26 December 2023: Original Paper
Effects of Preservation of Donor Liver Gastroduodenal Artery on Post-Transplant Biliary Complications in 187 Liver Transplant Recipients: A Retrospective Study
Qing Yan1BCEF, Ying Liu1BEF, Fei-Wen Deng1BE, Feng-jie Wang1BE, Huan-Wei Chen1AG*DOI: 10.12659/AOT.941699
Ann Transplant 2023; 28:e941699
Abstract
BACKGROUND: This retrospective study aimed to evaluate the effects of preservation of the donor liver gastroduodenal artery on post-transplant biliary complications in 187 liver transplant recipients.
MATERIAL AND METHODS: The clinical data of 187 liver transplantation recipients were retrospectively analyzed. Recipients were divided into conventional and modified groups. The technical point of the modified group is to preserve at least 2 cm of the distal gastroduodenal artery, and pay special attention to preserve the superior pancreaticoduodenal artery to ensure the distal blood supply to the common bile duct.
RESULTS: The modified group had significantly shorter operative time (7.17 vs 7.98) h (P<0.001) and less intraoperative blood loss (2715.40 vs 3434.93) ml (P=0.003) than the conventional group. The incidence of postoperative biliary complications (including anastomotic biliary leakage, ischemic bile duct stenosis, and anastomotic bile duct stenosis) in the modified group (4/114, 4.1%) was significantly lower (15/73, 20.5%) (P<0.001). There was no significant difference in the intraoperative cold and warm ischemia time and postoperative hospital stay length between the 2 groups. In addition, there was no significant difference in the effect of cardiac-death and brain-death sources on perioperative biliary complications, while the peak postoperative transaminase and total bilirubin were higher in patients receiving the donor liver of cardiac death (P<0.05).
CONCLUSIONS: Preserving the integrity of the donor gastroduodenal artery and surrounding tissue is beneficial to protect the blood supply of the extrahepatic bile duct, and can reduce the incidence of biliary complications.
Keywords: Liver Transplantation, Postoperative Complications
Background
Liver transplantation is the most effective means to cure hepatocellular carcinoma and various end-stage liver diseases [1]. Since the advent of liver transplantation, with decades of technological development, the safety and effectiveness of liver transplantation have improved significantly [2]. However, due to the high risk of the operation, unstable donor liver quality, complicated perioperative management, postoperative rejection, biliary anastomosis technology, and other reasons, the incidence of postoperative complications is still high, which strongly affects patient prognosis [3,4].
Biliary complications are one of the most common complications after liver transplantation, with incidence rates of 10.0% to 40.0% [3,5] and mortality rates of 6% to 12% [6]. Reducing the incidence of biliary complications has been an important focus of liver transplantation research. Previous studies reported that the main factors influencing biliary complications after liver transplantation are bile duct anastomosis technique, biliary blood supply, and rejection reaction [6]. The types of biliary complications include anastomotic biliary leakage, ischemic bile duct stenosis, and anastomotic bile duct stenosis [3]. With the development of bile duct anastomosis technique, the incidence of anastomotic biliary leakage and anastomotic bile duct stenosis have been decreased, but and the incidence of ischemic bile duct stricture remains high [7]. In view of the high incidence of previous biliary complications, we have improved the pruning method of the donor liver, focusing on protecting the blood supply of the extrahepatic bile ducts, especially at the distal end of the common bile duct, to reduce the occurrence of biliary complications after liver transplantation. The blood supply of the hilar bile duct mainly originates from the branches of the right hepatic artery and gallbladder artery, while the blood supply of the distal extrahepatic bile duct is mainly from the gastroduodenal artery and the branches of the posterior superior pancreaticoduodenal artery [8]. Through preserving the donor liver gastroduodenal artery and its branches, we could better ensure the blood supply to the lower part of the bile duct and reduce the occurrence of ischemic bile duct stenosis. The present study compared 2 methods of liver repair to explore the effect of preserving the donor liver gastroduodenal artery in reducing biliary complications. Therefore, this retrospective study aimed to evaluate the effects of preservation of the donor liver gastroduodenal artery on post-transplant biliary complications in 187 liver transplant recipients.
Material and Methods
CLINICAL DATA:
The clinical data of 187 patients who underwent orthotopic liver transplantation in our hospital from September 2011 to May 2022 were retrospectively analyzed. All patients signed the informed consent to participate in this project, which was approved by the Ethics Committee of our hospital. There were 165 males and 22 females, aged 31 to 71 years old, and the median age was 51 years old. The recipients were divided into a conventional group (73 cases) and a modified group (114 cases).
THE INCLUSION AND EXCLUSION CRITERIA FOR THIS STUDY WERE:
Inclusion criteria: 1) 18 to 75 years old; 2) Patients undergoing in situ liver transplantation due to liver malignancy, post-hepatitis cirrhosis, alcoholic cirrhosis, cholestatic cirrhosis, liver cirrhosis, or autoimmune cirrhosis; 3) The donor met the Chinese organ donation criteria, with brain death or heart death.
Exclusion criteria: 1) Perioperative death; 2) Undergoing internal drainage of a cholangioenterostomy.
CONVENTIONAL GROUP DONOR LIVER ACQUISITION AND PRUNING:
The acquisition of the donor liver used the traditional method of rapid liver and kidney combination acquisition, and the perfusion solution was Celsior fluid. During the acquisition process, the biliary tract was immediately fully flushed from the distal end of the common bile duct. The hepatic artery pruning was dissected starting from the abdominal aorta, noting the variant right hepatic artery derived from the superior mesenteric artery. If the variant right hepatic artery is thicker, it was anastomosed to the broken end of the splenic artery, and if the diameter was fine, it was anastomosed with the broken end of the gastroduodenal artery. The artery was trimmed to the bifurcation of the native hepatic artery and the gastroduodenal artery, and the blood vessel was ligated at the root of the gastroduodenal artery. Pruning the portal vein started at the level of the superior mesenteric venous perfusion, and the portal vein was retained as long as possible. The common bile duct was trimmed to the level of the upper duodenum, and excessive separation of the surrounding tissues was avoided to not affect the blood supply of the bile duct.
MODIFIED GROUP DONOR LIVER ACQUISITION AND PRUNING: This was based on the anatomical study of the extrahepatic bile duct feeding artery [9]. The source of blood supply to the extrahepatic bile duct is shown in the pattern diagram (Figure 1) and anatomical picture below (Figures 2, 3). The main changes in donor liver pruning emphasize the preservation of the gastroduodenal artery of at least 2 cm and ligation of the anterior branch of the superior pancreaticoduodenal artery entering the pancreas when the common bile duct finishes to the pancreatic segment. The posterior branch of the superior pancreaticoduodenal artery was preserved and the residual pancreatic tissue was completely removed. After the length of the required bile duct was determined during the operation, the common bile duct was cut sideways, and the integrity of the tissue between the distal end of the common bile duct and the stump of the gastroduodenal artery was preserved. This method protects the blood supply at the distal end of the common bile duct.
COMMON BILE DUCT ANASTOMOSIS IN THE CONVENTIONAL GROUP:
Common bile duct anastomosis was performed by end-to-end anastomosis, and the anterior and posterior walls were continuously sutured using 6-0 or 7-0 PDS-II suture. The bile duct of the recipient was left as long as possible, and the common bile duct of the donor liver was kept as short as possible to avoid having a bile duct that is too long and twisted or too short. If the diameter of the bile duct was too large, part of the too large bile duct was closed to ensure the consistent diameter of the bile duct of the donor–recipient.
COMMON BILE DUCT ANASTOMOSIS IN THE MODIFIED GROUP:
The common bile duct anastomosis was performed by end-to-end anastomosis, and the anterior and posterior walls were continuously sutured using 6-0 or 7-0 PDS-II suture. During anastomosis, attention was paid to keeping the donor bile duct as long as possible, and the bile duct of the recipient was kept as short as possible to avoid having a common bile duct that is too long and twisted or too short and has tension.
IMMUNOSUPPRESSIVE THERAPY:
In anhepatic phase, 250 mg methylprednisolone was administered intravenously. After reperfusion, 250 mg methylprednisolone was administered, and 20 mg basiliximab was given intravenously for immune induction before abdominal closure. On the fourth postoperative day, 20 mg basiliximab was given intravenously. Oral tacrolimus (1 mg/12 h) and mycophenolate sodium (360 mg/12 h) were started on postoperative day 1. After that, the concentration of tacrolimus was detected every Monday and Thursday and the early drug concentration was maintained at 5 to 10 ng/mL and adjusted according to the liver function examination results. After discharge, the patient was followed up monthly in the outpatient clinic. Blood drug concentration and liver function tests were performed, and the drug concentration was adjusted according to the examination results.
STATISTICAL ANALYSIS:
Data analysis was performed using SPSS 25.0 statistical software. The data of each group were tested for normality (Kolmogorov-Smirnov method). Measurement data with normal distribution are expressed as χ̄±s, and the t test was used for comparison of 2 groups. Non-normally distributed measurement data are expressed as M (Q1, Q3), and a non-parametric test (Mann-Whitney U test) was used. Count data are expressed as number of cases, and the rates of the 2 groups were compared using the chi-square test or Fisher exact probability method. P<0.05 was considered as a statistically significant difference. The intraoperative picture of bile duct anastomosis is shown in Figures 4 and 5.
Results
PATIENT BASIC CHARACTERISTICS:
The liver transplantation program was started in 2005, and the modified graft harvesting and transplantation methods were started in 2017. A total of 187 liver transplant patients were included from September 2011 to May 2022, and the basic characteristics of the 2 groups are shown in Table 1. Statistical analysis found no significant difference in the baseline data of the 2 groups (P>0.05).
POSTOPERATIVE COMPLICATIONS:
Statistical analysis found no significant differences in cold ischemia time, warm ischemia time, anhepatic phase, second warm ischemia time, or postoperative length of hospital stay between the 2 groups. The operation time and bleeding volume in the modified group were significantly less than those in the conventional group (P<0.05, Table 2). None of the patients had vascular-related complications such as arterial embolism or stenosis, which may be due to continuous improvement of the surgeon’s technique.
EFFECT OF DONOR SOURCE ON BILIARY COMPLICATIONS:
The occurrence of biliary complications in the 2 groups is shown in Table 3. In the conventional group, there were 15 postoperative biliary complications, and the incidence rate was 20.5% (15/73), and the average occurrence time was 18.5 days after surgery. Among them, 2 cases had anastomotic bile duct stenosis secondary to anastomotic biliary leakage, 1 case had hilar biliary ischemic biliary stenosis, 2 cases had simple anastomotic biliary stenosis, and 10 cases had ischemic non-anastomotic bile duct stenosis. In the modified group, there were 4 postoperative biliary complications, with an incidence of 5.3% (6/114), and the average occurrence time was 17 days after surgery. All patients had anastomotic stenosis, and none had ischemic non-anastomotic stenosis. There was no significant difference in the postoperative time of biliary complications between the 2 groups (P=0.450), but there was a significant difference between groups in incidence rates (χ2=10.429, P=0.001). The analysis of factors affecting biliary complications is shown in Table 4. The chi-square test showed that second warm ischemia time and liver pruning method were factors affecting biliary complications (P<0.05). We further performed the stratified chi-square test according the second warm ischemia time, and the results showed that the liver repair method was still associated with the occurrence of biliary tract complications (P<0.05) (Table 5).
Statistical analysis showed that donor source of cardiac death and brain death had no obvious effect on the incidence of postoperative biliary complications in patients. However, the peak postoperative transaminase and total bilirubin levels were significantly higher in patients receiving transplants from cardiac-death donors (P<0.05) (Table 6).
Discussion
We found that preserving the integrity of the donor gastroduodenal artery and surrounding tissue improved the incidence of postoperative biliary complications, and the incidence of ischemic bile duct stenosis was significantly decreased. It was previously reported that the blood supply of the distal extrahepatic bile duct is mainly derived from the gastroduodenal artery and the branches of the posterior superior pancreaticoduodenal artery [8,9]. In addition, studies have reported that the occurrence of biliary fistula and stenosis after postoperative biliary anastomosis has gradually decreased with technological progress, but the incidence of non-anastomosis complications is still high [7]. This is consistent with the conclusion of our study, which we believe is mainly due to biliary ischemia, and progress has been made in reducing postoperative biliary complications by improving donor liver pruning.
We found no significant difference in the effect of cardiac-death and brain-death sources on perioperative biliary complications, while the peak postoperative transaminase and total bilirubin were increased in patients receiving a liver from a cardiac-death donor. David et al reported that there was no significant difference in postoperative complications and length of stay[10]. However, there were also reports that livers from cardiac-death donors are associated with longer ischemia times [11], which appears to be the reason for the higher peak postoperative transaminase and total bilirubin levels.
Liver transplantation is the only effective method to cure end-stage liver diseases. With the advancement of surgical techniques, the overall complication rate of liver transplantation surgery has decreased significantly, especially postoperative infections and vascular complications. However, the incidence of postoperative biliary complications remains high [3]. Nemes et al [12] analyzed 45 related articles between 2008 and 2014, with a total of 14 411 cases – the biliary complication rate of liver transplantation was 23%, and the incidence of biliary complications in China was reported to range from 7.2% to 17.6% [13]. Biliary complications after liver transplantation usually show biliary stenosis and biliary leakage, with the incidence of anastomotic leakage ranging from 2% to 25% [14,15] and anastomotic stenosis ranging from 5% to 19% [5,16]. With the improvement of anastomotic technique and the use of absorbable surgical suture, the incidence of anastomotic leakage and anastomotic stenosis has decreased significantly, so the postoperative biliary complications are more manifested often as non-anastomotic stenosis, also known as ischemic biliary stenosis, which has incidence rates ranging from 3% to 16% [17,18]. In this study, except for 4 cases that were anastomotic stenosis or biliary fistula, all cases were extrahepatic biliary ischemic stenosis (11 cases), accounting for 73% (11/15) of all biliary complications in this group. Therefore, ensuring the blood supply of the extrahepatic bile ducts, especially that of the distal bile duct, is crucial to reduce the incidence of biliary complications. To achieve this, we preserved the patient’s gastroduodenal artery and surrounding tissue while pruning the liver and better preserving the blood supply of the distal extrahepatic bile duct. By comparing the occurrence of biliary complications in patients with 2 different methods of liver pruning, we found that this modified method can decrease the occurrence of biliary complications.
There are many factors affecting the occurrence of biliary complications after liver transplantation. In addition to the risk factors of the donor itself, such as advanced age, fatty liver, diabetes [19–22], and other influencing factors such as cold and warm ischemia time and ischemia and reperfusion injury [23–26], we believe that the reconstruction techniques of the biliary tract and the blood supply of the bile duct are the more important influencing factors. The application of microsurgical techniques in biliary anastomosis significantly reduces the incidence of anastomotic leakage and anastomotic stenosis. We usually continuously suture the anterior and posterior walls with a 6-0 or 7-0 PDS-II suture at a 2.5 × microscopic magnification. In terms of bile duct blood supply protection, some studies have found that the vascular network around the intrahepatic and hilar bile duct mainly originates from the branches of the right hepatic artery and gallbladder artery [9]. The bile duct vessel network below the common hepatic duct is more derived from the gastroduodenal artery and the branches of the posterior superior pancreaticoduodenal artery. Sixty percent of the blood supply of the extrahepatic bile duct was from the lower ascending gastroduodenal artery, the upper posterior pancreaticoduodenal artery and its branches, 38% from the right hepatic artery performed by the upper side, and 2% from the native hepatic artery. Through dissection of the posterior superior pancreatic duodenal artery of 15 cadavers, they found that the distance from the origin of the posterior superior pancreatic duodenal artery was 4.90 to 18.76 mm to the beginning of the gastroduodenal artery, with an average of (8.85±2.32) mm. In conventional orthotopic liver transplantation, the donor gastroduodenal artery was ligated, which means the donor liver extrahepatic bile duct can only rely on the right hepatic artery downward blood supply, which inevitably affects the blood supply of the extrahepatic bile duct. If the donor’s extrahepatic bile duct is retained for too long, ischemic changes of the bile duct will occur, which we believe is highly likely to be one of the important reasons for the high incidence of biliary ischemia-related complications in liver transplantation.
In conventional liver bile duct pruning, the donor bile duct is generally required to rely on the head of the common hepatic duct as far as possible, so as to reduce the effect of the gastroduodenal artery on the blood supply of the donor. However, the preserved short bile duct will inevitably increase the length of the recipient bile duct and the tension of the anastomosis. To solve this problem, we changed the donor liver pruning method, and paid attention to protecting the blood supply to the middle and lower segments of the common bile duct, which seeks to preserve at least 2 cm of the distal gastroduodenal artery and the integrity of the posterior superior pancreaticoduodenal artery and surrounding tissues. This could also avoid injury to the right hepatic artery from the distal end of the gastroduodenal artery and the variant right hepatic artery from the superior mesenteric artery. With the new method of pruning the donor–recipient bile duct, we found that the incidence of ischemic bile duct stenosis was significantly reduced after liver transplantation. At the same time, we can cut the donor bile duct at a lower position and reduce the tension of the anastomosis. However, attention should also be paid to avoiding the bile duct retained too long or twisted into an angle, and microtension bile duct anastomosis is preferred. Moreover, if the pancreas transplant is required, we suggest keeping the gastroduodenal artery to the pancreatic tissue side to maintain its blood supply.
This study has several limitations. First, as a retrospective study, the influence of measured and unmeasured confounders on biliary complications is inevitable. Second, patients were included between 2011 and 2022, which is a relatively long time and there may be some changes in the surgical team and operative instruments. Third, this study was performed in a single center, the number of patients included in the analysis was limited, and there may be some influence on the results due to insufficient sample size. Future prospective and large-scale multicenter study are needed to verify our findings.
Conclusions
In conclusion, preserving the integrity of the donor gastroduodenal artery is beneficial to protect the blood supply of the extrahepatic bile duct and can reduce the incidence of biliary complications after liver transplantation caused by extrahepatic bile duct ischemia.
Figures
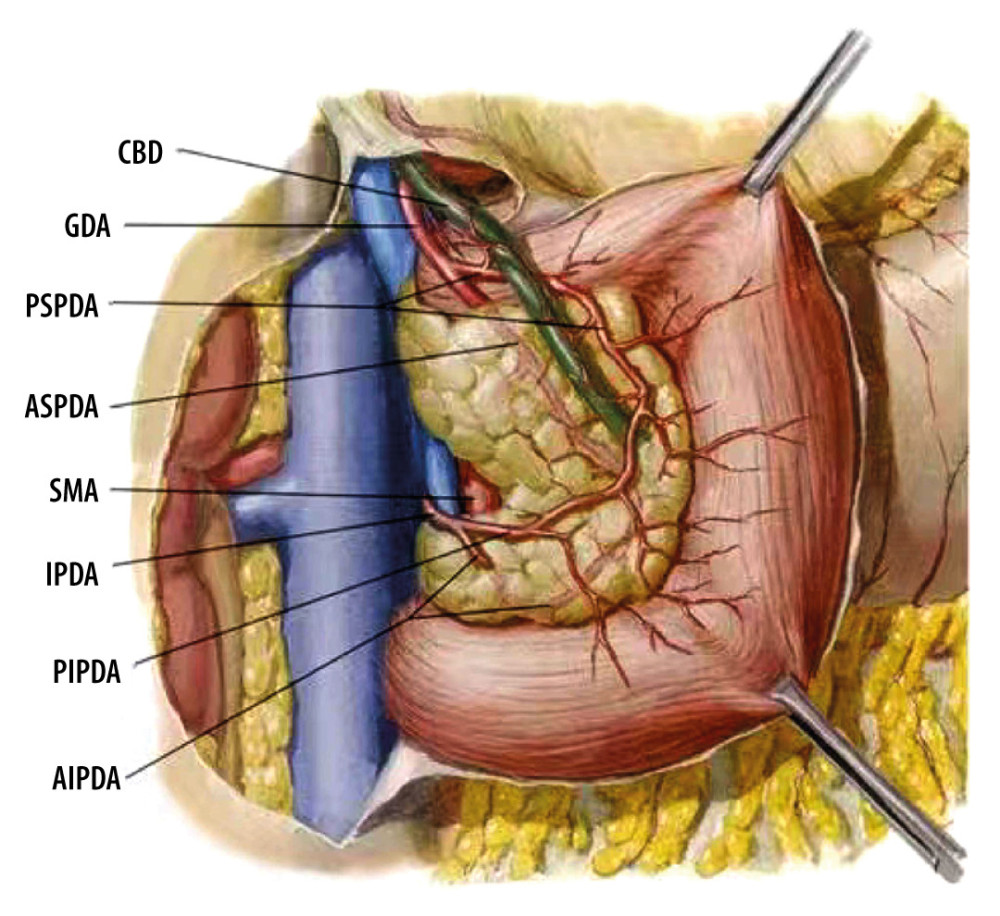 Figure 1. Common bile duct blood supply pattern diagram. The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the anterior superior pancreaticoduodenal artery and posterior superior pancreaticoduodenal artery. CBD – common bile duct; GDA – gastroduodenal artery; PSPDA – posterior superior pancreaticoduodenal artery; ASPDA – anterior superior pancreaticoduodenal artery; SMA – superior mesenteric artery; IPDA – inferior pancreaticoduodenal artery; PIPDA – posterior inferior pancreaticoduodenal artery; AIPDA – anterior inferior pancreaticoduodenal artery.
Figure 1. Common bile duct blood supply pattern diagram. The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the anterior superior pancreaticoduodenal artery and posterior superior pancreaticoduodenal artery. CBD – common bile duct; GDA – gastroduodenal artery; PSPDA – posterior superior pancreaticoduodenal artery; ASPDA – anterior superior pancreaticoduodenal artery; SMA – superior mesenteric artery; IPDA – inferior pancreaticoduodenal artery; PIPDA – posterior inferior pancreaticoduodenal artery; AIPDA – anterior inferior pancreaticoduodenal artery. 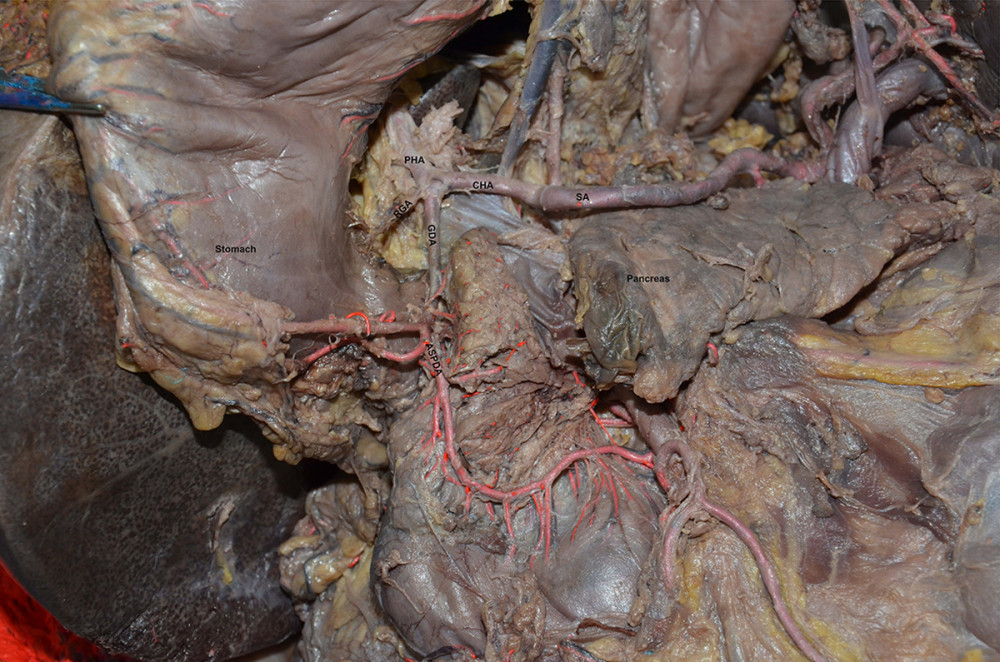 Figure 2. Anatomical diagram of extrahepatic duct blood supply (anterior view). PHA – proper hepatic artery; CHA – common hepatic artery; RGA – right gastric artery; SA – splenic artery.
Figure 2. Anatomical diagram of extrahepatic duct blood supply (anterior view). PHA – proper hepatic artery; CHA – common hepatic artery; RGA – right gastric artery; SA – splenic artery. 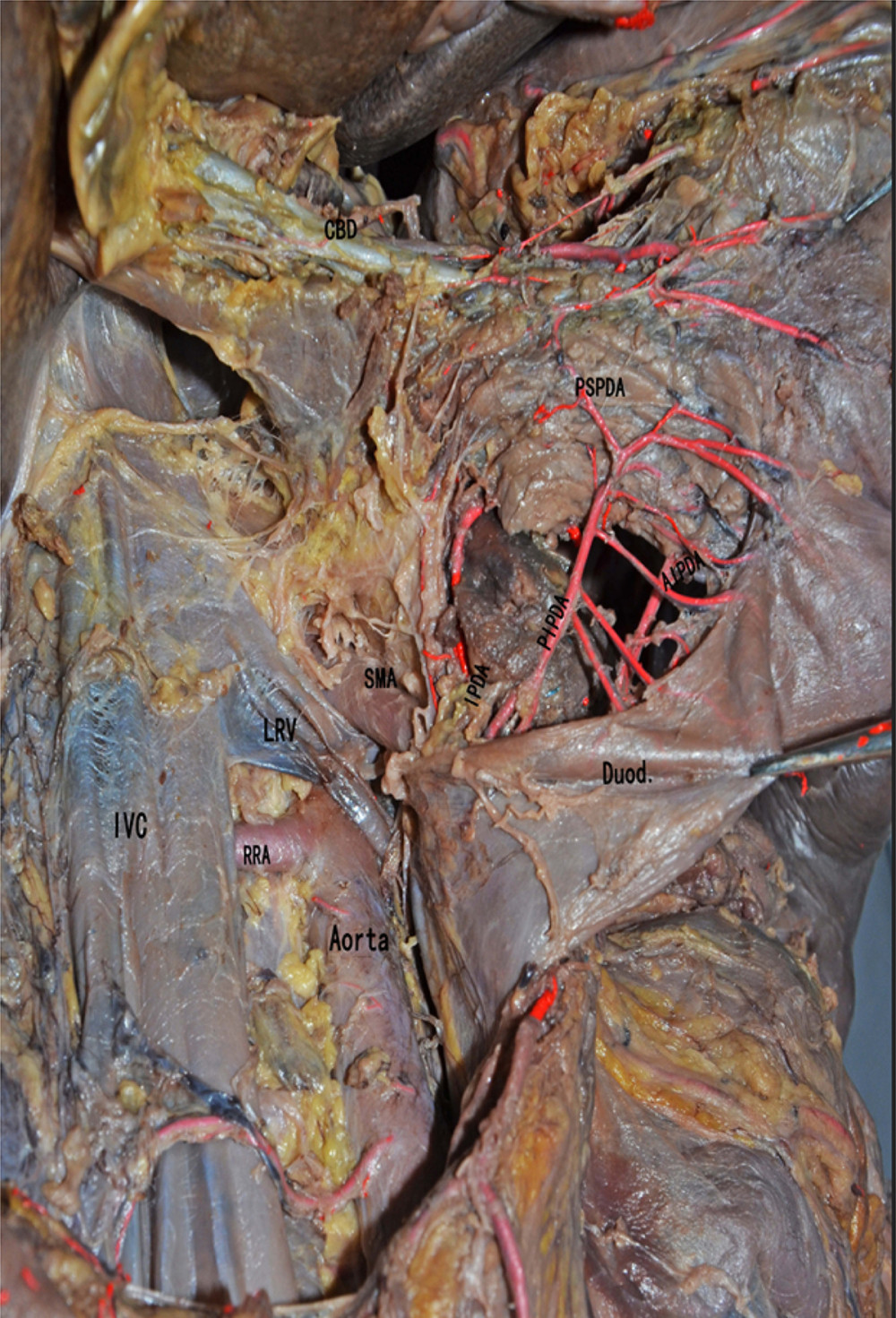 Figure 3. The anatomical diagram of extrahepatic duct blood supply (posterior view). The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the posterior superior pancreaticoduodenal artery and the anterior superior pancreaticoduodenal artery. IVC – inferior vena cava; LRV – left renal vein; RRA – right renal artery.
Figure 3. The anatomical diagram of extrahepatic duct blood supply (posterior view). The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the posterior superior pancreaticoduodenal artery and the anterior superior pancreaticoduodenal artery. IVC – inferior vena cava; LRV – left renal vein; RRA – right renal artery. 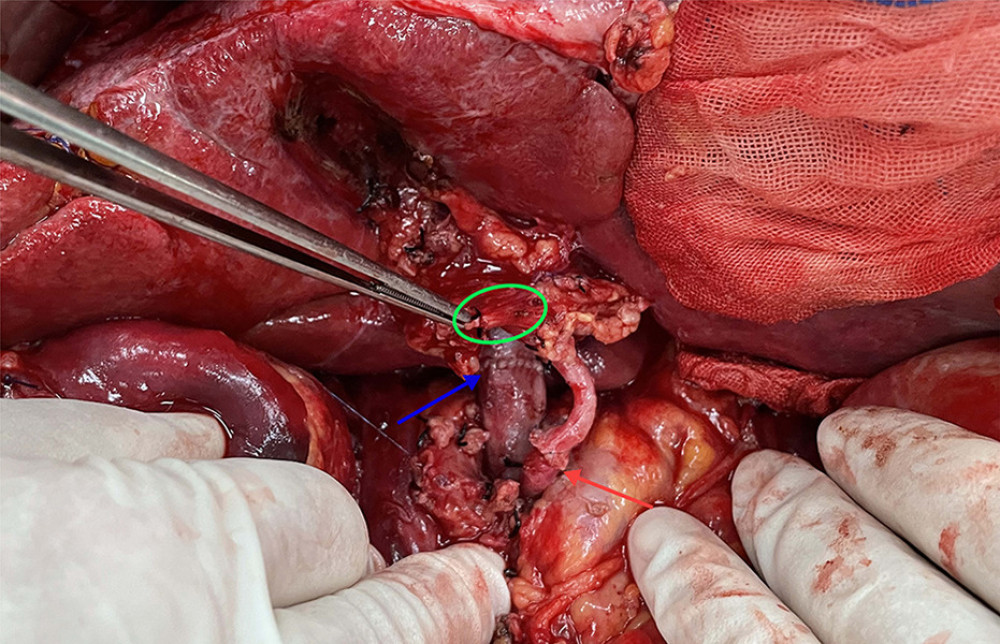 Figure 4. The picture of intraoperative hilar region before bile duct anastomosis. The vessel of the forceps lift was the donor-derived pancreaticoduodenal artery (green circle). The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis.
Figure 4. The picture of intraoperative hilar region before bile duct anastomosis. The vessel of the forceps lift was the donor-derived pancreaticoduodenal artery (green circle). The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis. 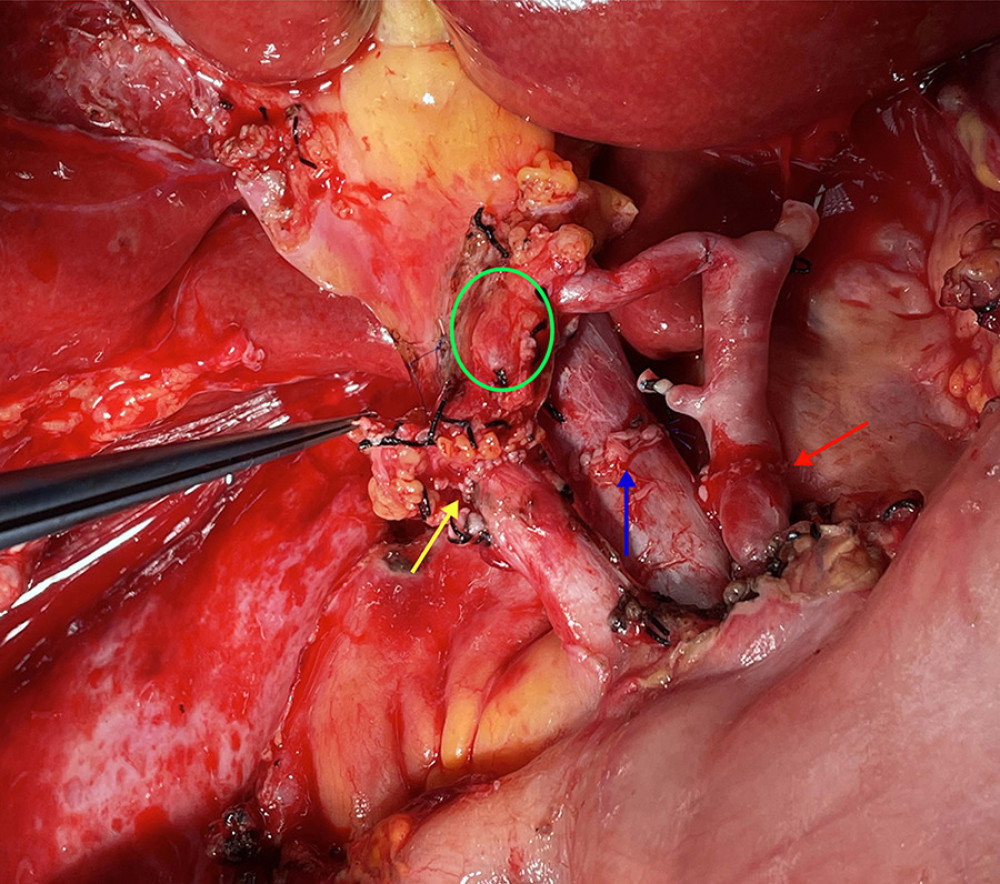 Figure 5. The picture of intraoperative hilar region after bile duct anastomosis. The green circle indicates donor-derived pancreaticoduodenal artery, and the yellow allow indicates the bile duct anastomosis. The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis.
Figure 5. The picture of intraoperative hilar region after bile duct anastomosis. The green circle indicates donor-derived pancreaticoduodenal artery, and the yellow allow indicates the bile duct anastomosis. The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis. Tables
Table 1. Basic characteristics of included patients.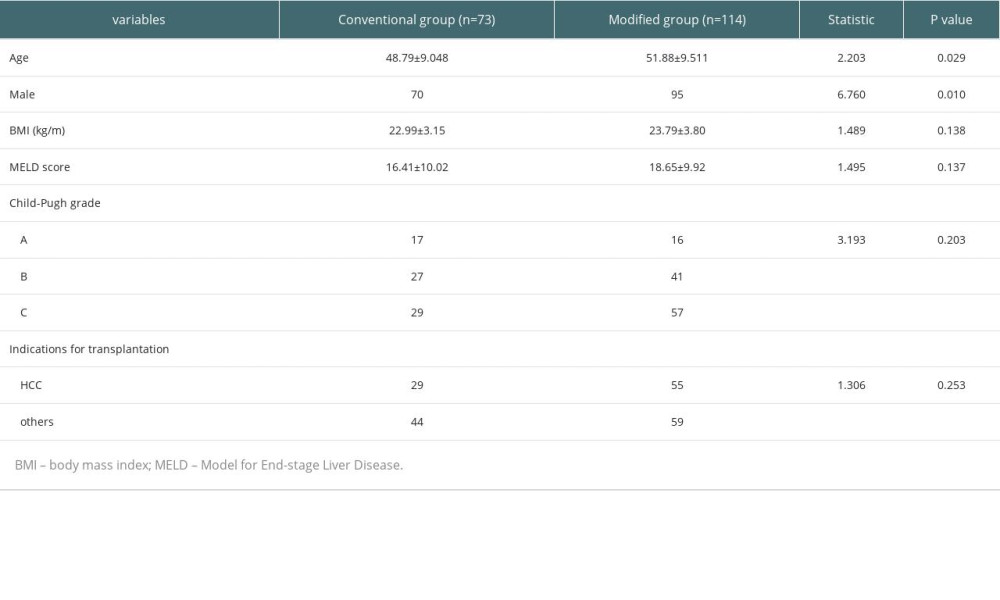 Table 2. Comparison of the surgical status in the 2 groups of liver transplant patients.
Table 2. Comparison of the surgical status in the 2 groups of liver transplant patients.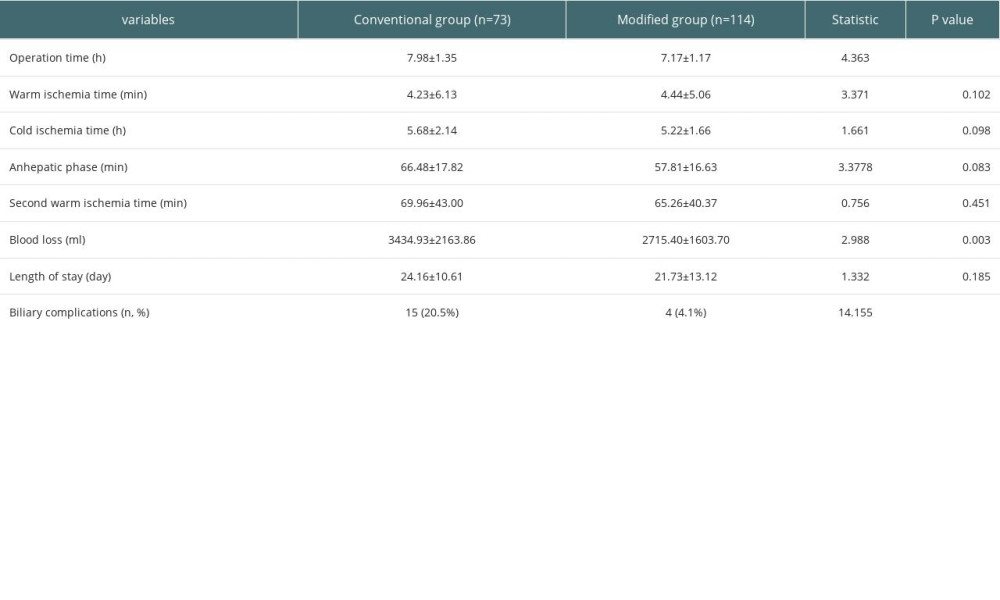 Table 3. The occurrence of biliary complications in 2 groups.
Table 3. The occurrence of biliary complications in 2 groups.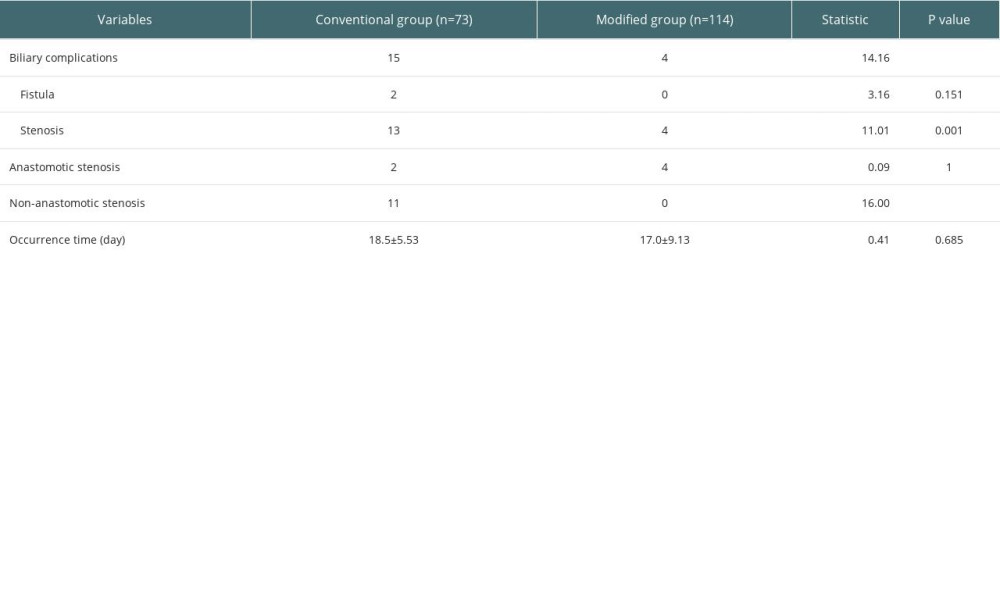 Table 4. Analysis of factors affecting biliary complications.
Table 4. Analysis of factors affecting biliary complications.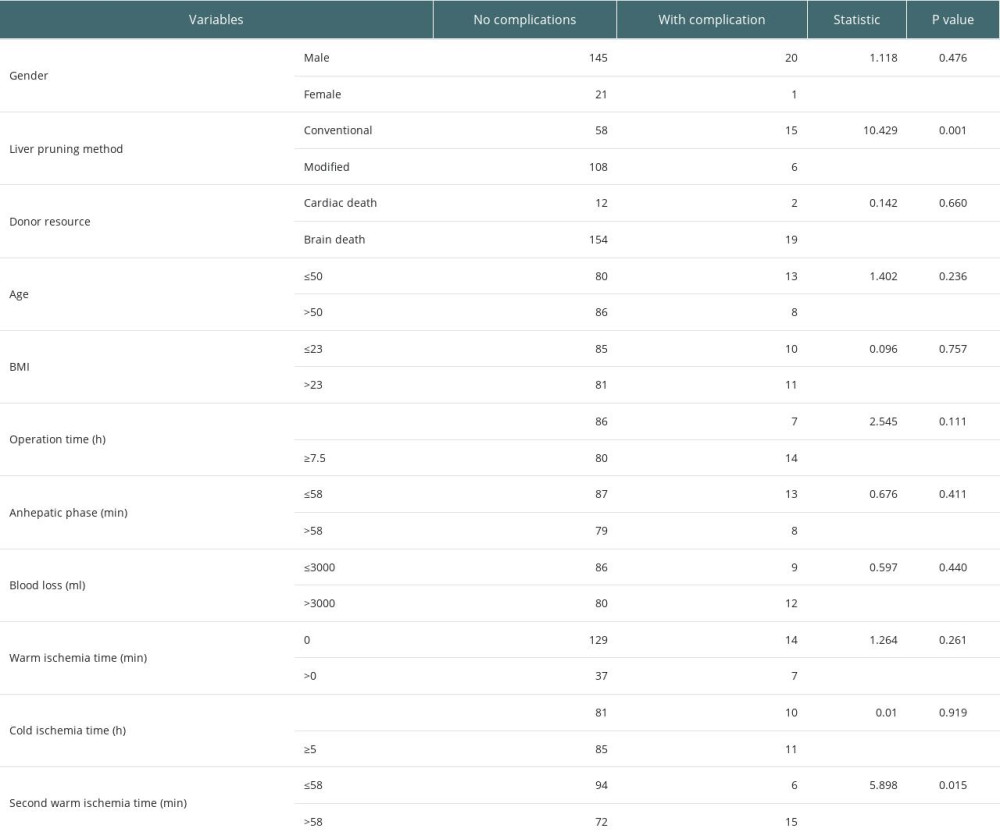 Table 5. Stratified chi-square test to analyze the effect of liver pruning method on biliary complications.
Table 5. Stratified chi-square test to analyze the effect of liver pruning method on biliary complications.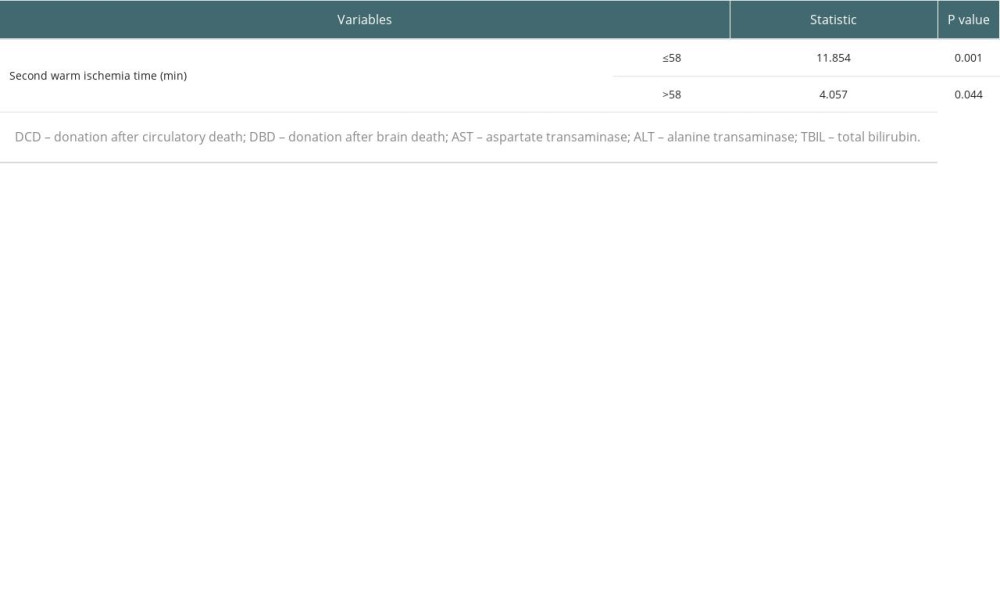 Table 6. Effect of donor source on postoperative liver function indicators.
Table 6. Effect of donor source on postoperative liver function indicators.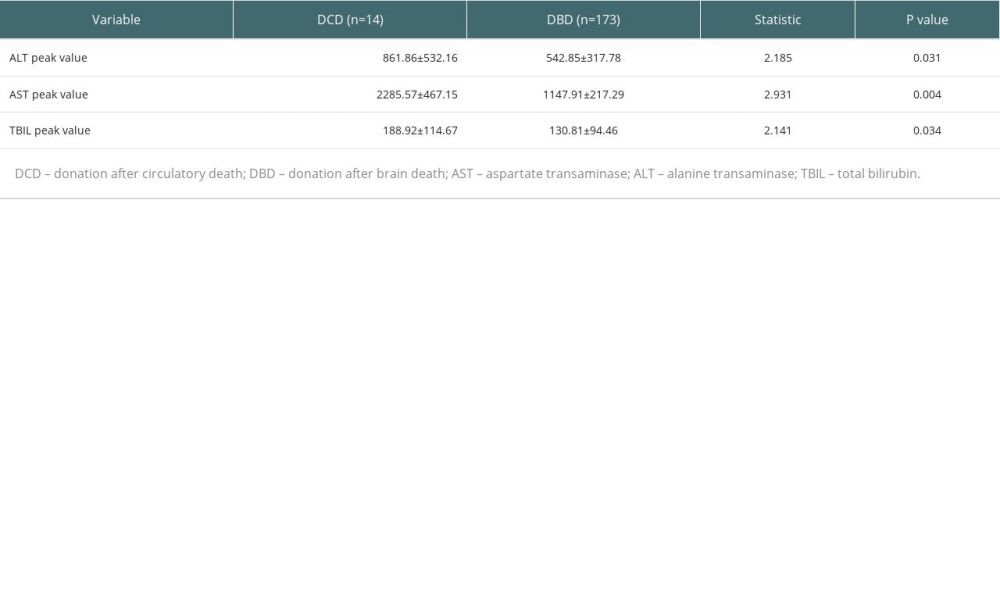
References
1. Bodzin AS, Baker TB, Liver transplantation today: Where we are now and where we are going: Liver Transpl, 2018; 24(10); 1470-75
2. Meirelles RF, Salvalaggio P, Rezende MB, Liver transplantation: History, outcomes and perspectives: Einstein (Sao Paulo), 2015; 13(1); 149-52
3. Kochhar G, Parungao JM, Hanouneh IA, Parsi MA, Biliary complications following liver transplantation: World J Gastroenterol, 2013; 19(19); 2841-46
4. Forde JJ, Bhamidimarri KR, Management of biliary complications in liver transplant recipients: Clin Liver Dis, 2022; 26(1); 81-99
5. Kienlein S, Schoening W, Andert A, Biliary complications in liver transplantation: Impact of anastomotic technique and ischemic time on short- and long-term outcome: World J Transplant, 2015; 5(4); 300-9
6. Mejía GA, Olarte-Parra C, Pedraza A, Biliary complications after liver transplantation: Incidence, risk factors and impact on patient and graft survival: Transplant Proc, 2016; 48(2); 665-68
7. Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P, Biliary complications after liver transplantation: Old problems and new challenges: Am J Transplant, 2013; 13(2); 253-65
8. Dai J, Wu XF, Yang C, Study of relationship between the blood supply of the extrahepatic bile duct and duct supply branches from gastroduodenal artery on imaging and anatomy: Chin Med J (Engl), 2015; 128(3); 322-26
9. Sheng-Jun CS-QF, Shan-Zhang T, Anatomical study of the application of extrahepatic bile duct supply arteries in clinical liver transplantation: Chin J Organ Transplant, 2005; 26(2); 103-4
10. Singhal A, Wima K, Hoehn RS, Hospital resource use with donation after cardiac death allografts in liver transplantation: A matched controlled analysis from 2007 to 2011: J Am Coll Surg, 2015; 220(5); 951-58
11. Hou X, Sui W, Che W, Current status and recent advances in liver transplant using organs donated after cardiac death: Exp Clin Transplant, 2015; 13(1); 6-18
12. Nemes B, Gámán G, Doros A, Biliary complications after liver transplantation: Expert Rev Gastroenterol Hepatol, 2015; 9(4); 447-66
13. Xuanl LF, Lin L, Etiology, diagnosis and treatment of biliary complications after liver transplantation: Chin J Hepatobiliary Surg, 2013; 19(6); 469-72
14. Thuluvath PJ, Pfau PR, Kimmey MB, Ginsberg GG, Biliary complications after liver transplantation: The role of endoscopy: Endoscopy, 2005; 37(9); 857-63
15. Londoño MC, Balderramo D, Cárdenas A, Management of biliary complications after orthotopic liver transplantation: The role of endoscopy: World J Gastroenterol, 2008; 14(4); 493-97
16. Sharma S, Gurakar A, Jabbour N, Biliary strictures following liver transplantation: Past, present and preventive strategies: Liver Transpl, 2008; 14(6); 759-69
17. Jay CL, Lyuksemburg V, Ladner DP, Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: A meta-analysis: Ann Surg, 2011; 253(2); 259-64
18. O’Neill S, Roebuck A, Khoo E, A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation: Transpl Int, 2014; 27(11); 1159-74
19. Detry O, Donckier V, Lucidi V, Liver transplantation from donation after cardiac death donors: Initial Belgian experience 2003–2007: Transpl Int, 2010; 23(6); 611-18
20. DeOliveira ML, Jassem W, Valente R, Biliary complications after liver transplantation using grafts from donors after cardiac death: Results from a matched control study in a single large volume center: Ann Surg, 2011; 254(5); 716-22 discussion 722–23
21. Frongillo F, Lirosi MC, Sganga G, Graft steatosis as a risk factor of ischemic-type biliary lesions in liver transplantation: Transplant Proc, 2014; 46(7); 2293-94
22. Axelrod DA, Dzebisashvili N, Lentine KL, Variation in biliary complication rates following liver transplantation: Implications for cost and outcome: Am J Transplant, 2015; 15(1); 170-79
23. Guichelaar MM, Benson JT, Malinchoc M, Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation: Am J Transplant, 2003; 3(7); 885-90
24. Chan EY, Olson LC, Kisthard JA, Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors: Liver Transpl, 2008; 14(5); 604-10
25. de Vera ME, Lopez-Solis R, Dvorchik I, Liver transplantation using donation after cardiac death donors: Long-term follow-up from a single center: Am J Transplant, 2009; 9(4); 773-81
26. Chen CL, Concejero AM, Lin TS, Outcome of routine use of microsurgical biliary reconstruction in pediatric living donor liver transplantation: J Hepatobiliary Pancreat Sci, 2013; 20(5); 492-97
Figures
 Figure 1. Common bile duct blood supply pattern diagram. The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the anterior superior pancreaticoduodenal artery and posterior superior pancreaticoduodenal artery. CBD – common bile duct; GDA – gastroduodenal artery; PSPDA – posterior superior pancreaticoduodenal artery; ASPDA – anterior superior pancreaticoduodenal artery; SMA – superior mesenteric artery; IPDA – inferior pancreaticoduodenal artery; PIPDA – posterior inferior pancreaticoduodenal artery; AIPDA – anterior inferior pancreaticoduodenal artery.
Figure 1. Common bile duct blood supply pattern diagram. The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the anterior superior pancreaticoduodenal artery and posterior superior pancreaticoduodenal artery. CBD – common bile duct; GDA – gastroduodenal artery; PSPDA – posterior superior pancreaticoduodenal artery; ASPDA – anterior superior pancreaticoduodenal artery; SMA – superior mesenteric artery; IPDA – inferior pancreaticoduodenal artery; PIPDA – posterior inferior pancreaticoduodenal artery; AIPDA – anterior inferior pancreaticoduodenal artery. Figure 2. Anatomical diagram of extrahepatic duct blood supply (anterior view). PHA – proper hepatic artery; CHA – common hepatic artery; RGA – right gastric artery; SA – splenic artery.
Figure 2. Anatomical diagram of extrahepatic duct blood supply (anterior view). PHA – proper hepatic artery; CHA – common hepatic artery; RGA – right gastric artery; SA – splenic artery. Figure 3. The anatomical diagram of extrahepatic duct blood supply (posterior view). The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the posterior superior pancreaticoduodenal artery and the anterior superior pancreaticoduodenal artery. IVC – inferior vena cava; LRV – left renal vein; RRA – right renal artery.
Figure 3. The anatomical diagram of extrahepatic duct blood supply (posterior view). The vascular network around the hilar bile originates mainly from the branches of the right hepatic artery and the gallbladder artery, and the bile vessel network below the common hepatic duct is more derived from the gastroduodenal artery and its branches including the posterior superior pancreaticoduodenal artery and the anterior superior pancreaticoduodenal artery. IVC – inferior vena cava; LRV – left renal vein; RRA – right renal artery. Figure 4. The picture of intraoperative hilar region before bile duct anastomosis. The vessel of the forceps lift was the donor-derived pancreaticoduodenal artery (green circle). The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis.
Figure 4. The picture of intraoperative hilar region before bile duct anastomosis. The vessel of the forceps lift was the donor-derived pancreaticoduodenal artery (green circle). The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis. Figure 5. The picture of intraoperative hilar region after bile duct anastomosis. The green circle indicates donor-derived pancreaticoduodenal artery, and the yellow allow indicates the bile duct anastomosis. The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis.
Figure 5. The picture of intraoperative hilar region after bile duct anastomosis. The green circle indicates donor-derived pancreaticoduodenal artery, and the yellow allow indicates the bile duct anastomosis. The blue arrow indicates the portal vein anastomosis and the red arrow indicates the arterial anastomosis. Tables
 Table 1. Basic characteristics of included patients.
Table 1. Basic characteristics of included patients. Table 2. Comparison of the surgical status in the 2 groups of liver transplant patients.
Table 2. Comparison of the surgical status in the 2 groups of liver transplant patients. Table 3. The occurrence of biliary complications in 2 groups.
Table 3. The occurrence of biliary complications in 2 groups. Table 4. Analysis of factors affecting biliary complications.
Table 4. Analysis of factors affecting biliary complications. Table 5. Stratified chi-square test to analyze the effect of liver pruning method on biliary complications.
Table 5. Stratified chi-square test to analyze the effect of liver pruning method on biliary complications. Table 6. Effect of donor source on postoperative liver function indicators.
Table 6. Effect of donor source on postoperative liver function indicators. Table 1. Basic characteristics of included patients.
Table 1. Basic characteristics of included patients. Table 2. Comparison of the surgical status in the 2 groups of liver transplant patients.
Table 2. Comparison of the surgical status in the 2 groups of liver transplant patients. Table 3. The occurrence of biliary complications in 2 groups.
Table 3. The occurrence of biliary complications in 2 groups. Table 4. Analysis of factors affecting biliary complications.
Table 4. Analysis of factors affecting biliary complications. Table 5. Stratified chi-square test to analyze the effect of liver pruning method on biliary complications.
Table 5. Stratified chi-square test to analyze the effect of liver pruning method on biliary complications. Table 6. Effect of donor source on postoperative liver function indicators.
Table 6. Effect of donor source on postoperative liver function indicators. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








