13 September 2022: Original Paper
Diagnostic Role of Tumor Markers for Hepatocellular Carcinoma in Liver Transplantation Candidates: An Analysis Using the Korean Organ Transplantation Registry Database
Woo-Hyoung KangDOI: 10.12659/AOT.936937
Ann Transplant 2022; 27:e936937
Abstract
BACKGROUND: This study analyzed pretransplant alpha-fetoprotein (AFP) and proteins induced by vitamin K absence or antagonist-II (PIVKA-II) in liver transplantation (LT) candidates.
MATERIAL AND METHODS: A total of 3,273 LT recipients enrolled at the Korean Organ Transplantation Registry were divided according to hepatocellular carcinoma (HCC) status and background liver disease, and AFP and PIVKA-II were compared.
RESULTS: In all patients, the median AFP and PIVKA-II were 6.3 ng/mL and 29 mAU/mL in the viable-HCC group and 3.3 ng/mL and 35 mAU/mL, respectively, in the no-HCC group (P<0.001 for AFP and p=0.037 for PIVKA-II). In patients with hepatitis B virus infection, they were 6.0 ng/mL and 26 mAU/mL in the HCC group and 3.2 ng/mL and 21 mAU/mL in the no-HCC group, respectively (P<0.001 and P<0.001). In patients with hepatitis C virus infection, they were 10.7 ng/mL and 37 mAU/mL in the HCC group and 2.6 ng/mL and 21 mAU/mL in the no-HCC group, respectively (P<0.001 and P=0.117). In alcoholic liver disease patients, they were 5.2 ng/mL and 61 mAU/mL in the HCC group and 6.4 ng/mL and 75 mAU/mL in the no-HCC group, respectively (P<0.001 and P=0.419). In patients with other diseases, they were 7.1 ng/mL and 32 mAU/mL in the HCC group and 3.3 ng/mL and 28 mAU/mL in the no-HCC group, respectively (P<0.001 and P=0.822).
CONCLUSIONS: The results of the present study indicate that pretransplant serum AFP and PIVKA-II were highly variably expressed in LT candidates with end-stage liver diseases; therefore, their values should be cautiously interpreted because their role in HCC diagnosis is limited.
Keywords: Liver Neoplasms, hepatocellular carcinoma, tumor marker, Liver Transplantation, carcinogenesis, viral hepatitis, Biomarkers, Tumor, Carcinoma, Hepatocellular, Humans, Liver Transplantation, Protein Precursors, Prothrombin, Registries, Republic of Korea, alpha-Fetoproteins
Background
The hepatocellular carcinoma (HCC) tumor markers alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II or des-gamma-carboxy prothrombin) are variably expressed in patients with HCC [1–6]. These tumor markers also can be expressed even in patients without HCC, especially in those with chronic hepatitis or liver cirrhosis. As a result, the reliability of these tumor markers for diagnosis of HCC is much lower in patients with end-stage liver diseases waiting for liver transplantation (LT) compared with the general population [3]. It was reported that alcoholic liver cirrhosis is associated with high expression of PIVKA-II in patients without HCC [7]. To assess the diagnostic role of HCC tumor markers during pretransplant recipient workup, it is necessary to analyze the expression patterns of serum AFP and PIVKA-II in LT candidates with or without HCC, regardless of background liver diseases.
Material and Methods
This was a retrospective multi-center observational study on the expression of HCC tumor markers in LT candidates. The LT database of the Korean Organ Transplantation Registry (KOTRY) was searched to identify adult patients aged 19 years or older who underwent primary LT between April 2014 and December 2020. The exclusion criteria were re-transplantation, HCC combined with other malignancy (intrahepatic cholangiocarcinoma and combined HCC-cholangiocarcinoma), and unavailability of pretransplant AFP and PIVKA-II values.
For this study, 3273 LT recipients were selected. They were divided according to the pathological diagnosis of HCC and background liver diseases. HCC status was divided into 3 groups according to the explant liver pathology, as viable-HCC, non-viable-HCC, and no-HCC groups. Non-viable-HCC was defined as HCC showing complete pathological response following pretransplant locoregional treatment [8]. Background liver diseases were divided into 4 groups, as hepatitis B virus (HBV)-associated liver cirrhosis, hepatitis C virus (HCV)-associated liver cirrhosis, alcoholic liver disease (ALD), and other diseases. The institutional review board of participating institutions approved this study protocol (Asan Medical Center No. 2014-0898), which waived the requirement for informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
The normal cutoff values for serum AFP and PIVKA-II used in the present study were 7.5 ng/mL and 40 mAU/mL, respectively [8]. Numerical data are presented as medians with 25–75 percentiles or means with standard deviation. Continuous variables were compared using the
Results
PROFILES IN ALL PATIENTS:
The number of patients with viable-HCC, non-viable-HCC, and no-HCC were 2063 (63.0%), 261 (8.0%), and 949 (29.0%), respectively. Patient numbers according to the HCC status and background liver disease were summarized at Table 1. Deceased donor and living donor LTs were performed in 412 (12.6%) and 2861 (87.4%) patients, respectively.
The mean and median serum AFP levels were 330.7±3422.6 ng/mL and 6.3 ng/mL (25–75 percentiles: 3.1–24.9), respectively, in the viable-HCC group; 14.0±45.2 ng/mL and 4.0 ng/mL (25–75 percentiles: 2.4–7.4), respectively, in the non-viable-HCC group; and 17.8±73.8 ng/mL and 3.3 ng/mL (25–75 percentiles: 2.0–6.5), respectively, in the no-HCC group (P<0.001; viable-HCC vs no-HCC, P<0.001; viable-HCC vs non-viable-HCC, P=0.135; non-viable-HCC vs no-HCC, P=0.425; Figure 1A).
The mean and median serum PIVKA-II levels were 686.8±5381.3 mAU/mL and 29 mAU/mL (25–75 percentiles: 18–77), respectively, in the viable-HCC group; 65.6±167.3 mAU/mL and 22 mAU/mL (25–75 percentiles: 16–36), respectively, in the non-viable-HCC group; and 387.4±1422.9 mAU/mL and 35 mAU/mL (25–75 percentiles: 18–154), respectively, in the no-HCC group (P=0.037; viable-HCC vs no-HCC, P=0.091; viable-HCC vs non-viable-HCC, P=0.062; non-viable-HCC vs no-HCC, P<0.001; Figure 1B).
Expression of AFP and PIVKA-II in the no-viable-HCC group showed equivocal findings between the viable-HCC and no-HCC groups; thus, the non-viable-HCC group was excluded from further analysis.
ROC curve analyses of AFP for HCC diagnosis showed that the area under the ROC curve (AUC) was 0.666 (P<0.001) and application of a cutoff of 7.5 ng/mL resulted in a sensitivity of 44.9% and specificity of 79.7%. ROC AUC for PIVKA-II was 0.544 (P=0.002) and application of a cutoff of 40 mAU/mL resulted in a sensitivity of 62.6% and specificity of 46.9% (Table 2 and Figure 1C).
The correlation analysis of AFP and PIVKA-II showed a correlation coefficient ρ of 0.229 (P<0.001) in the viable-HCC group and ρ of 0.037 (P=0.250) in the no-HCC group (Figure 2).
PROFILES IN PATIENTS WITH HBV-ASSOCIATED LIVER CIRRHOSIS:
In patients with HBV-associated liver cirrhosis, the mean and median AFP levels were 400.7±3905.5 ng/mL and 6.0 ng/mL (25–75 percentiles: 2.9–28.2), respectively, in the viable-HCC group; and 22.9±64.8 ng/mL and 3.2 ng/mL (25–75 percentiles: 1.7–9.6), respectively, in the no-HCC group (P<0.001; Figure 3A).
The mean and median PIVKA-II levels were 727.5±5937.7 mAU/mL and 26 mAU/mL (25–75 percentiles: 18–60), respectively, in the viable-HCC group; and 154.4±1002.1 mAU/mL and 21 mAU/mL (25–75 percentiles: 14–44), respectively, in the no-HCC group (P<0.001; Figure 3B).
ROC curve analyses of AFP for HCC diagnosis showed that the AUC was 0.636 (P < 0.001) and application of a cutoff of 7.5 ng/mL resulted in a sensitivity of 44.4% and specificity of 70.6%. ROC AUC for PIVKA-II was 0.585 (P<0.001) and application of a cutoff of 40 mAU/mL resulted in a sensitivity of 32.9% and specificity of 73.6% (Table 2 and Figure 3C).
PROFILES IN PATIENTS WITH HCV-ASSOCIATED LIVER CIRRHOSIS:
In patients with HCV-associated liver cirrhosis, the mean and median AFP levels were 217.3±1524.1 ng/mL and 10.7 ng/mL (25–75 percentiles: 4.6–37.4), respectively, in the viable-HCC group; and 4.0±3.4 ng/mL and 2.6 ng/mL (25–75 percentiles: 2.1–5.0), respectively, in the no-HCC group (P<0.001; Figure 4A).
The mean and median PIVKA-II levels were 290.6±1459.0 mAU/mL and 37 mAU/mL (25–75 percentiles: 18–113), respectively, in the viable-HCC group; and 132.4±228.4 mAU/mL and 21 mAU/mL (25–75 percentiles: 14–151), respectively, in the no-HCC group (p=0.117; Figure 4B).
ROC curve analyses of AFP for HCC diagnosis showed that the AUC was 0.809 (P<0.001) and application of a cutoff of 7.5 ng/mL resulted in a sensitivity of 58.0% and specificity of 89.3%. ROC AUC for PIVKA-II was 0.592 (P=0.157) and application of a cutoff of 40 mAU/mL resulted in a sensitivity of 49.5% and specificity of 64.3% (Table 2 and Figure 4C).
PROFILES OF PATIENTS WITH ALD-ASSOCIATED LIVER CIRRHOSIS:
In patients with ALD-associated liver cirrhosis, the mean and median AFP levels were 59.3±388.8 ng/mL and 5.2 ng/mL (25–75 percentiles: 3.4–10.7), respectively, in the viable-HCC group; and 6.4±28.7 ng/mL and 3.5 ng/mL (25–75 percentiles: 2.1–5.6), respectively, in the no-HCC group (P<0.001; Figure 5A).
The mean and median PIVKA-II levels were 825.1±3694.7 mAU/mL and 61 mAU/mL (25–75 percentiles: 26–206), respectively, in the viable-HCC group; and 420.9±1187.9 mAU/mL and 75 mAU/mL (25–75 percentiles: 25–253), respectively, in the no-HCC group (P=0.419; Figure 5B).
ROC curve analyses of AFP for HCC diagnosis showed that the AUC was 0.671 (P<0.001) and application of a cutoff of 7.5 ng/mL resulted in a sensitivity of 36.1% and specificity of 86.2%. ROC AUC for PIVKA-II was 0.519 (P=0.413) and application of a cutoff of 40 mAU/mL resulted in a sensitivity of 42.1% and specificity of 62.3% (Table 2 and Figure 5C).
PROFILES OF PATIENTS WITH LIVER CIRRHOSIS OF OTHER CAUSES:
In patients with liver cirrhosis of other etiology, the mean and median AFP levels were 78.2±344.7 ng/mL and 7.1 ng/mL (25–75 percentiles: 3.5–23.6), respectively, in the viable-HCC group; and 37.9±128.3 ng/mL and 3.3 ng/mL (25–75 percentiles: 2.0–7.9) respectively in the no-HCC group (P<0.001; Figure 6A).
The mean and median PIVKA-II levels were 490.7±3680.4 mAU/mL and 32 mAU/mL (25–75 percentiles: 18–76), respectively, in the viable-HCC group; and 595.9±2132.2 mAU/mL and 28 mAU/mL (25–75 percentiles: 18–108), respectively, in the no-HCC group (P=0.822; Figure 6B).
ROC curve analyses of AFP for HCC diagnosis showed that the AUC was 0.665 (P<0.001) and application of a cutoff of 7.5 ng/mL resulted in a sensitivity of 47.8% and specificity of 74.3%. ROC AUC for PIVKA-II was 0.507 (P=0.819) and application of a cutoff of 40 mAU/mL resulted in a sensitivity of 42.0% and specificity of 62.2% (Table 2 and Figure 6C).
COMPARISON OF AFP AND PIVKA-II ACCORDING TO BACKGROUND LIVER DISEASE AND HCC:
In patients with viable HCC, median AFP and PIVKA-II levels were 6.0 ng/mL (25–75 percentiles: 2.9–28.2) and 26 mAU/mL (25–75 percentiles: 18–60), respectively, in HBV-associated liver cirrhosis; 10.7 ng/mL (25–75 percentiles: 4.6–37.4) and 37 mAU/mL (25–75 percentiles: 18–113), respectively, in HCV-associated liver cirrhosis; 5.2 ng/mL (25–75 percentiles: 3.4–10.7) and 61 mAU/mL (25–75 percentiles: 26–206), respectively, in patients with ALD-associated liver cirrhosis; and 7.1 ng/mL (25–75 percentiles: 3.5–23.6) and 32 mAU/mL (25–75 percentiles: 18–76), respectively, in patients with liver cirrhosis of other etiology (
In patients with no HCC, median AFP and PIVKA-II levels were 3.2 ng/mL (25–75 percentiles: 1.7–9.6) and 21 mAU/mL (25–75 percentiles: 14–44), respectively, in HBV-associated liver cirrhosis; 2.6 ng/mL (25–75 percentiles: 2.1–5.0) and 21 mAU/mL (25–75 percentiles: 14–151), respectively, in HCV-associated liver cirrhosis; 3.5 ng/mL (25–75 percentiles: 2.1–5.6) and 75 mAU/mL (25–75 percentiles: 25–253), respectively, in patients with ALD-associated liver cirrhosis; and 3.3 ng/mL (25–75 percentiles: 2.0–7.9) and 28 mAU/mL (25–75 percentiles: 18–108), respectively, in patients with liver cirrhosis of other etiology (
Discussion
The results of the present study revealed that pretransplant serum AFP and PIVKA-II were quite variably expressed according to the status of HCC and background liver diseases. Expression of PIVKA-II showed low diagnostic predictability for HCC due to its high production in the non-viral hepatitis livers without HCC.
AFP is a glycoprotein that is produced in early fetal life by the liver. It can be produced by many tumors, including HCC, hepatoblastoma, and non-seminomatous germ-cell tumors of the ovary and testis. Tumor cells synthesize fetal proteins through de-differentiation of adult hepatocytes [9]. During fetal life, AFP is synthesized at first by the yolk sac, then by the liver. Although AFP production is markedly reduced after birth, its production continues at a low level during adulthood [10]. AFP can increase temporarily in cases of liver injury or regeneration, particularly after liver resection, during fulminant viral hepatitis, or chronic viral hepatitis [11]. Serum AFP levels increase by 20–80% in patients with HCC and are closely related to aggressive tumor biology [12,13]. In 1984, PIVKA-II was found to be significantly increased in the serum of HCC patients and it could serve as a new serum marker for HCC [14]. Many studies suggested that the combined detection of PIVKA-II and AFP may improve HCC diagnosis compared to the use of each biomarker alone [15]. The diagnostic value of PIVKA-II is controversial and it is still debated whether there is a correlation between PIVKA-II and AFP and whether PIVKA-II can completely replace or supplement the role of AFP in HCC diagnosis [15,16].
It is reported that serum AFP (>15 or 20 ng/mL) as a screening test for HCC had sensitivity between 39% and 64%, specificity between 76% and 91%, and positive predictive value between 9% and 33% [17,18]. A case-control study of 340 cirrhotic patients showed that AFP levels >20 ng/mL had sensitivity of 60% and specificity of 91% to diagnose HCC. At this threshold, 40% of all HCC patients would be missed [19]. Although the prognostic value of AFP in LT seems to be established, there is an issue regarding the cutoff value used to evaluate the level of AFP. There is no clear consensus regarding the expression level of AFP above which patients should not be eligible LT candidates [20].
An Italian multi-center study with 388 patients with chronic liver disease reported that the overall ROC AUC values for AFP and PIVKA-II were 0.698 (
The expression patterns of PIVKA-II in patients with ALD and other non-viral liver diseases were unique in the present study, in which PIVKA-II had no role in diagnosis of HCC. A Korean study with 2528 patients without HCC revealed that ALD and antibiotics use may be confounding factors when interpreting high serum PIVKA-II levels in patients without HCC. Therefore, serum PIVKA-II levels in patients with ALD should be interpreted with caution [7]. The detailed mechanism of PIVKA-II elevation is not known yet. A Japanese study suggested that the time course for elevation of serum PIVKA-II levels in ALD was different from that of HCC. In HCC, serum PIVKA-II levels continued to increase until treatment. In contrast, its increase was transient and its levels returned to baseline in ALD [23]. To investigate the mechanism by which elevation of serum PIVKA-II in patients with ALD occurred, the effect of vitamin K on production of PIVKA-II by hepatocytes was investigated. PIVKA-II production was inhibited by addition of vitamin K in a dose-dependent manner, and elevation of serum PIVKA-II in ALD patients was suppressed by administration of vitamin K. Taken together, these results suggest that vitamin K may have a role in the mechanism of PIVKA-II elevation in sera of these patients. However, there was no correlation observed between serum vitamin K concentration and PIVKA-II in ALD patients. This result suggests that elevation of serum PIVKA-II in ALD patients may not be due to vitamin K deficiency [23,24].
The results of the present study reveal that the expression pattern of AFP appeared to be consistently expressed in patients with viable HCC regardless of the background liver diseases. In contrast, the expression pattern of PIVKA-II seems to be more influenced by the background liver diseases. In patients with ALD and other non-viral liver diseases, PIVKA-II was paradoxically more produced in those without HCC than in those with HCC. Abnormally high expression of AFP and PIVKA-II is closely associated with the diseased livers and HCC; thus, the majority of patients showed normalization of these tumor markers after liver transplantation performing complete removal of the native liver.
This study has certain limitation. This was a retrospective multi-center cohort study in an HBV-endemic country, and the majority of HCCs in the present study had thus developed in HBV-infected livers. The severity of liver diseases and the stage of HCC were not included in analysis. Pretransplant HCC treatment was also not taken into account.
Conclusions
The results of the present study indicate that pretransplant serum AFP and PIVKA-II were highly variably expressed in LT candidates with end-stage liver diseases; thus, their values should be cautiously interpreted because their role in HCC diagnosis is limited.
Figures
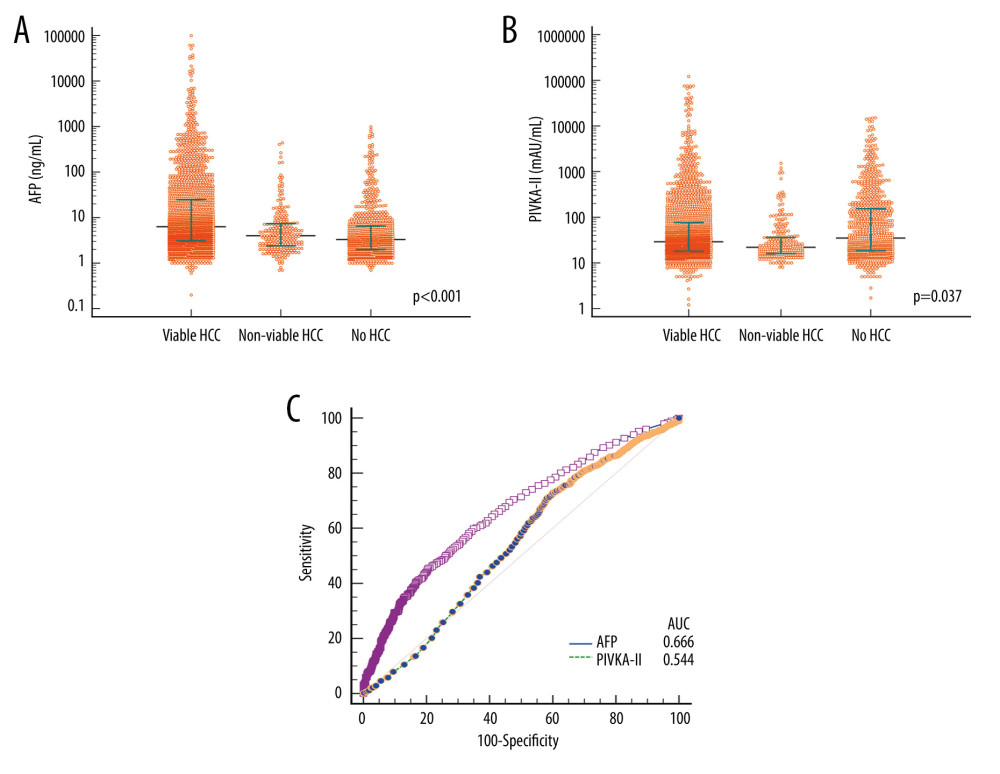 Figure 1. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to the status of hepatocellular carcinoma (HCC) in all patients. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis for diagnosis of HCC. AUC indicates the area under the curve.
Figure 1. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to the status of hepatocellular carcinoma (HCC) in all patients. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis for diagnosis of HCC. AUC indicates the area under the curve. 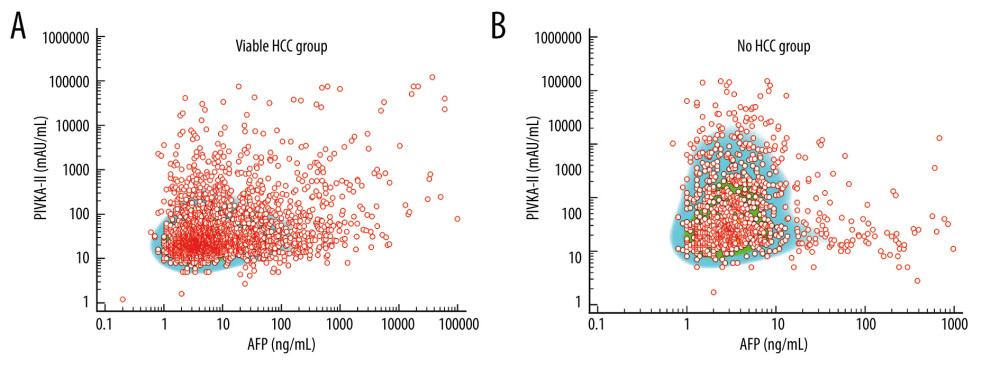 Figure 2. Scatter plots on distribution of alpha-fetoprotein (AFP) and vitamin K absence or antagonist-II (PIVKA-II) values in patients with viable (A) and no (B) hepatocellular carcinoma (HCC).
Figure 2. Scatter plots on distribution of alpha-fetoprotein (AFP) and vitamin K absence or antagonist-II (PIVKA-II) values in patients with viable (A) and no (B) hepatocellular carcinoma (HCC). 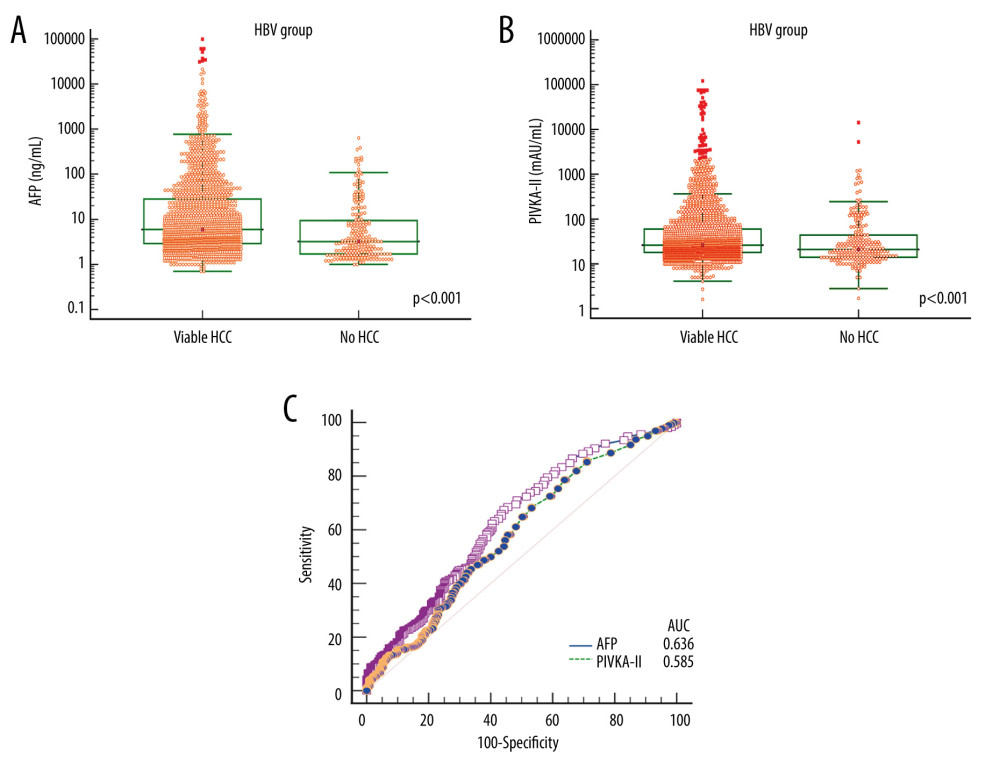 Figure 3. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 3. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. 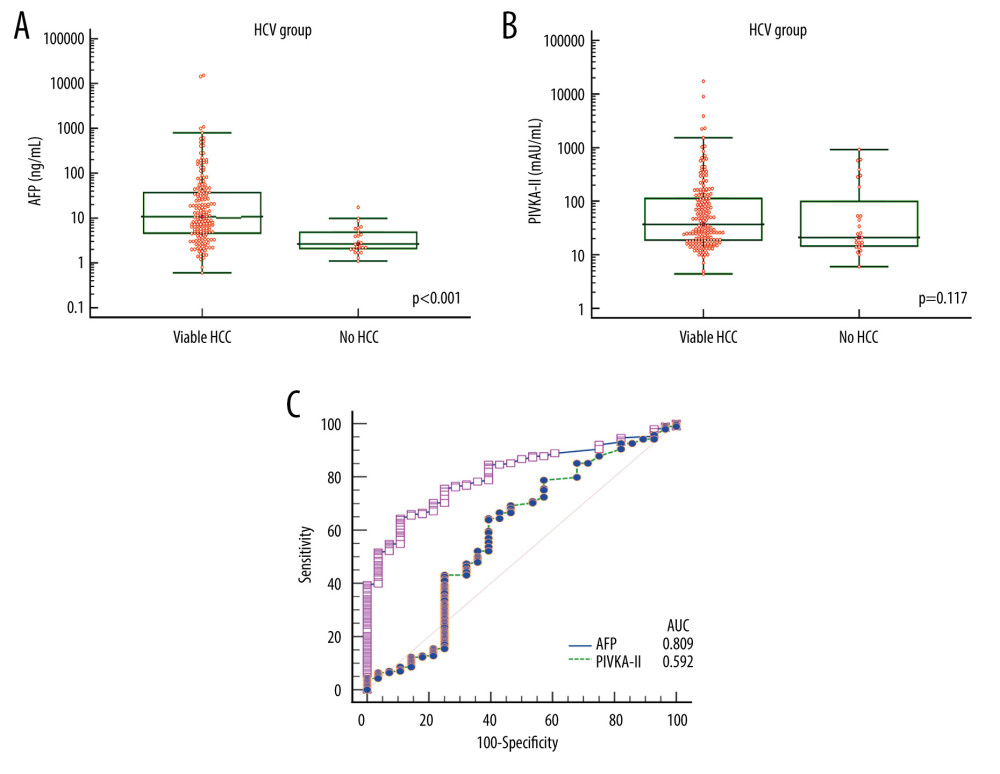 Figure 4. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 4. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. 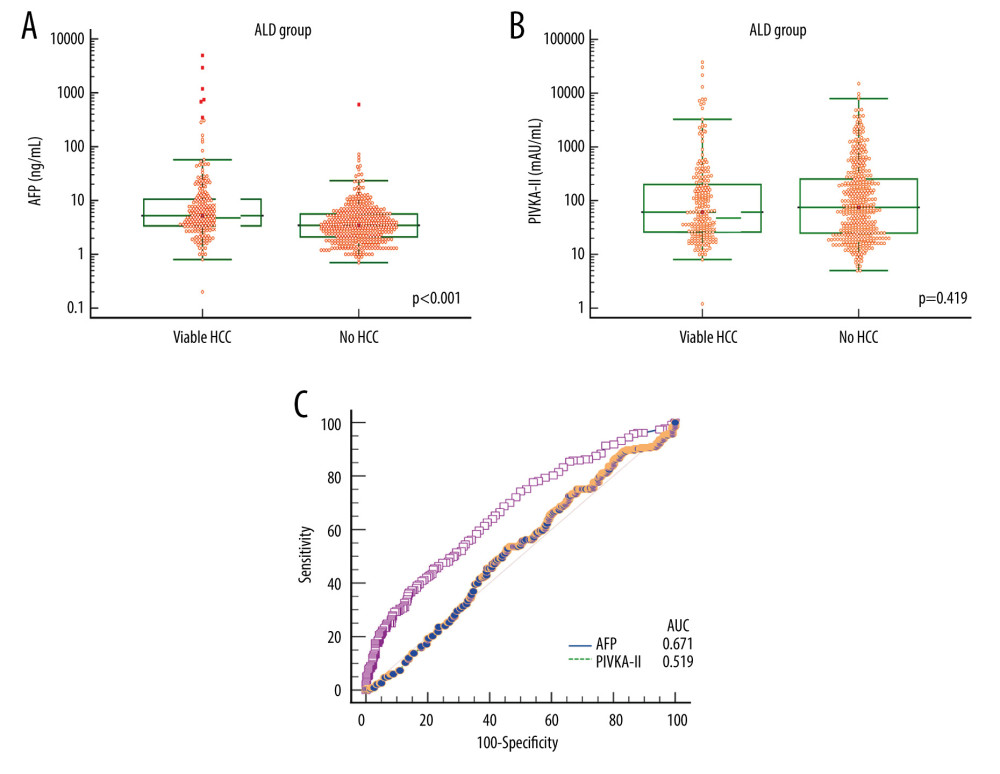 Figure 5. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with alcoholic liver disease (ALD). Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 5. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with alcoholic liver disease (ALD). Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. 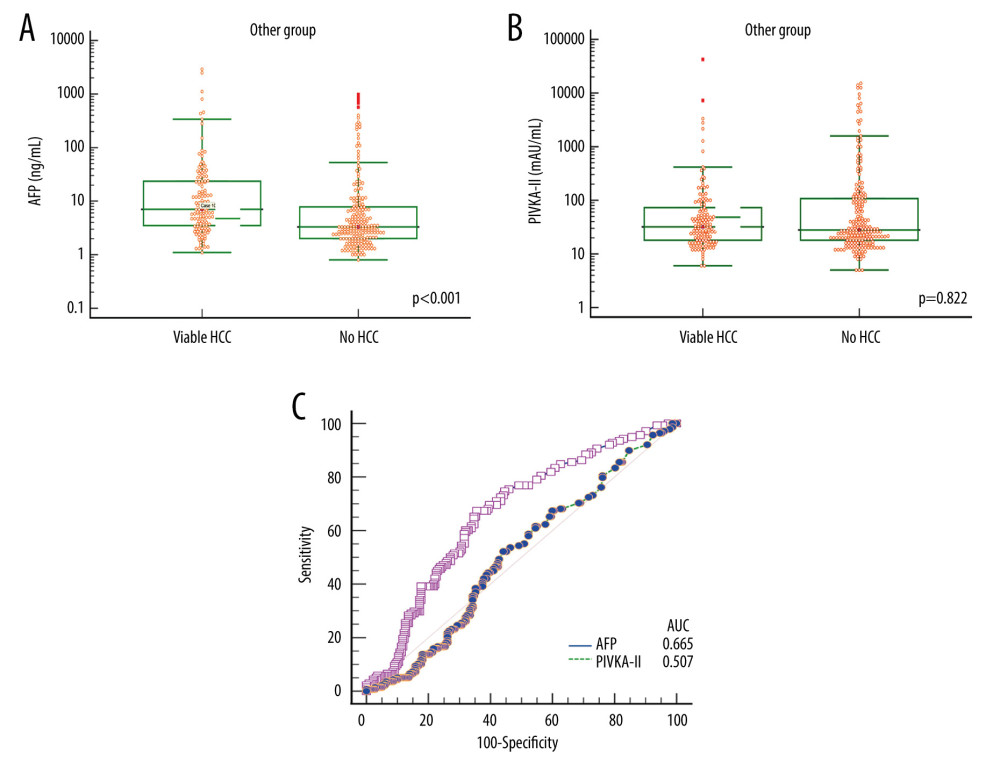 Figure 6. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with liver cirrhosis of other etiology. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 6. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with liver cirrhosis of other etiology. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. References
1. Ha TY, Hwang S, Kim KH, Expression pattern analysis of hepatocellular carcinoma tumor markers in viral hepatitis B and C patients undergoing liver transplantation and resection: Transplant Proc, 2014; 46; 888-93
2. Hwang S, Song GW, Lee YJ, Multiplication of tumor volume by two tumor markers is a post-resection prognostic predictor for solitary hepatocellular carcinoma: J Gastrointest Surg, 2016; 20; 1807-20
3. Arrigoni A, Andriulli A, Gindro T, Pattern analysis of serum alpha-fetoprotein in the early diagnosis of hepatocellular carcinoma in liver cirrhosis: Int J Biol Markers, 1988; 3; 172-76
4. Tang W, Miki K, Kokudo N, Des-gamma-carboxy prothrombin in cancer and non-cancer liver tissue of patients with hepatocellular carcinoma: Int J Oncol, 2003; 22; 969-75
5. Nakamura S, Nouso K, Sakaguchi K, Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size: Am J Gastroenterol, 2006; 101; 2038-43
6. Nagaoka S, Yatsuhashi H, Hamada H, The des-gammacarboxy prothrombin index is a new prognostic indicator for hepatocellular carcinoma: Cancer, 2003; 98; 2671-77
7. Kang KH, Kim JH, Kang SH, The influence of alcoholic liver disease on serum PIVKA-II levels in patients without hepatocellular carcinoma: Gut Liver, 2015; 9; 224-30
8. Kang WH, Hwang S, Song GW, Prognostic effect of transarterial chemoembolization-induced complete pathological response in patients undergoing liver resection and transplantation for hepatocellular carcinoma: Liver Transpl, 2017; 23; 781-90
9. Sell S, Alpha-fetoprotein, stem cells and cancer: How study of the production of alpha-fetoprotein during chemical hepatocarcinogenesis led to reaffirmation of the stem cell theory of cancer: Tumour Biol, 2008; 29; 161-80
10. Tomasi TB, Structure and function of alpha-fetoprotein: Annu Rev Med, 1977; 28; 453-65
11. Behne T, Copur MS, Biomarkers for hepatocellular carcinoma: Int J Hepatol, 2012; 2012; 859076
12. Sherman M, The resurrection of alphafetoprotein: J Hepatol, 2010; 52; 939-40
13. Liu C, Xiao GQ, Yan LN, Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma: World J Gastroenterol, 2013; 19; 1811-19
14. Liebman HA, Furie BC, Tong MJ, Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma: N Engl J Med, 1984; 310; 1427-3
15. Kobayashi M, Ikeda K, Kawamura Y, High serum des-gamma-carboxy prothrombin level predicts poor prognosis after radiofrequency ablation of hepatocellular carcinoma: Cancer, 2009; 115; 571-80
16. Zinkin NT, Grall F, Bhaskar K, Serum proteomics and biomarkers in hepatocellular carcinoma and chronic liver disease: Clin Cancer Res, 2008; 14; 470-77
17. Sherman M, Peltekian KM, Lee C, Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: Incidence and prevalence of hepatocellular carcinoma in a North American urban population: Hepatology, 1995; 22; 432-38
18. Tsukuma H, Hiyama T, Tanaka S, Risk factors for hepatocellular carcinoma among patients with chronic liver disease: N Engl J Med, 1993; 328; 1797-801
19. Trevisani F, D’Intino PE, Morselli-Labate AM, Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: Influence of HBsAg and anti-HCV status: J Hepatol, 2001; 34; 570-75
20. Charrière B, Maulat C, Suc B, Muscari F, Contribution of alpha-fetoprotein in liver transplantation for hepatocellular carcinoma: World J Hepatol, 2016; 8; 881-90
21. Ricco G, Cavallone D, Cosma C, Impact of etiology of chronic liver disease on hepatocellular carcinoma biomarkers: Cancer Biomark, 2018; 21; 603-12
22. Park CS, Chung YK, Kang SH, Hwang S, Diagnostic predictability of hepatocellular carcinoma tumor markers in patients waiting for living donor liver transplantation: Ann Liver Transplant, 2022; 2; 34-42
23. Sakizono K, Oita T, Eto MStudies on the mechanism of elevation of serum PIVKA-II levels in alcoholic liver cirrhosis: Rinsho Byori, 2002; 50; 289-95 [in Japanese]
24. Ohhira M, Ohtake T, Saito H, Increase of serum des-gamma-carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma: Alcohol Clin Exp Res, 1999; 23(4 Suppl); 67S-70S
Figures
 Figure 1. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to the status of hepatocellular carcinoma (HCC) in all patients. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis for diagnosis of HCC. AUC indicates the area under the curve.
Figure 1. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to the status of hepatocellular carcinoma (HCC) in all patients. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis for diagnosis of HCC. AUC indicates the area under the curve. Figure 2. Scatter plots on distribution of alpha-fetoprotein (AFP) and vitamin K absence or antagonist-II (PIVKA-II) values in patients with viable (A) and no (B) hepatocellular carcinoma (HCC).
Figure 2. Scatter plots on distribution of alpha-fetoprotein (AFP) and vitamin K absence or antagonist-II (PIVKA-II) values in patients with viable (A) and no (B) hepatocellular carcinoma (HCC). Figure 3. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 3. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. Figure 4. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 4. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-associated liver cirrhosis. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. Figure 5. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with alcoholic liver disease (ALD). Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 5. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with alcoholic liver disease (ALD). Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. Figure 6. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with liver cirrhosis of other etiology. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve.
Figure 6. Comparison of alpha-fetoprotein (AFP; A) and protein induced by vitamin K absence or antagonist-II (PIVKA-II; B) levels according to viable and no hepatocellular carcinoma (HCC) in patients with liver cirrhosis of other etiology. Bars indicate 25–75 percentiles. C) Receiver operating characteristic curve analysis of AFP and PIVKA-II for diagnosis of HCC. AUC indicates the area under the curve. Tables
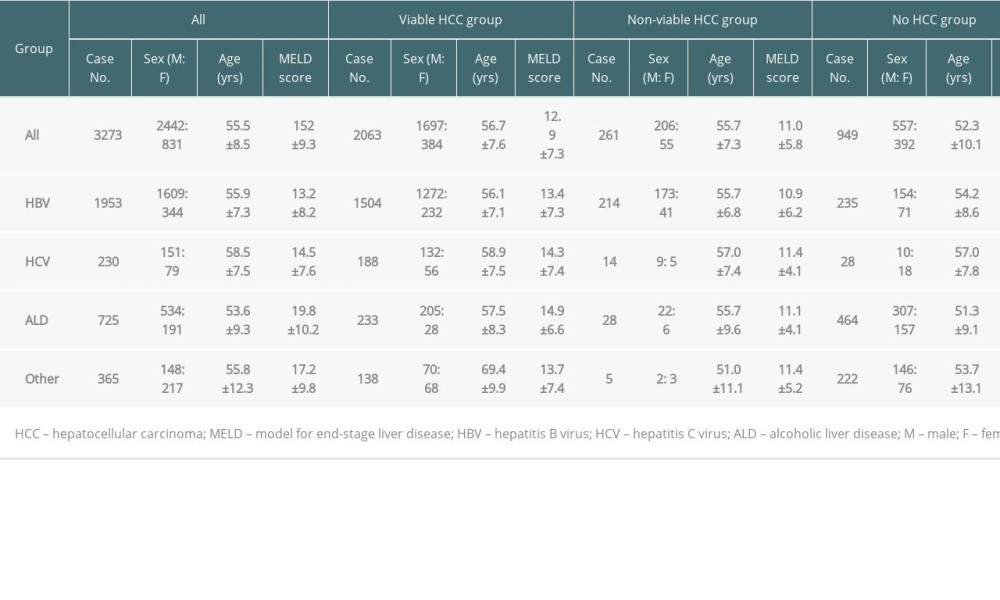 Table 1. Patient profiles according to the status of HCC and background liver diseases.
Table 1. Patient profiles according to the status of HCC and background liver diseases.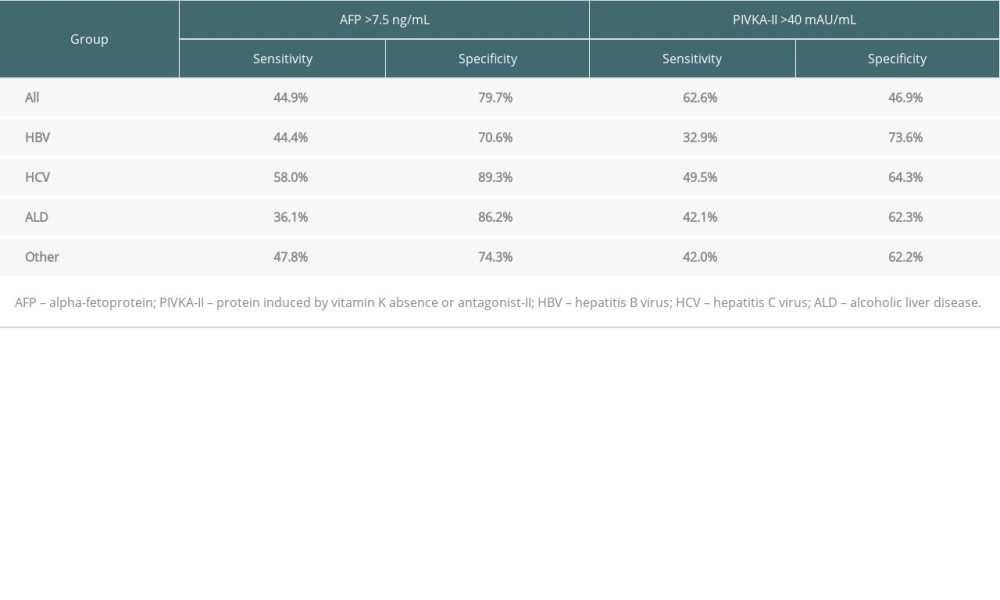 Table 2. Comparison of diagnostic predictability of tumor markers for hepatocellular carcinoma.
Table 2. Comparison of diagnostic predictability of tumor markers for hepatocellular carcinoma. Table 1. Patient profiles according to the status of HCC and background liver diseases.
Table 1. Patient profiles according to the status of HCC and background liver diseases. Table 2. Comparison of diagnostic predictability of tumor markers for hepatocellular carcinoma.
Table 2. Comparison of diagnostic predictability of tumor markers for hepatocellular carcinoma. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








