15 December 2023: Original Paper
Clinical Outcomes of Administration of Rituximab for Desensitization in Liver Transplant Patients with Preformed Donor-Specific Antibodies: A Single-Center Experience
Masato ShizukuDOI: 10.12659/AOT.941456
Ann Transplant 2023; 28:e941456
Abstract
BACKGROUND: The management and fate of liver transplant (LT) recipients with preformed donor-specific antibodies (pDSA) remain controversial. The aim of this study was to evaluate the clinical impact of rituximab desensitization on pDSA in LT recipients.
MATERIAL AND METHODS: This retrospective observational study enrolled 120 LT patients aged ≥18 years. Patients with pDSA were administered 500 mg/body rituximab 1-21 days before LT, except for those who had an active infection or had insufficient time to receive rituximab. We allocated patients to groups with or without pDSA, and then divided patients with pDSA into rituximab (+) and rituximab (–) groups for further analysis.
RESULTS: Twenty-three patients (19.2%) with pDSA were identified. Of these, 18 received rituximab and 5 did not receive rituximab. No patients developed adverse events related to rituximab. In both groups, the levels of pDSA class I in all patients were decreased immediately after LT, whereas those of pDSA class II decreased slowly. There were no significant differences in pathology findings and overall survival between patients with pDSA who were rituximab (+) or rituximab (–), and between patients with or without pDSA.
CONCLUSIONS: Rituximab desensitization for LT patients with pDSA was managed successfully without significant complications. Due to the small sample size, we could not demonstrate the benefit of rituximab desensitization for LT patients compared with the rituximab (–) group. Additionally, clinical outcomes in patients with pDSA, with or without rituximab, were similar to those without pDSA. Rituximab desensitization might be not essential for LT.
Keywords: Graft Rejection, Desensitization, Immunologic, Liver Transplantation, rituximab
Background
The importance of preformed donor-specific antibodies (pDSA) in liver transplantation recipients was reported recently, similar to other solid organ transplantations, including the kidney. The prevalence of pDSA was reported to be approximately 10–20% in liver transplantation recipients [1,2], which cannot be ignored for the management of liver transplantation. pDSA in liver transplantation recipients was reported to increase the risk of chronic graft rejection [3,4] and acute antibody-mediated rejection (AMR) [5,6]. Moreover, pDSA decreased graft survival [7] and contributed to graft fibrosis [8] and early mortality [1,9]. Therefore, to remove pDSA before liver transplantation, a combination of rituximab, plasma exchange, and immunoglobulin has been attempted as desensitization therapy [10], although no effective therapy has been established. Although many studies reported unfavorable outcomes related to pDSA, some studies reported that pDSA in liver transplantation recipients was not related to graft function, pathology findings [11], patient survival [5], or clinical impact [12]. Therefore, the clinical implication of pDSA in liver transplantation recipients remains controversial.
In other solid organ transplantations, especially the kidney, intravenous immunoglobulin, rituximab, plasmapheresis, and thymoglobulin were reported to prevent adverse events in pDSA-related disorders, such as chronic rejection or AMR [13–15]. However, few studies have investigated therapeutic interventions to control pDSA in liver transplantation recipients.
Although some therapeutic agents were reported to be beneficial for the management of pDSA in liver transplantation recipients, the present study focused on rituximab desensitization for liver transplant patients with pDSA because rituximab is commonly used in kidney transplantation and evidence has suggested its benefit [10]. The aim of this study was to evaluate the clinical impact of rituximab desensitization on liver transplant patients with pDSA.
Material and Methods
PATIENT POPULATION:
This study was a retrospective observational study. From October 2014 to December 2021, 120 liver transplantation patients at Nagoya University Hospital were enrolled. Patients who died within 30 days after liver transplantation or pediatric patients under 18 years old were excluded from this study. All patients were evaluated for pDSA at their initial assessment before liver transplantation. We allocated patients to groups with or without pDSA, and then divided patients with pDSA into rituximab (+) and rituximab (−) groups and compared their clinical outcomes for further evaluation.
SCHEDULE OF THE ADMINISTRATION OF RITUXIMAB AND IMMUNOSUPPRESSIVE PROTOCOL FOR PATIENTS WITH PDSA:
Based on our desensitization protocol (Figure 1), patients with pDSA at the initial assessment received rituximab at 500 mg/body 1–21 days before liver transplantation. No other immunosuppressive agents were administered for desensitization before liver transplantation. Patients with an infectious disease or those with insufficient time to receive rituximab because of the deceased donor liver transplant schedule underwent liver transplantation without rituximab desensitization. The decision to administer rituximab was determined after considering the patient’s clinical condition prior to liver transplantation because this was not a randomized controlled study.
For the postoperative immunosuppression protocol, tacrolimus was started from postoperative day 1, and the target trough level of tacrolimus was 8.0–12.0 ng/ml for the first 2 weeks. Thereafter, it was tapered and adjusted to a target trough level of 5.0–8.0 ng/ml. Mycophenolate mofetil was started at 500–1000 mg/day within 5 days of the transplant in most patients and adjusted according to adverse effects as necessary. Intravenous methylprednisolone 10 mg/body weight (kg) was administered intraoperatively before portal perfusion. The dose of methylprednisolone was tapered (1 mg/kg, days 1–3; 0.5 mg/kg, days 4–6; 0.3 mg/kg, day 7). Oral prednisolone was tapered from 0.3 mg/kg until 3–6 months post liver transplantation. We did not perform splenectomy unless a patient needed graft inflow modulation or a splenic artery aneurysm was confirmed.
DSA DETECTION:
Screening anti-HLA tests were performed with LABScreen PRA beads (One Lambda, Inc., CA, USA) at the initial assessment. When pDSA was detected, LABScreen Single beads were used to determine their specificity (One Lambda, Inc.). The results of pDSA levels normalized to background values are reported as the mean fluorescence index (MFI). The positive cutoff value was determined as MFI >1000 for a single DSA. MFI >10 000 was considered strongly positive. In cases where more than 1 pDSA was positive, the cumulative MFI value was used for the total pDSA.
POST-TRANSPLANTATION FOLLOW-UP FOR PDSA:
To evaluate the change in pDSA after liver transplantation, we used LABScreen PRA beads at 2 weeks, 1, 3, 6, and 12 months, and every year after liver transplantation. If pDSA were detected, we added LABScreen Single beads and identified pDSA alleles. In addition, we evaluated pathology findings by protocol and episodic biopsy at the same time as checking for pDSA.
COMPLEMENT COMPONENT 4D (C4D) STAINING:
We performed C4d staining of tissues obtained from a liver biopsy after liver transplantation for patients with pDSA from October 2014 to December 2018.
STATISTICAL ANALYSIS:
Categorial variables were compared using Fisher’s exact test or χ2 test. The normality of distribution was evaluated by the Shapiro-Wilk test. If the data did not show a normal distribution, the Mann-Whitney U test or Kruskal-Wallis test were used. For a normal distribution, the homogeneity of variance was evaluated by Levene’s test. If the data showed homogeneity of variance, the t test or analysis of variance was used. An estimate of overall survival was evaluated by the Kaplan–Meier method. P values <0.05 were considered statistically significant. All statistical analyses were performed using EZR (Easy R; Division of Hematology, Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [16].
ETHICS APPROVAL:
This study followed the ethics guidelines of the Declaration of Helsinki and was approved by the institutional review board of Nagoya University Hospital (No. 2021–0381). All patients provided written informed consent for the administration of rituximab for desensitization and the use of their clinical data for retrospective analysis in the future.
Results
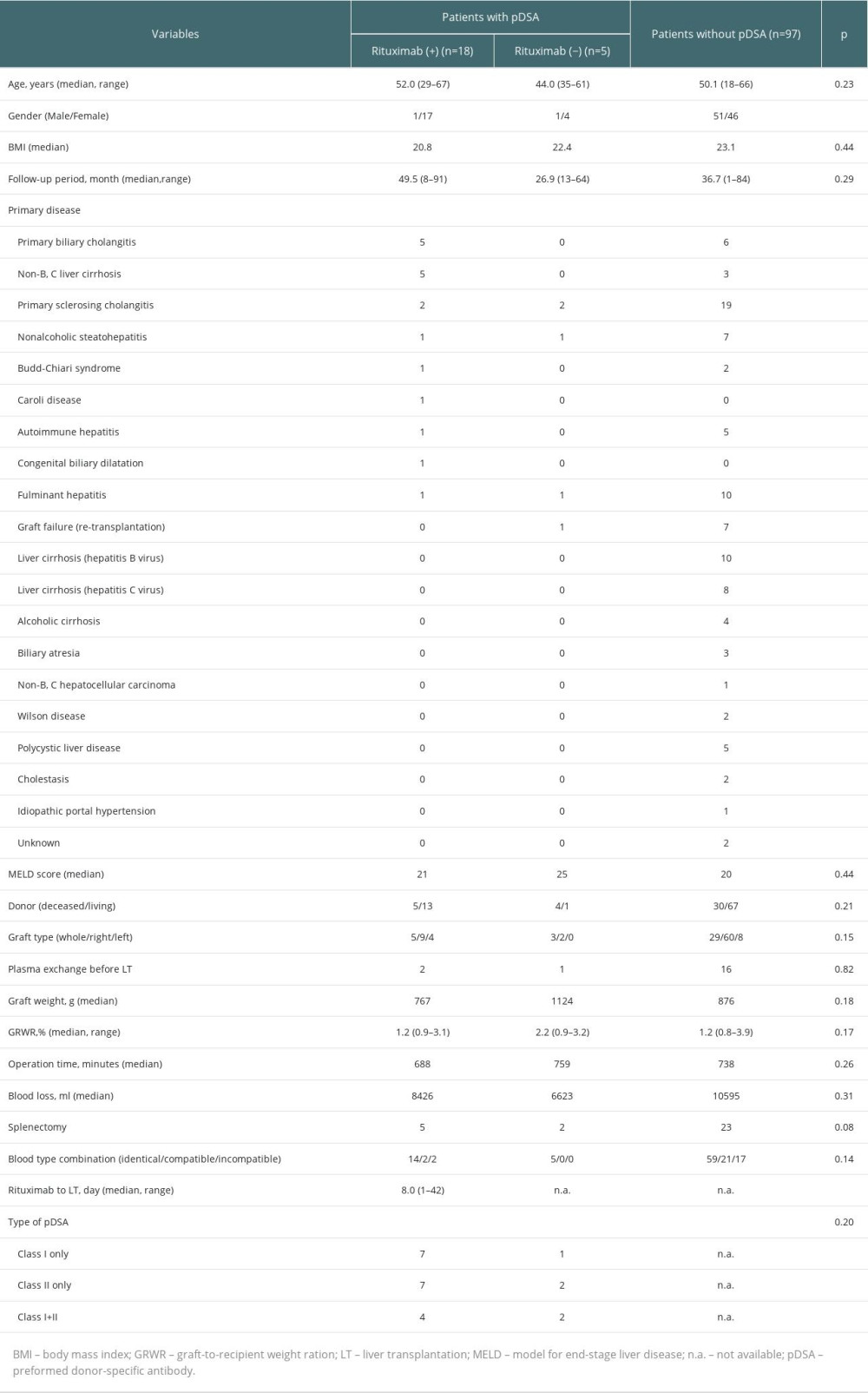
PATIENT CHARACTERISTICS:
Of 120 patients, 97 (80.8%) were negative for pDSA and 23 (19.2%) were positive for pDSA before liver transplantation (Table 1). Of these patients, 18 received rituximab and 5 did not receive rituximab (4 patients did not have sufficient time to receive rituximab before the transplant and 1 patient had a potential infection related to spontaneous bacterial peritonitis). There were no significant differences between the characteristics of patients with or without pDSA.
CHANGES IN THE MFI LEVEL OF PDSA BEFORE AND AFTER LIVER TRANSPLANTATION:
In the rituximab (+) group (patients with pDSA class I only, n=7; pDSA class II only, n=7; pDSA class I+II, n=4), the MFI level of pDSA class I in all patients (11/11 patients, 100%) was decreased 1 month after liver transplantation compared with preoperative MFI levels, and the levels in 9 patients decreased substantially to a negative MFI (<1000), whereas the MFI level of pDSA class II was decreased in 9/11 patients (81.8%) compared with preoperative MFI levels (Figure 2A, 2B). In the rituximab (−) group (patients with pDSA class I only, n=1; pDSA class II only, n=2; pDSA class I+II, n=2), the MFI level of pDSA class I in all patients was decreased at 1 month after liver transplantation, whereas the MFI level of pDSA class II was decreased in 2/4 patients (50.0%) (Figure 2C, 2D). Changes in the MFI level of pDSA class II in both groups decreased slowly compared with pDSA class I. The changes in pDSA were similar in both groups regardless of treatment with or without rituximab.
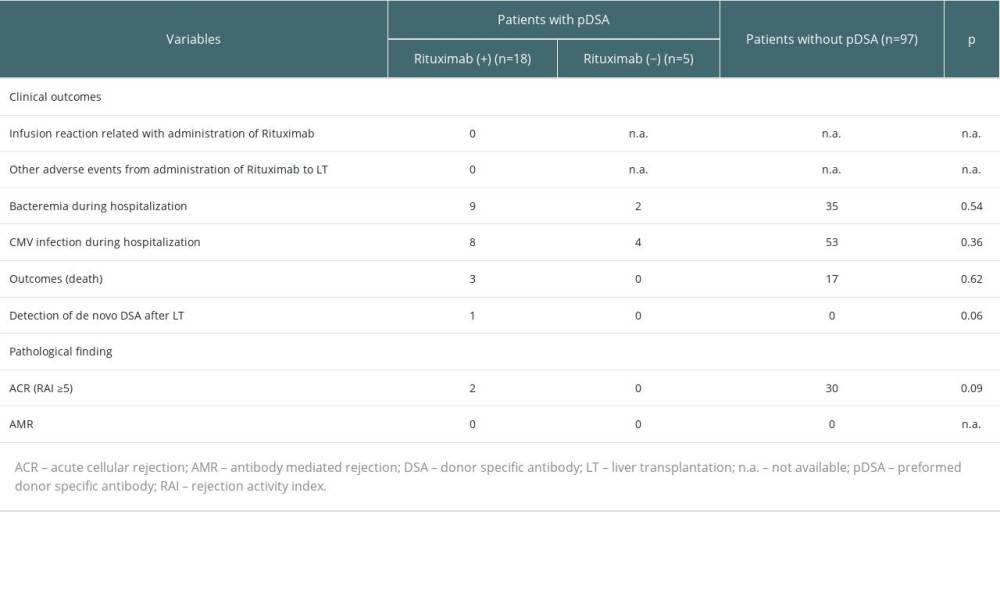
CLINICAL OUTCOMES OF LIVER TRANSPLANT PATIENTS WITH OR WITHOUT PDSA:
No immediate adverse events related to the administration of rituximab were observed in the rituximab (+) group between drug administration and liver transplantation (Table 2). Cytomegalovirus (CMV) was detected in 8 patients in the rituximab (+) group, 4 in the rituximab (−) group after liver transplantation, and in 53 patients without pDSA. The incidence of CMV infection was not significantly different among these groups. Additionally, de novo DSA was detected in one patient in the rituximab (+) group 10 months after liver transplantation, but the patient had no complications nor clinical and pathology findings after the appearance of de novo DSA. In this study, there were no significant differences in patient survival between the rituximab (+) and rituximab (−) groups (Figure 3), although the groups had a small sample size, especially the rituximab (−) group. Moreover, there were no significant differences in the overall survival of patients with or without pDSA (Figure 3).
There were 3 deaths in the rituximab (+) group. One patient underwent living donor liver transplantation because of nonalcoholic steatohepatitis (Model of End-stage Liver Disease (MELD) score 29; Child Pugh score 12, Graft-to-Recipient Weight Ratio (GRWR) 1.51). Sequential biliary complications appeared and liver function recovery was delayed because of an infection. Although pDSA and other de novo DSA were not detected postoperatively, this patient died 10 months after liver transplantation. Another patient died because of traumatic intracranial hemorrhage more than 4 years after liver transplantation, and pDSA and anti-HLA antibodies detected before liver transplantation were persistently positive after transplantation; however, their impact on the graft was not observed. We performed protocol biopsies 3 times and all results were normal. The third patient, whose underlying disease was primary sclerosing cholangitis, underwent living donor liver transplantation (MELD score 20; Child Pugh score 10; GRWR 0.98). This patient also had biliary complications with deteriorating graft function. pDSA and other de novo DSA were not detected after surgery. Despite multidisciplinary therapy, the clinical course did not improve. Furthermore, this patient experienced gastrointestinal bleeding related to CMV duodenitis. As a result, the patient died of multi-organ failure 4 months after liver transplantation. The causes of death in these 3 cases were not directly related to pDSA or desensitization, but there might have been an indirect relationship to the strong immunosuppression induced by desensitization.
PATHOLOGY OUTCOMES IN LIVER TRANSPLANT PATIENTS WITH PDSA:
Regarding the pathology findings, acute cellular rejection (≥moderate) or AMR were not significantly different between the 3 groups (Table 2).
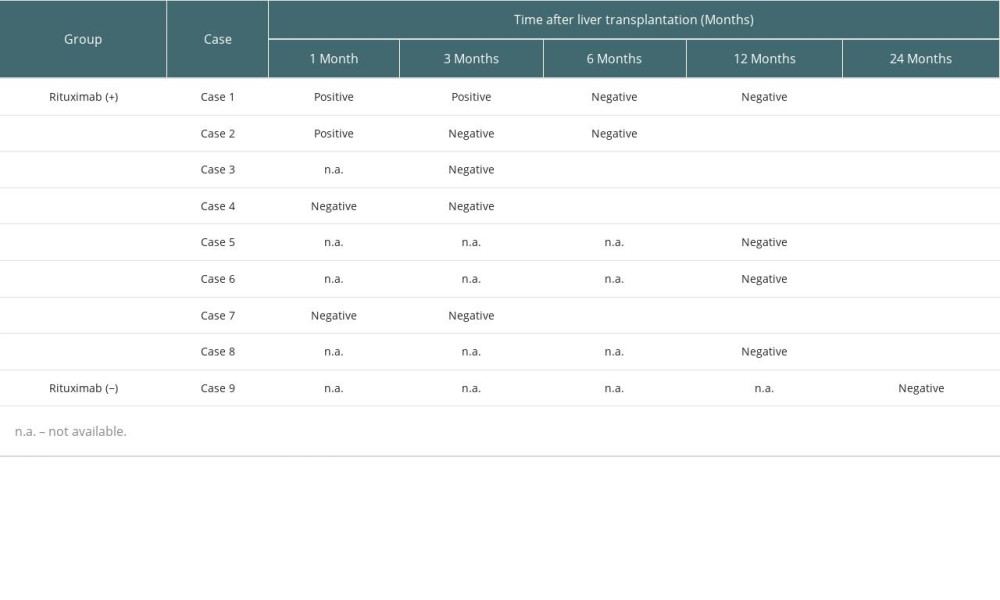
C4D STAINING OF LIVERS FROM PATIENTS WITH PDSA:
We performed C4d staining for 9 patients with pDSA who underwent liver transplantation from October 2014 to December 2018 (8 cases, 16 samples in the rituximab (+) group; 1 case, 1 sample in the rituximab (−) group). Although a liver biopsy could not be performed for some patients and the timing of C4d staining was not completely consistent, we found that 2 patients had positive C4d staining 1 month after liver transplantation, and almost all cases were negative for C4d staining at 6 or 12 months after transplantation (Table 3). No AMR findings were observed in any samples.
Discussion
To avoid unfavorable outcomes, plasma exchange or the use of rituximab for desensitization have become common prophylactic strategies for solid organ transplant patients with DSA. However, the desensitization strategy for liver transplant patients with pDSA is still controversial because bilateral studies reported that pDSA led to poor clinical outcomes but no negative outcomes. The present study showed that the administration of rituximab as desensitization for liver transplant patients with pDSA led to favorable outcomes, whereas patients who did not receive rituximab desensitization experienced no negative impact on their clinical and pathology outcomes during the study period. Therefore, although a very small number of patients were enrolled, the present study suggests rituximab might not be essential for desensitization in liver transplantation recipients.
Whereas the MFI of pDSA class I decreased immediately, the decrease in the MFI of pDSA class II was relatively slow (Figure 2). This tendency toward a slow decrease in the MFI was similar between the rituximab (+) and rituximab (−) groups, but different from those undergoing kidney transplantation [17,18]. This tendency was similar to results in a previous study [19]. These findings showed that the manner in which MFI declines might differ between class I and class II, although its clinical significance is unknown. We assume that these results may be related to the absorption of pDSA by the liver graft, the binding of pDSA with secreted soluble HLA from the liver graft, or the regeneration of hepatocytes [20], rather than the direct effects of rituximab.
A number of studies have reported that rituximab controlled unfavorable outcomes during liver transplantation related to pDSA, including rejection [4,5,15], graft dysfunction [15,21], and patient survival [1,19,22,23]. Of note, rituximab increased the risk of infectious complications [24,25]. In this study, cases of CMV and bacteremia were not significantly different among the 3 groups. Therefore, although a very small number of cases were enrolled in this study, our results suggest the administration of rituximab is acceptable in terms of risk of perioperative infections. Moreover, no immediate adverse events were observed and no significant differences in pathology findings, clinical outcomes, or incidence of de novo DSA were observed (Table 2). In addition, the overall survival after liver transplantation among the 3 groups was not significantly different (Figure 3). Therefore, we could not demonstrate a clinical or pathological advantage of the administration of rituximab. Although there were no significant differences clinically, 3 patients in the rituximab (+) group died in this study (1 died because of traumatic intracranial hemorrhage that was unrelated to rituximab). Another patient who died was progressing favorably until a biliary leakage complication occurred 2 months after the liver transplantation. Despite appropriate bile drainage, the bile leakage did not improve, an intra-abdominal abscess developed, and the graft function decreased gradually. pDSA was not detected during hospitalization. The postoperative course in the other patient was not favorable. The graft failed and biliary leakage complications occurred 2 weeks after the liver transplantation. The patient sequentially developed severe infections, including bacterial, mycolic, and CMV, with impaired graft functioning. The patient ultimately developed multi-organ failure and died. A direct association between the causes of death and administration of rituximab is unclear; however, we assumed that rituximab may have contributed to the deaths, especially in the latter patient.
In this study, we performed C4d staining in samples from some patients (Table 3). Interestingly, some samples from the rituximab (+) group had positive C4d staining 1 or 3 months after liver transplantation, although there were no findings of rejection. Subsequently, the C4d staining became negative 3 or 6 months after liver transplantation. In addition, there were no findings of AMR in any cases. We discontinued performing C4d staining because there were no significant changes in AMR even though C4d staining was positive. It is unclear what the clinical implications of this finding are; however, further investigation is needed to interpret this result.
This study had some limitations. First, it was not a randomized controlled trial and we could not exclude selection bias. Second, there was a relatively small number of patients, especially in the rituximab (−) group. Therefore, we could not demonstrate significant implications related to rituximab and caution is needed when interpreting our results. To validate the impact of rituximab on liver transplant patients with pDSA more accurately, a large-scale study is needed.
Conclusions
In conclusion, we demonstrated that rituximab desensitization for liver transplant patients with pDSA was managed successfully; however, we could not validate the administration of rituximab for liver transplant patients with pDSA. Although the number of patients with pDSA was very small and caution is needed when interpreting our results, rituximab desensitization for liver transplant patients with pDSA might be unnecessary. Further studies are necessary to demonstrate whether rituximab desensitization for liver transplantation recipients with pDSA is an absolute requirement to avoid AMR and achieve better outcomes.
Figures
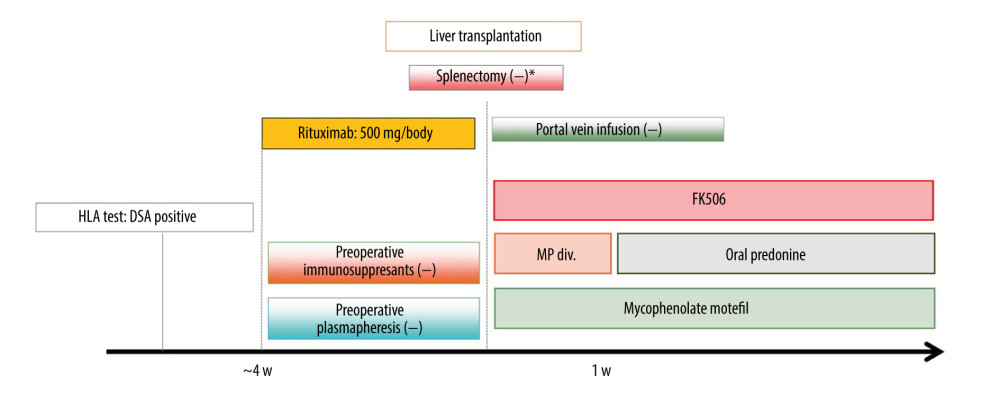 Figure 1. Desensitization protocol for liver transplant recipients with pDSA. FK506 – tacrolimus; HLA – human leukocyte antigen; MP – methylprednisolone; pDSA – preformed donor-specific antibody. * Splenectomy was performed when portal hypertension was present.
Figure 1. Desensitization protocol for liver transplant recipients with pDSA. FK506 – tacrolimus; HLA – human leukocyte antigen; MP – methylprednisolone; pDSA – preformed donor-specific antibody. * Splenectomy was performed when portal hypertension was present. 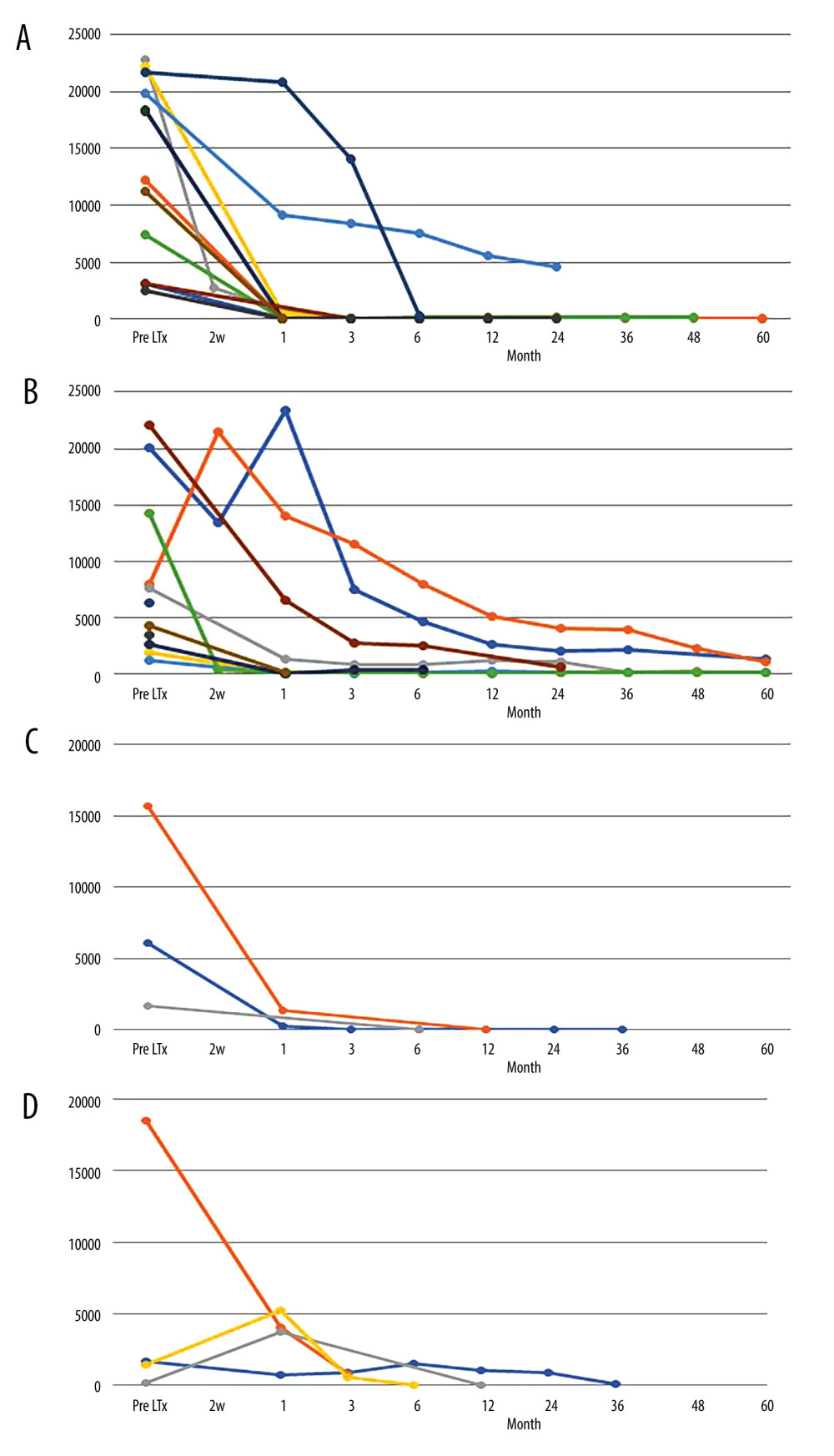 Figure 2. MFI changes of pDSA after liver transplantation. (A) MFI changes in pDSA class I in the rituximab (+) group. (B) MFI changes in pDSA class II in the rituximab (+) group. (C) MFI changes in pDSA class I in the rituximab (−) group. (D) MFI changes in pDSA class II in rituximab (−) group. MFI – mean fluorescence index; pDSA – preformed donor-specific antibody.
Figure 2. MFI changes of pDSA after liver transplantation. (A) MFI changes in pDSA class I in the rituximab (+) group. (B) MFI changes in pDSA class II in the rituximab (+) group. (C) MFI changes in pDSA class I in the rituximab (−) group. (D) MFI changes in pDSA class II in rituximab (−) group. MFI – mean fluorescence index; pDSA – preformed donor-specific antibody. 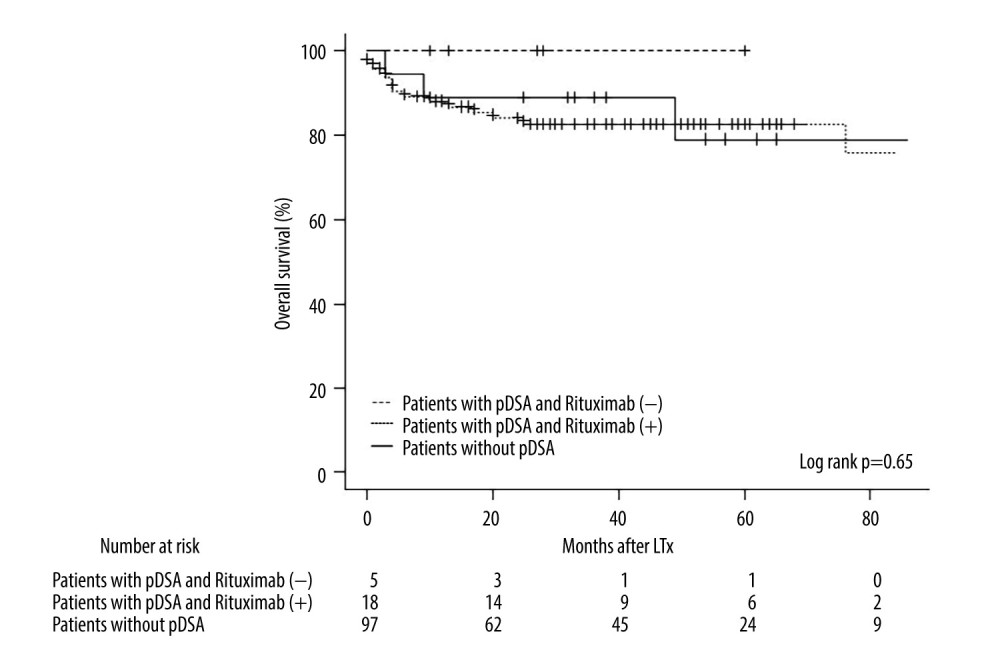 Figure 3. Kaplan-Meier curve for the overall survival of patients in the rituximab (+) and rituximab (−) groups and patients without pDSA. LTx – liver transplantation; pDSA – preformed donor-specific antibody.
Figure 3. Kaplan-Meier curve for the overall survival of patients in the rituximab (+) and rituximab (−) groups and patients without pDSA. LTx – liver transplantation; pDSA – preformed donor-specific antibody. References
1. Tamura K, Tohyama T, Watanabe J, Preformed donor-specific antibodies are associated with 90-day mortality in living-donor liver transplantation: Hepatol Res, 2019; 49; 929-41
2. O’Leary JG, Kaneku H, Jennings LW, Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation: Liver Transpl, 2013; 19; 973-80
3. O’Leary JG, Kaneku H, Susskind BM, High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant: Am J Transplant, 2011; 11; 1868-76
4. O’Leary JG, Klintmalm GB, Impact of donor-specific antibodies on results of liver transplantation: Curr Opin Organ Transpl, 2013; 18; 279-84
5. Del Bello A, Neau-Cransac M, Lavayssiere L, Outcome of liver transplant patients with preformed donor-specific anti-human leukocyte antigen antibodies: Liver Transpl, 2020; 26; 256-67
6. Cuadrado A, San Segundo D, Lopez-Hoyos M, Clinical significance of donor-specific human leukocyte antigen antibodies in liver transplantation: World J Gastroenterol, 2015; 21; 11016-26
7. Kaneku H, O’Leary JG, Taniguchi M, Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with Chronic rejection and graft loss after liver transplantation: Liver Transpl, 2012; 18; 984-92
8. Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts: Liver Transpl, 2012; 18; 1333-42
9. McCaughan JA, Robertson V, Falconer SJ, Preformed donor-specific HLA antibodies are associated with increased risk of early mortality after liver transplantation: Clin Transplant, 2016; 30; 1538-44
10. Montgomery RA, Loupy A, Segev DL, Antibody-mediated rejection: New approaches in prevention and management: Am J Transplant, 2018; 18; 3-17
11. Kim H, Yi NJ, Song EY, Preformed donor-specific antibodies do not affect the 1-year allograft survival in living donor liver transplantation: Clin Transplant, 2018; 32; 10
12. Taner T, Gandhi MJ, Sanderson SO, Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year: Am J Transplant, 2012; 12; 1504-10
13. Loupy A, Suberbielle-Boissel C, Zuber J, Combined posttransplant prophylactic IVIg/anti-CD 20/plasmapheresis in kidney recipients with preformed donor-specific antibodies: A pilot study: Transplantation, 2010; 89; 1403-10
14. Parajuli S, Joachim E, Alagusundaramoorthy S, Subclinical antibody-mediated rejection after kidney transplantation: treatment outcomes: Transplantation, 2019; 103; 1722-29
15. Kubal CA, Mangus RS, Saxena R, Crossmatch-positive liver transplantation in patients receiving thymoglobulin-rituximab induction: Transplantation, 2014; 97; 56-63
16. Kanda Y, Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics: Bone Marrow Transpl, 2013; 48(3); 452-58
17. Sicard A, Amrouche L, Suberbielle C, Outcome of kidney transplantations performed with preformed donor-specific antibodies of unknown etiology: Am J Transplant, 2014; 14; 193-201
18. Itabashi Y, Aikawa A, Muramatsu M, Living-donor kidney transplant with preformed donor-specific antibodies: Exp Clin Transplant, 2019; 17; 43-49
19. Akamatsu N, Hasegawa K, Sakamoto S, Rituximab desensitization in liver transplant recipients with preformed donor-specific HLA antibodies: A Japanese nationwide survey: Transplant Direct, 2021; 7; e729
20. Taner T, Stegall MD, Heimbach JK, Antibody-mediated rejection in liver transplantation: Current controversies and future directions: Liver Transpl, 2014; 20; 514-27
21. Levitsky J, Kaneku H, Jie C, Walsh RC, Donor-specific HLA antibodies in living versus deceased donor liver transplant recipients: Am J Transplant, 2016; 16; 2437-44
22. Yoshizawa A, Egawa H, Yurugi K, Significance of semiquantitative assessment of preformed donor-specific antibody using luminex single bead assay in living related liver transplantation: Clin Dev Immunol, 2013; 2013; 972705
23. Sakamoto S, Akamatsu N, Hasegawa K, The efficacy of rituximab treatment for antibody-mediated rejection in liver transplantation: A retrospective Japanese nationwide study: Hepatol Res, 2021; 51; 990-99
24. Hayashi H, Takamura H, Tajima H, Infectious complications in adult ABO-incompatible liver transplantation: Our preliminary experience: International Surgery, 2019; 104; 176-81
25. Habicht A, Broker V, Blume C, Increase of infectious complications in ABO-incompatible kidney transplant recipients-a single centre experience: Nephrol Dial Transplant, 2011; 26; 4124-31
Figures
 Figure 1. Desensitization protocol for liver transplant recipients with pDSA. FK506 – tacrolimus; HLA – human leukocyte antigen; MP – methylprednisolone; pDSA – preformed donor-specific antibody. * Splenectomy was performed when portal hypertension was present.
Figure 1. Desensitization protocol for liver transplant recipients with pDSA. FK506 – tacrolimus; HLA – human leukocyte antigen; MP – methylprednisolone; pDSA – preformed donor-specific antibody. * Splenectomy was performed when portal hypertension was present. Figure 2. MFI changes of pDSA after liver transplantation. (A) MFI changes in pDSA class I in the rituximab (+) group. (B) MFI changes in pDSA class II in the rituximab (+) group. (C) MFI changes in pDSA class I in the rituximab (−) group. (D) MFI changes in pDSA class II in rituximab (−) group. MFI – mean fluorescence index; pDSA – preformed donor-specific antibody.
Figure 2. MFI changes of pDSA after liver transplantation. (A) MFI changes in pDSA class I in the rituximab (+) group. (B) MFI changes in pDSA class II in the rituximab (+) group. (C) MFI changes in pDSA class I in the rituximab (−) group. (D) MFI changes in pDSA class II in rituximab (−) group. MFI – mean fluorescence index; pDSA – preformed donor-specific antibody. Figure 3. Kaplan-Meier curve for the overall survival of patients in the rituximab (+) and rituximab (−) groups and patients without pDSA. LTx – liver transplantation; pDSA – preformed donor-specific antibody.
Figure 3. Kaplan-Meier curve for the overall survival of patients in the rituximab (+) and rituximab (−) groups and patients without pDSA. LTx – liver transplantation; pDSA – preformed donor-specific antibody. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860