Baimakhanov Z, Soyama A, Takatsuki M, Effective balloon-occluded retrograde transvenous obliteration of the superior mesenteric vein-inferior vena cava shunt in a patient with hepatic encephalopathy after living donor liver transplantation: Clin J Gastroenterol, 2014; 7(4); 342-45
27. Saad WE, Chick JFB, Srinivasa RN, Two-year outcomes of balloon-occluded retrograde transvenous obliteration of gastric varices in liver transplant recipients: A multi-institutional study: Diagn Interv Imaging, 2017; 98(11); 801-8
28. Frischtak HL, Davis JP, Shah NL, Therapeutic options for bleeding oesophageal varices: Cyanoacrylate and balloon-occluded retrograde obliteration (BRTO): BMJ Case Rep, 2017; 2017; bcr2017219615
29. Nakai M, Sato M, Sahara S, Transhepatic catheter-directed thrombolysis for portal vein thrombosis after partial splenic embolization in combination with balloon-occluded retrograde transvenous obliteration of splenorenal shunt: World J Gastroenterol Aug 21, 2006; 12(31); 5071-74
30. Moon DB, Lee SG, Ahn C, Application of intraoperative cine-portogram to detect spontaneous portosystemic collaterals missed by intraoperative doppler exam in adult living donor liver transplantation: Liver Transpl, 2007; 13(9); 1279-84
31. Moon DB, Lee SG, Kim KH, The significance of complete interruption of large spontaneous portosystemic collaterals in adult living donor liver transplantation as a graft salvage procedure: Transpl Int, 2008; 21(7); 698-700
32. Reddy MS, Rela M, Portosystemic collaterals in living donor liver transplantation: What is all the fuss about?: Liver Transpl, 2017; 23(4); 537-44
Figures
 Figure 1. Criteria for spontaneous portosystemic shunts (SPSS) ligation in our departmentFirst, we considered whether postoperative interventional radiology (IVR) for SPSS was possible. If postoperative IVR was impossible, SPSS were ligated during the operation. Next, we observed the hepatopetal flow during liver transplantation (LT). If the hepatopetal flow was poor, we ligated the SPSS. After LT, if the hepatopetal flow was poor, IVR was performed to occlude the SPSS. In case of hepatic encephalopathy, IVR was also performed, even if the hepatopetal flow was sufficient. SPSS was defined as venous communication between the portal and venous systems with a largest diameter of ≥10 mm.
Figure 1. Criteria for spontaneous portosystemic shunts (SPSS) ligation in our departmentFirst, we considered whether postoperative interventional radiology (IVR) for SPSS was possible. If postoperative IVR was impossible, SPSS were ligated during the operation. Next, we observed the hepatopetal flow during liver transplantation (LT). If the hepatopetal flow was poor, we ligated the SPSS. After LT, if the hepatopetal flow was poor, IVR was performed to occlude the SPSS. In case of hepatic encephalopathy, IVR was also performed, even if the hepatopetal flow was sufficient. SPSS was defined as venous communication between the portal and venous systems with a largest diameter of ≥10 mm. Figure 2. Overall survival of patients with spontaneous portosystemic shunts (SPSS) in ligation and non-ligation groupsThe overall survival rate at 1, 3, and 5 years were 80%, 80%, and 80%, respectively, in the ligation group, and 76%, 68%, and 55%, respectively, in the non-ligation group. This difference was not statistically significant (P=0.17).
Figure 2. Overall survival of patients with spontaneous portosystemic shunts (SPSS) in ligation and non-ligation groupsThe overall survival rate at 1, 3, and 5 years were 80%, 80%, and 80%, respectively, in the ligation group, and 76%, 68%, and 55%, respectively, in the non-ligation group. This difference was not statistically significant (P=0.17). Figure 3. Regression of spontaneous portosystemic shunts (SPSS)(A) Gastrorenal shunt: The gastrorenal shunt had shrunk remarkably at 2 years after liver transplantation (LT). (B) Left gastric-azygous shunt: The left gastric shunt was too thin to detect 6 months after LT.
Figure 3. Regression of spontaneous portosystemic shunts (SPSS)(A) Gastrorenal shunt: The gastrorenal shunt had shrunk remarkably at 2 years after liver transplantation (LT). (B) Left gastric-azygous shunt: The left gastric shunt was too thin to detect 6 months after LT. Tables
 Table 1. Patient characteristics (ligation group vs non-ligation group).
Table 1. Patient characteristics (ligation group vs non-ligation group). Table 2. The feature of spontaneous portosystemic shunts (SPSS) and the perioperative level of NH3.
Table 2. The feature of spontaneous portosystemic shunts (SPSS) and the perioperative level of NH3. Table 3. Characteristics of the non-ligation group (survivors vs non-survivors/re-transplantation).
Table 3. Characteristics of the non-ligation group (survivors vs non-survivors/re-transplantation). Table 4. Features of spontaneous portosystemic shunts (SPSS), perioperative level of NH3, and postoperative interventional radiology (IVR) in the non-ligation group.
Table 4. Features of spontaneous portosystemic shunts (SPSS), perioperative level of NH3, and postoperative interventional radiology (IVR) in the non-ligation group. Table 1. Patient characteristics (ligation group vs non-ligation group).
Table 1. Patient characteristics (ligation group vs non-ligation group). Table 2. The feature of spontaneous portosystemic shunts (SPSS) and the perioperative level of NH3.
Table 2. The feature of spontaneous portosystemic shunts (SPSS) and the perioperative level of NH3. Table 3. Characteristics of the non-ligation group (survivors vs non-survivors/re-transplantation).
Table 3. Characteristics of the non-ligation group (survivors vs non-survivors/re-transplantation). Table 4. Features of spontaneous portosystemic shunts (SPSS), perioperative level of NH3, and postoperative interventional radiology (IVR) in the non-ligation group.
Table 4. Features of spontaneous portosystemic shunts (SPSS), perioperative level of NH3, and postoperative interventional radiology (IVR) in the non-ligation group. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








