23 December 2022: Original Paper
Condition of the Oral Cavity in Patients After Heart Transplantation: A Preliminary Report
Katarzyna Olek1ABCDEFG, Agnieszka Kuczaj2DEF*, Olaf Gruca3ABC, Marcin OlekDOI: 10.12659/AOT.937734
Ann Transplant 2022; 27:e937734
Abstract
BACKGROUND: The constant impairment of the immune system caused by lifelong use of immunosuppressive drugs in patients after heart transplantation has a significant impact on oral cavity health. The aim of this study was to analyze the health of the oral cavity in patients after heart transplantation, with particular regard to occurring pathogens.
MATERIAL AND METHODS: The study included 25 patients after heart transplantation. The research scheme was divided into 2 parts. The first part consisted of a survey on general health and oral hygiene habits. The second part of the examination consisted of an analysis of the health of the oral cavity: the mucosa, periodontium, and hard dental tissues. Particular attention was paid to PET (test for the presence of pathogens causing periodontitis/periimplantitis) and CAT (diagnostic test for the presence of Candida in the oral cavity), which are real-time PCR tests used to detect pathogens causing periodontitis and microorganisms present in oral candidiasis.
RESULTS: The conducted research and in-depth analysis of the results showed that the oral health condition in patients after heart transplantation is not satisfactory, regardless of the time that has elapsed since the surgery, sex, age, hygiene habits, or the type of immunosuppression used. The oral cavity of patients after heart transplantation is colonized with Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, and Candida albicans.
CONCLUSIONS: The cooperation of the dentist with the attending physician at each stage of the treatment should play an unquestionable role.
Keywords: Heart Transplantation, Microbiota, Humans, periodontitis, Porphyromonas gingivalis, Treponema denticola, Oral Health
Background
The recommended method of treatment in end-stage heart failure patients without absolute contraindications is heart transplantation. This procedure is the treatment of last resort in patients who are refractory to medical or device therapy. After the transplantation, the recipient has to take lifelong immunosuppressive drugs enabling graft protection from host alloimmunity. These drugs have a number of significant adverse effects, including susceptibility to oncogenic and infectious factors. Permanent impairment of the immune system also influences the health of the oral cavity. The microbiome of the gastrointestinal tract and the oral cavity may play a significant role in modulation of the recipient’s inflammatory processes. Particularly noteworthy in this context is the assessment of the health of hard tooth tissues, oral mucosa, and periodontium [1–4]]. Currently, periodontitis is classified as a “complex” disease, characterized by the interaction of genes with environmental factors. Risk factors for periodontitis include genetic polymorphism, obesity, smoking, general diseases, sex, age, and race. In turn, bacteria linked to poor oral health (eg,
The key to achieving the balance of the condition of the oral cavity and therapeutic success is to combine the maintenance of proper oral hygiene by the patient with professional dental care [7–9].
The aim of this study was to evaluate the condition of the oral cavity in patients after heart transplantation and to assess pathogens colonizing their dental pockets. We divided the whole group of patients in 2 subgroups according to time elapsed since transplantation. We hypothesized that long-term exposition to immunosuppressive drugs significantly affects the oral microbiota. We assessed the long-term oral flora changes in patients after transplantation, and whether the duration of taking immunosuppressive drugs affects the oral microbiome. According to ISHLT [10], the mean survival of patients after heart transplantation is 12.5 years. Patients after 10 years may have beneficial factors improving survival. The role of the microbiome in graft rejection and systemic inflammation is still being discussed. We assessed whether there were significant differences between these 2 groups.
We used PET (test for the presence of pathogens causing periodontitis/periimplantitis) and CAT (diagnostic test for the presence of
Material and Methods
INCLUSION CRITERIA:
We included consecutive adult patients after heart transplantation, who regularly attended an outpatient clinic in a regional transplant center. The participants signed written informed consent for participation in the study. The study was performed in accordance with the Declaration of Helsinki. The Bioethics Committee of the Medical University of Silesia approved the study (decision No. KNW/0022/KB1/35/I/19).
EXCLUSION CRITERIA:
The project did not involve patients who did not consent to participate in the study, patients who did not show willingness to cooperate in the study, incapacitated people, soldiers, prisoners, persons with business or private ties with the person conducting the examination, or persons undergoing orthodontic treatment. Toothless patients, patients with active gingival bleeding, patients with insufficient number of teeth to assess oral hygiene indicators and to conduct PET and CAT tests were also excluded from the study.
STATISTICAL METHODS:
We presented categorical variables as counts and percentages. Continuous variables are expressed as means and standard deviations for normally distributed data or median with lower and upper quartiles. To verify the normal distribution of data, we used the Shapiro-Wilk test. The chi-square test was utilized to compare categorical variables, and the
Results
Mean age of the 13 patients (3 women) up to 10 years after transplantation was 45.8±16.2 (Group 1.). The mean age of 12 patients (4 women) over 10 years after transplantation was 55.8±15.0 years (Group 2). The mean time from transplantation in Group 1 was 7.2±2.2 and in Group 2 it was 14.0±3.8 years. In Group 1, 11 (92.3%) patients received tacrolimus and 1 (7.7%) received cyclosporine A as a basic regimen. In Group 2, 8 (58.3%) patients received tacrolimus and 5 (41.7%) received cyclosporine. None of the investigated patients received steroids, anticonvulsants, dihydropyridine calcium channel blockers, verapamil, or diltiazem. Clinical characteristics of the 2 groups are presented in Table 2.
Statistical analysis of hygiene habits shows that the groups were comparable. No significance difference between groups was noted in use of dental floss or mouthwash (
No statistically significant (
In the case of the remaining indices, API and OHI the differences between Group 1 and Group 2 were not statistically significant (0.7 vs 0.6, 1.6 vs 1.4;
The degree of mobility of the teeth was not significantly different between groups (
All patients taking Cyclosporin A developed hyperplastic gingivitis (1 in Group 1 and 5 in Group 2). Pathologic pockets (defined as periodontal fissure >3 mm in depth) were present despite the type of basic immunosuppressive regimen. However, in patients taking tacrolimus, it was present in 6 (50.0%) patients in Group 1 vs 5 (71.4%) in Group 2, but it was present in all patients taking cyclosporine A in both groups. These data are summarized in Table 5.
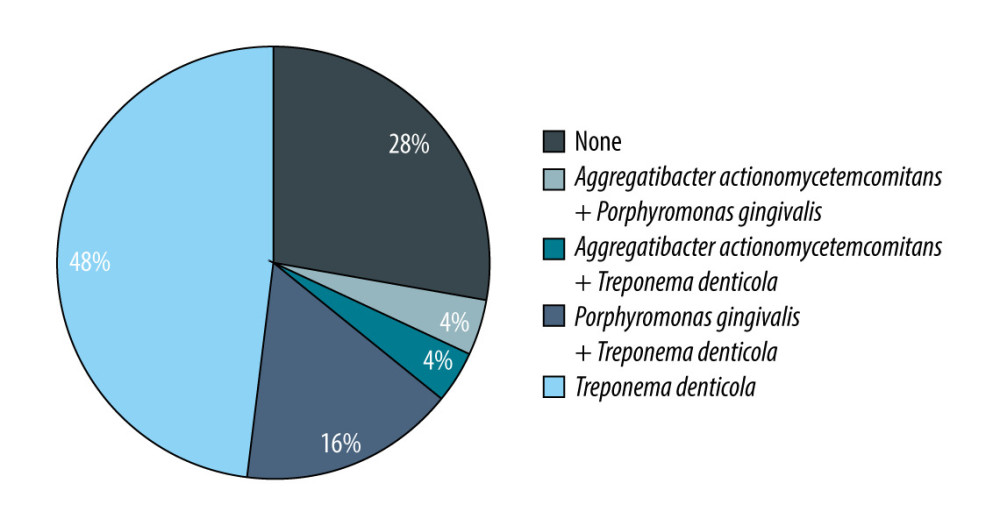
The presence of bacterial and fungal infections in both analyzed groups is presented in Table 6.
In Group 1, all the bacterial infections coexisted with fungal infection. In 6 patients, 2 bacterial pathogens coexisted: in 4 cases
The analysis of the depth of the gingival pockets – 3.9 (0.8) mm in Group 1 vs 3.9 (0.8) mm in Group 2 – and the correlating loss of connective tissue attachment indicated no statistically significant differences (
The values of gingival pockets were also statistically analyzed in patients with negative and positive PET results in relation to all detected pathogens (
Subjects with more than 1 bacterial pathogen versus the remaining group showed only a trend towards deeper pockets – 4.3 (0.6) mm vs other patients 3.7 (0.6) (
Discussion
STUDY LIMITATIONS:
A significant limitation of the study is its small sample size. Due to this fact some correlations did not reach statistical significance. In more numerous groups we could have performed age- and sex-matched analyses. It would be also interesting to investigate the whole gastrointestinal tract microbiota in relation to graft tolerance.
Conclusions
The obtained results show that oral hygiene and the condition of hard tooth tissues and periodontium in patients after heart transplantation is not satisfactory. More than once, the respondents noted full involvement in the underlying disease, without a sufficient focus on the health of the oral cavity. Impairment of the immune system caused by the continued intake of immunosuppressants increases the likelihood of oral fungal, bacterial, or viral infections. The detected pathogens in patients after heart transplantation were
Proper preparation of the patient’s oral cavity before starting immunosuppressive therapy and appropriate dental care after heart transplantation will reduce the incidence of complications and minimize the risk of their generalization. The cooperation of the dentist with the attending physician at each stage of treatment should play an important role.
Tables
Table 1. Diagnostic tests for the presence of pathogens causing periodontitis (PET) and diagnostic tests for the presence of Candida (CAT) (data from the manufacturer of the tests).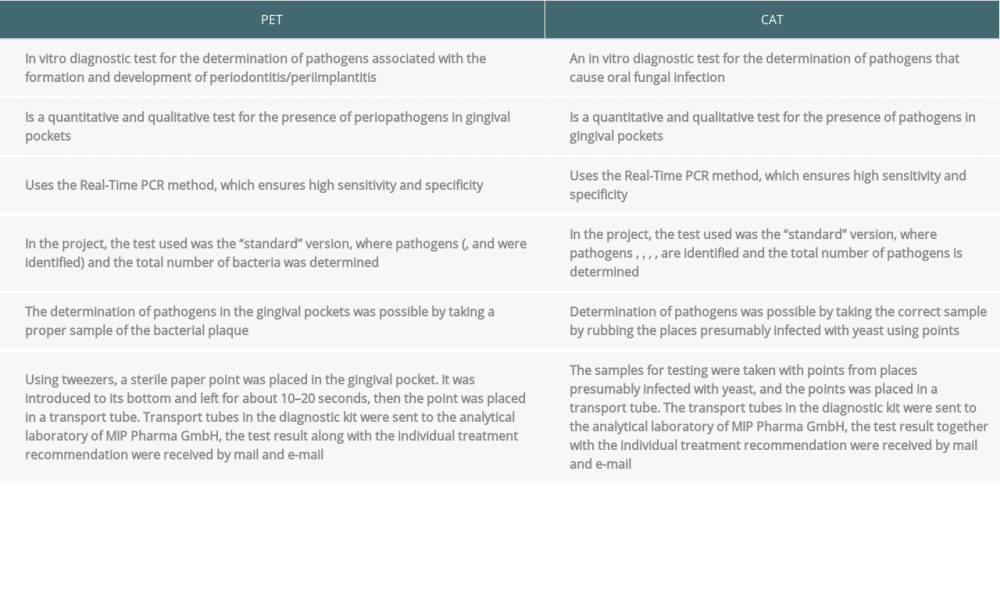 Table 2. Clinical characteristics of the 2 analyzed groups.
Table 2. Clinical characteristics of the 2 analyzed groups.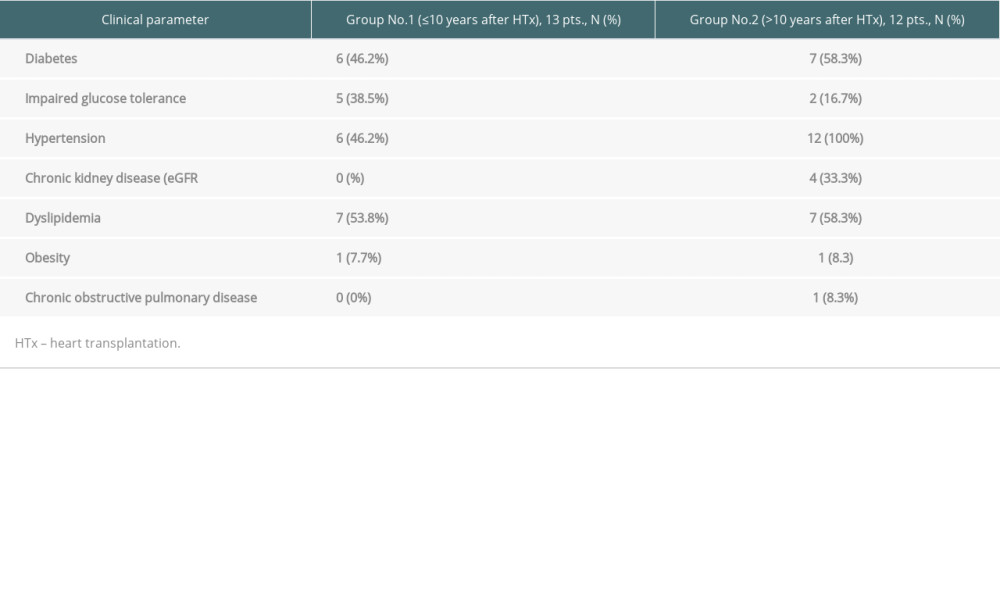 Table 3. Data on hygiene habits.
Table 3. Data on hygiene habits.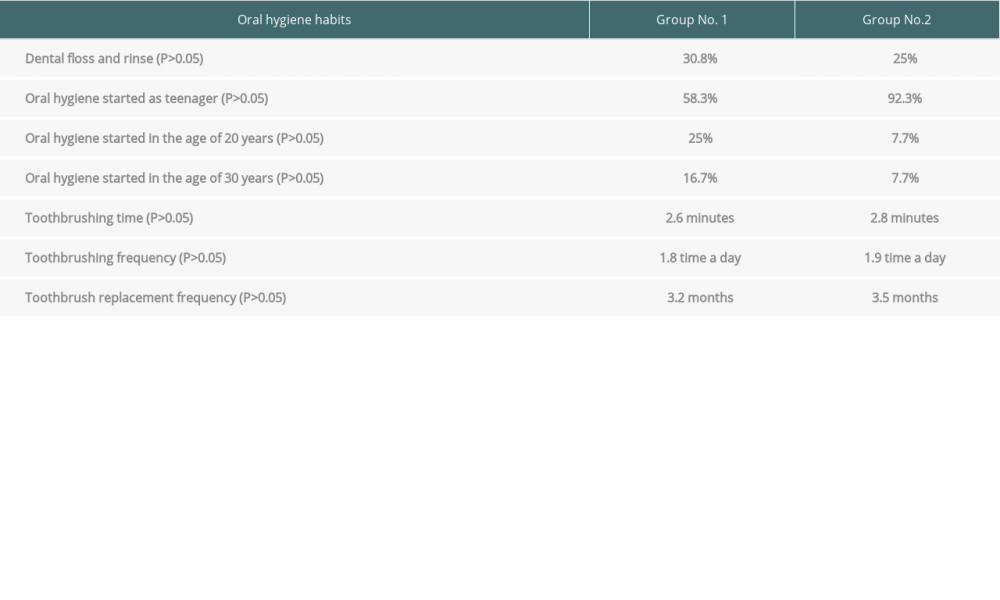 Table 4. Oral health indicators assessment.
Table 4. Oral health indicators assessment.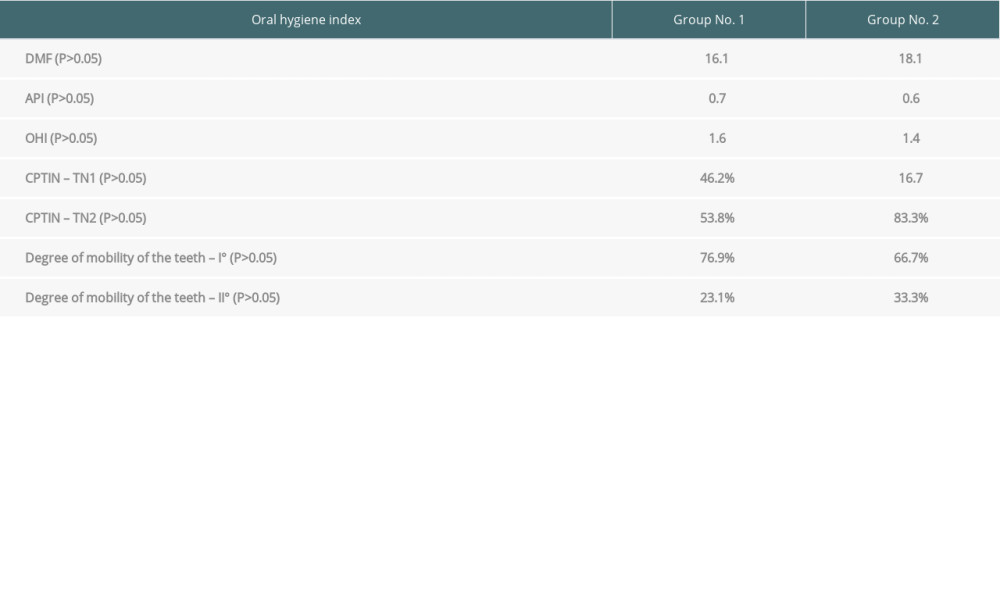 Table 5. The prevalence of hyperplastic gingivitis and pathological pockets in the analyzed groups depending on the type of immunosuppression.
Table 5. The prevalence of hyperplastic gingivitis and pathological pockets in the analyzed groups depending on the type of immunosuppression.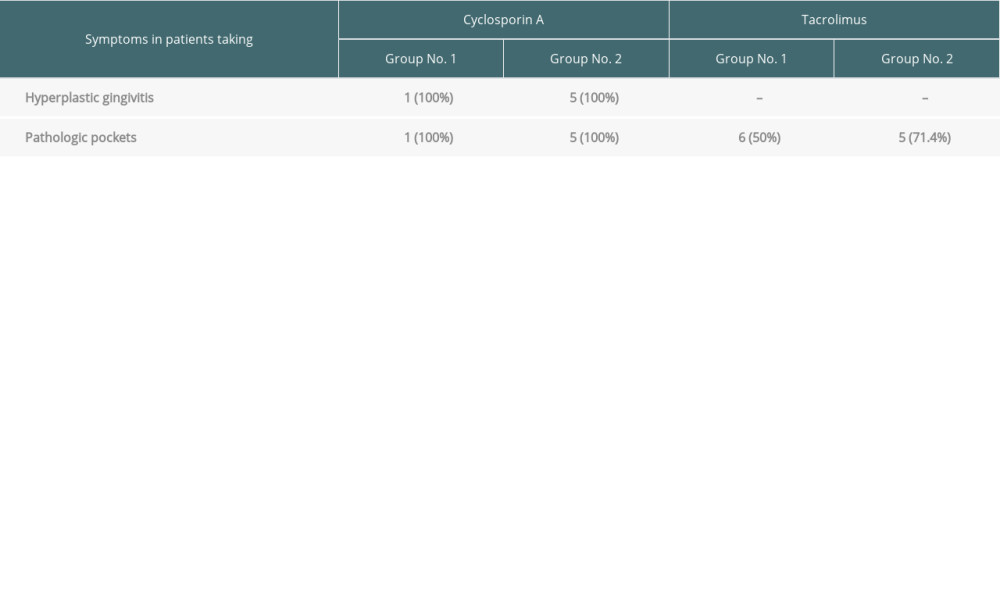 Table 6. The percentage distribution of the bacteria and Candida albicans detected in the analyzed groups.
Table 6. The percentage distribution of the bacteria and Candida albicans detected in the analyzed groups.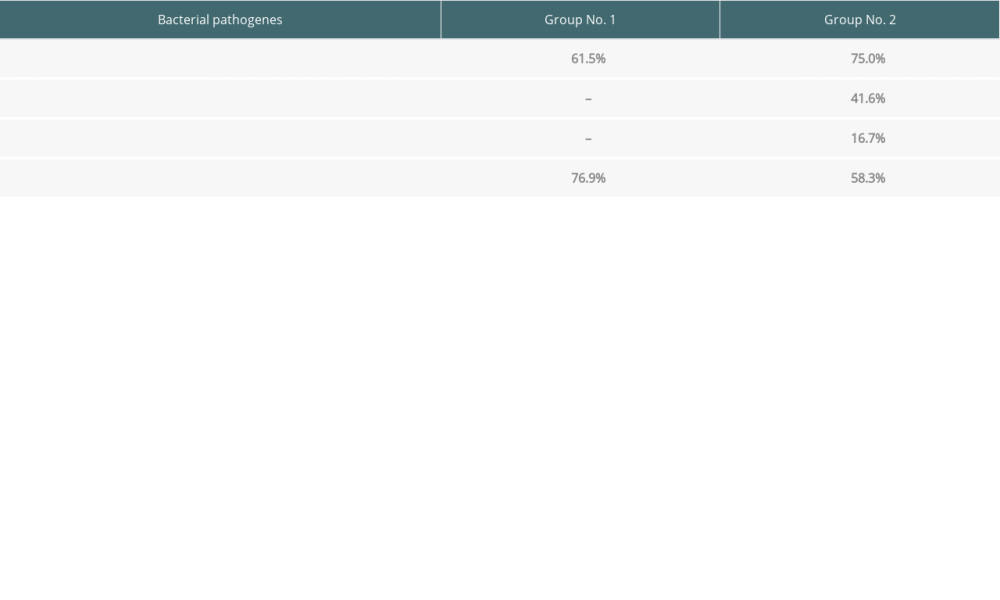 Table 7. Depth of gingival pockets according to pathogen in patients with negative and positive PET results.
Table 7. Depth of gingival pockets according to pathogen in patients with negative and positive PET results.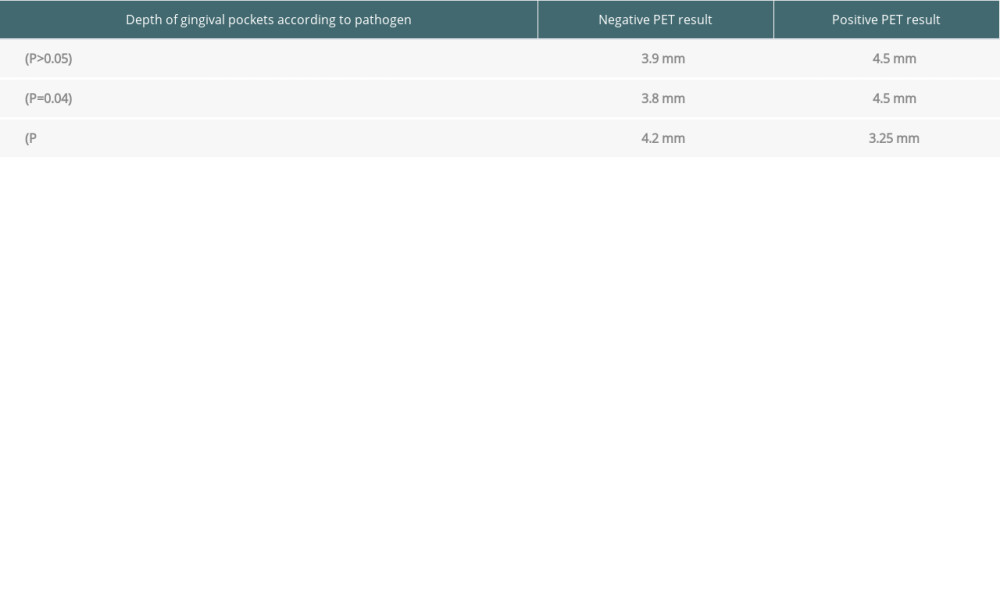
References
1. Gackowska M, Gackowski A, Chomyszyn-Gajewska MPrinciples of dental management in heart transplant recipients: Poradnik Stomatologiczny, 2003; 3; 32-34 [in Polish]
2. Wagoner LE, Management of the cardiac transplant recipient: Roles of the transplant cardiologist and primary care physician: Am J Med Sci, 1997; 314(3); 173-84
3. Golder DT, Drinnan AJ, Dental aspects of cardiac transplantation: Transplant Proc, 1993; 25(3); 2377-80
4. Dominiak A, Interewicz B, Swoboda E, Olszewski WL, Endogeneous sources of infection in transplant recipients: Ann Transplant, 2006; 11(4); 30-37
5. Bohn B, Pinsino A, Braghieri L, Association between oral microbiome, inflammation, endotoxemia, and heart failure severity: J Heart Lung Transplant, 2022; 41(4); S202-S3
6. Dela Cruz M, Littmann E, Nayak R, The gut microbiome in heart transplantation: A prospective pilot study: J Heart Lung Transplant, 2021; 40(4); S25
7. Miskiewicz A, Szparecki G, Nowak M, Górska REpidemiological analysis of colonization and drug resistance in patients with prosthetic stomatopathy: Borgis – Nowa Stomatologia, 2013; 3; 141-44 [in Polish]
8. Niedzielska I, Wziątek-Kuczmik DThe influence of odontogenic foci of infection on diseases of other organs – a review the literature: Chirurgia Polska (Polish Surgery), 2007; 9(2); 92-96 [in Polish]
9. Dominiak M, Zapała J, Gedrange T, 2013, Warsaw, EDRA Urban [in Polish]
10. Khush KK, Cherikh WS, Chambers DCISHLT. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation, Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation: J Heart Lung Transplant, 2018; 37(10); 1155
11. Lange DE, Plagmann HC, Eenboom A, Promesberger A, Clinical methods for the objective evaluation of oral hygiene: Dtsch Zahnarztl Z, 1977; 32(1); 44-47
12. Greene JC, Vermillion JR, The simplified oral hygiene index: J Am Dent Assoc, 1964; 68; 7-13
13. Ainamo J, Barmes D, Beagrie G, Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN): Int Dent J, 1982; 32(3); 281-91
14. Górska R, Konopka T: Periodontologia współczesna, 2013, Otwock, Med Tour Press International [in Polish]
15. Kurzeja M, Grabowska E, Grabowska MThe evaluation of periodontal tissues and treatment needs in patients after heart transplantation: Borgis – Nowa Stomatologia, 2002; 2; 99-104 [in Polish]
16. Borakowska M, Preiskorn M, Stawicka ROral lesions in kidney or renal transplant patients receiving immunosuppressive therapy: Nowa Stomatologia, 1999; 3; 31-33 [in Polish]
17. Bedra B, Grenda R, Olczak-Kowalczyk DOral changes in patients after solid organ transplantation depending on the type of immunosuppression used: a pilot study: Czasopismo Stomatologiczne: Organ Polskiego Towarzystwa Stomatologicznego, 2006; 11; 759-68 [in Polish]
18. De Iudicibus S, Castronovo G, Gigante A, Role of MDR1 gene polymorphisms in gingival overgrowth induced by cyclosporine in transplant patients: J Periodontal Res, 2008; 43(6); 665-72
19. Siahi-Benlarbi R, Nies SM, Sziegoleit A, Caries-, Candida- and Candida antigen/antibody frequency in children after heart transplantation and children with congenital heart disease: Pediatr Transplant, 2010; 14(6); 715-21
20. Szymańska M, Węgorska DUnusual changes in the oral mucosa in a patient treated with Cyclosporin A: Czasopismo Stomatologiczne, 1994; 12; 809-14 [in Polish]
21. Dongari-Bagtzoglou A, Dwivedi P, Ioannidou E, Oral Candida infection and colonization in solid organ transplant recipients: Oral Microbiol Immunol, 2009; 24(3); 249-54
22. Magliocca KR, Harris DB, Schain DC, Cutaneous and oral mucosal lesions in a cardiac transplant recipient: J Am Dent Assoc, 2012; 143(6); e25-e28
23. Sasaoka T, Kato TS, Oda N, Common occurrence of everolimus-associated aphthous stomatitis in Japanese heart transplant recipients: Transplant Proc, 2010; 42(9); 3700-3
24. Hollander SA, Yasnovsky JR, Reinhartz O, Behcet’s disease and heart transplantation: A word of caution: J Heart Lung Transplant, 2010; 29(11); 1306-8
25. Shah AT, Wu E, Wein RO, Oral squamous cell carcinoma in post-transplant patients: Am J Otolaryngol, 2013; 34(2); 176-79
26. Pakosz K, Zakliczyński M, Król W, Association of transforming growth factor β1 (TGF-β1) with gingival hyperplasia in heart transplant patients undergoing cyclosporine-A treatment: Ann Transplant, 2012; 17(2); 45-52
27. Shaqman M, Ioannidou E, Burleson J, Periodontitis and inflammatory markers in transplant recipients: J Periodontol, 2010; 81(5); 666-72
28. McGaw T, Lam S, Coates J, Cyclosporin-induced gingival overgrowth: correlation with dental plaque scores, gingivitis scores, and cyclosporin levels in serum and saliva: Oral Surg Oral Med Oral Pathol, 1987; 64(3); 293-97
29. Dery KJ, Kadono K, Hirao H, Microbiota in organ transplantation: An immunological and therapeutic conundrum?: Cell Immunol, 2020; 351; 104080
30. McIntosh CM, Chen L, Shaiber A, Gut microbes contribute to variation in solid organ transplant outcomes in mice: Microbiome, 2018; 6(1); 96
Tables
 Table 1. Diagnostic tests for the presence of pathogens causing periodontitis (PET) and diagnostic tests for the presence of Candida (CAT) (data from the manufacturer of the tests).
Table 1. Diagnostic tests for the presence of pathogens causing periodontitis (PET) and diagnostic tests for the presence of Candida (CAT) (data from the manufacturer of the tests). Table 2. Clinical characteristics of the 2 analyzed groups.
Table 2. Clinical characteristics of the 2 analyzed groups. Table 3. Data on hygiene habits.
Table 3. Data on hygiene habits. Table 4. Oral health indicators assessment.
Table 4. Oral health indicators assessment. Table 5. The prevalence of hyperplastic gingivitis and pathological pockets in the analyzed groups depending on the type of immunosuppression.
Table 5. The prevalence of hyperplastic gingivitis and pathological pockets in the analyzed groups depending on the type of immunosuppression. Table 6. The percentage distribution of the bacteria and Candida albicans detected in the analyzed groups.
Table 6. The percentage distribution of the bacteria and Candida albicans detected in the analyzed groups. Table 7. Depth of gingival pockets according to pathogen in patients with negative and positive PET results.
Table 7. Depth of gingival pockets according to pathogen in patients with negative and positive PET results. Table 1. Diagnostic tests for the presence of pathogens causing periodontitis (PET) and diagnostic tests for the presence of Candida (CAT) (data from the manufacturer of the tests).
Table 1. Diagnostic tests for the presence of pathogens causing periodontitis (PET) and diagnostic tests for the presence of Candida (CAT) (data from the manufacturer of the tests). Table 2. Clinical characteristics of the 2 analyzed groups.
Table 2. Clinical characteristics of the 2 analyzed groups. Table 3. Data on hygiene habits.
Table 3. Data on hygiene habits. Table 4. Oral health indicators assessment.
Table 4. Oral health indicators assessment. Table 5. The prevalence of hyperplastic gingivitis and pathological pockets in the analyzed groups depending on the type of immunosuppression.
Table 5. The prevalence of hyperplastic gingivitis and pathological pockets in the analyzed groups depending on the type of immunosuppression. Table 6. The percentage distribution of the bacteria and Candida albicans detected in the analyzed groups.
Table 6. The percentage distribution of the bacteria and Candida albicans detected in the analyzed groups. Table 7. Depth of gingival pockets according to pathogen in patients with negative and positive PET results.
Table 7. Depth of gingival pockets according to pathogen in patients with negative and positive PET results. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860