20 September 2022: Original Paper
Long-Term Patency of All-in-One Sleeve Patch Graft Venoplasty in 16 Patients Who Underwent Living Donor Liver Transplantation with a Right Liver Graft: A 10-Year, Single-Center, Retrospective Study
Byeong-Gon NaDOI: 10.12659/AOT.936888
Ann Transplant 2022; 27:e936888
Abstract
BACKGROUND: This retrospective study from a single center aimed to evaluate the long-term patency of all-in-one sleeve venoplasty (ASV) in 16 patients who underwent living donor liver transplantation (LDLT) with a right liver graft (RLG) between 2009 and 2019. ASV unifies the right hepatic vein (RHV), short hepatic vein (SHV), and middle hepatic vein (MHV) of an RLG. ASV enables wide side-to-side anastomosis to the recipient inferior vena cava (IVC).
MATERIAL AND METHODS: Of 2875 patients who underwent LDLT with an RLG from August 2009 to July 2019, 16 (0.5%) patients underwent ASV. We analyzed the ASV techniques applied to these patients, as well as patient long-term outcomes.
RESULTS: Type 1 ASV unified 1 RHV, 1 IRHV, and 1 MHV conduit (n=12 [75.0%]). Type 2 ASV unified 1 RHV, multiple IRHVs, and 1 MHV conduit (n=4 [25.0%]). All patients are currently alive, with a mean follow-up period of 70.1±41.9 months. No patient underwent retransplantation. Follow-up computed tomography showed SHV occlusion in 1 (6.3%) patient at 4 months, resulting in 1-, 3-, and 5-year SHV patency rates of 93.8% each. MHV occlusion was identified in 6 (37.5%) patients, with 1-, 3-, and 5-year MHV patency rates of 81.3%, 68.8%, and 68.8%, respectively (P=0.037). No patient underwent endovascular stenting of the SHV or MHV. Patency rates were significantly higher for SHV than MHV (P=0.037).
CONCLUSIONS: ASV using various vascular patches is a useful technique enabling secure reconstruction of an RLG in grafts with complex hepatic vein anatomy or recipients with poor IVC condition.
Keywords: Liver Transplantation, Hepatic Vein Outflow Obstruction, Hepatic vein, Allograft, Liver Grafting, Humans, Liver Diseases, Living Donors, Plastic Surgery Procedures
Background
Graft outflow vein reconstruction is the most important procedure for successful implantation of a right liver graft (RLG) in living donor liver transplantation (LDLT) [1,2]. The hepatic venous drainage pathways of RLGs include the right hepatic vein (RHV), middle hepatic vein (MHV), and short hepatic vein (SHV) including the inferior RHV (IRHV) [2]. Each of these hepatic veins has its own drainage territory, thus making complete reconstruction of these outflow veins essential to prevent hepatic venous congestion [3,4].
Many innovative surgical techniques have been devised to reconstruct graft outflow veins in LDLT. All-in-one sleeve venoplasty (ASV) is a unique method of unifying the RHV, MHV, and SHV of an RLG [5–7], thereby enabling wide side-to-side anastomosis, a technique similar to double inferior vena cava (IVC) anastomosis in deceased donor liver transplantation. ASV is a technique that simplifies the reconstruction of multiple hepatic veins and can reduce warm ischemia time in LDLT using an RLG [6]. ASV can be made through quilt unification venoplasty (QUV) using various homograft vessels [8–10].
This 10-year retrospective study from a single center aimed to evaluate the long-term patency and technical details of ASV in 16 patients who underwent LDLT using an RLG between 2009 and 2019.
Material and Methods
PATIENT SELECTION:
The LT database of Asan Medical Center was searched to identify patients who underwent LDLT using an RLG with ASV over an 11-year period from August 2009 to July 2019. Of the 2875 patients who underwent LDLT using an RLG during this period, 16 (0.5%) patients underwent ASV. The medical records of these 16 patients were retrospectively reviewed, with all patients followed up until August 2021.
This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013. The study protocol was approved by the Institutional Review Board of Asan Medical Center (No. 2021-1347), which waived the requirement for informed consent due to the retrospective nature of this study.
INDICATION OF ALL-IN-ONE SLEEVE VENOPLASTY:
Because SHVs in RLGs varies in number, size and location, our institutional guidelines were established for reconstructing SHVs in relation to RHV [9,10]. QUV was performed in patients with 2 or more scattered SHVs, enabling a single anastomosis to the IVC. An interposition MHV conduit could be reconstructed separately to the IVC or integrated into the QUV orifice to make an ASV orifice. The decision on whether to integrate an MHV conduit depended primarily on the size and length of the MHV conduit. If the MHV conduit was large and long enough, separate MHV reconstruction was preferred because it was technically easier than outflow vein reconstruction with ASV. If the MHV conduit was relatively small or short, ASV was indicated because it is vulnerable to outflow obstruction (Figure 1). The availability of sizable vascular homografts was the most important factor enabling ASV, because these homografts could not be replaced by prosthetic vascular grafts.
SURGICAL TECHNIQUE FOR ALL-IN-ONE SLEEVE VENOPLASTY:
All of the outflow vein branches of an RLG, including RHV, SHV, and MHV branches, could be unified at the wide sleeve patch corresponding to the IVC. The RHV and IRHV/SHVs were unified at the large-sized vascular homograft patch, followed by anastomosis of an MHV conduit or segment VIII hepatic vein (V8) opening to the large sleeve patch graft (Figures 2, 3). This technique resulted in the formation of a wide orifice, resembling a half-sliced IVC at the RLG, thus enabling side-to-side cavo-caval anastomosis.
Side-to-side anastomosis usually involves deep side-clamping of the recipient IVC, which induces near-total clamping of the IVC flow. The alternative shallow side-clamping is more demanding than conventional deep clamping. If the recipient IVC is completely isolated, total separate clamping of the retrohepatic IVC at the supra- and infrahepatic portions is convenient for wide anastomosis [9].
PATENCY EVALUATION OF THE MHV AND SHV:
After LT, dynamic computed tomography (CT) was performed weekly during hospitalization, every 3–6 months at the outpatient clinic for the first 3 years, annually for 5 years, and biannually thereafter. Follow-up CT scans were performed more frequently in patients diagnosed with hepatocellular carcinoma.
Occlusion of the MHV conduit was defined as non-visualization of blood flow in the vascular conduit between the V8 (or segment V hepatic vein [V5] when only V5 was reconstructed) and the IVC on liver dynamic CT. If V5 was occluded but V8 remained patent, the MHV conduit was regarded as patent. If a CT scan could not be performed owing to impaired renal function, Doppler ultrasonography was performed. Occlusion of the SHV was defined as absence of continuity between the intrahepatic SHV and IVC on liver dynamic CT.
STATISTICAL ANALYSIS:
All numerical data are presented as means and standard deviations. Survival rates were estimated using the Kaplan-Meier method and compared using the log-rank test. A
Results
PATIENT PROFILES:
The clinical profiles of the 16 patients who underwent LDLT using an RLG with ASV are summarized in Table 1. The mean recipient age was 53.6±6.7 years. Hepatitis B virus-associated liver cirrhosis was the most common disease leading to LT (n=8 [50.0%]). One (6.3%) patient was diagnosed with Budd-Chiari syndrome. Two (12.5%) patients underwent a second LDLT due to graft failure at 90 and 197 months, respectively, after the first LDLT. The mean model for end-stage liver disease (MELD) score was 15.5±9.5. Three (18.8%) patients underwent ABO-incompatible LDLT. The mean graft-to-recipient weight ratio was 1.11±0.29%.
CONFIGURATIONS OF ALL-IN-ONE SLEEVE VENOPLASTY:
The configuration of ASV was stratified into 2 types. Type 1, a combination of 1 RHV, 1 SHV, and 1 MHV conduit or V8, was performed in 12 (76.0%) patients (Figure 3A–3C). Type 2, a combination of 1 RHV, multiple SHVs, and 1 MHV conduit or V8 was performed in 4 (25.0%) patients (Figures 2, 3D). All available vascular patches, including the cryopreserved iliac arteries/veins and aortas, and autologous saphenous veins, were used for QUV.
The mean cold ischemic time including bench work was 105.6±29.1 min; the mean warm ischemic time was 51.7±24.9 min; and mean total ischemic time was 157.4±40.9 min (Table 1).
SURVIVAL OUTCOMES:
The mean follow-up period in these 16 patients was 70.1±41.9 months. All 16 study patients are currently alive. Two (12.5%) patients experienced recurrence of hepatocellular carcinoma. None underwent retransplantation.
ANALYSES OF PATENCY OF THE SHV AND MHV:
During post-transplant CT follow-up, SHV occlusion was identified in 1 (6.3%) patient at 4 months, resulting in 1-, 3-, and 5-year SHV patency rates of 93.8% each (Figure 4A). MHV occlusion was identified in 6 (37.5%) patients, with the 1-, 3-, and 5-year MHV patency rates of 81.3%, 68.8% and 68.8%, respectively (Figure 4B). No patient underwent endovascular stenting of the SHV or MHV. The patency rate was significantly higher in SHV than in MHV (P=0.037).
Discussion
Every outflow vein of the liver has its own drainage territory. It is generally accepted that SHV or MHV branches greater than 5 mm are indicated for revascularization during LDLT using an RLG. It was reported that 44.1% of RLGs have sizable SHVs requiring reconstruction [10]. Donor SHV anatomy can vary widely, with the presence of variant SHVs making their reconstruction difficult [7]. Based on experience, our institution has established guidelines for reconstructing SHVs in relation to the RHV [9,10]. QUV for multiple SHVs is a simple method to attach a new IVC wall cuff to an RLG. This method not only reduces the risk of anastomotic stenosis or torsion of SHVs, but also enhances donor safety because there is no need for wide excision of the donor IVC wall. The surgical procedure of QUV at the back table takes a longer time than conventional bench work, which would be shortened along the learning curve [8–10].
The majority of RLGs have sizable V5 and V8 branches at the liver cut surface, with these branches also requiring revascularization. MHV reconstruction using a vascular conduit has been regarded as a standard technique for LDLT using an RLG [11]. Such an MHV conduit can be reconstructed separately to the IVC or integrated into the QUV orifice to form an ASV. The decision on whether to implant the MHV conduit separately or to form an ASV was based on the size and length of the MHV conduit and the availability of sizable vascular homograft patches. A recipient IVC in poor condition, such as Budd-Chiari syndrome or those undergoing retransplantation after LDLT, is a good indication of ASV, as the latter can reduce the risk of graft outflow vein obstruction [12]. An abnormally small RHV with relatively large multiple SHVs is also an eligible indication of ASV, particularly type 2 ASV.
SHV reconstruction using a cryopreserved IVC patch was devised in the early era of adult LDLT [5]. Because the barrel of an IVC homograft is large enough to accommodate multiple SHVs, it can effectively accommodate every variant of SHV anatomy. However, sizable IVC homografts can be recovered only from deceased tissue donors and it is nearly impossible to obtain from deceased multi-organ donors. Rather than using IVC homograft patches, we have used every available vascular patch, including cryopreserved iliac arteries/veins and aortas, as well as autologous saphenous veins.
Although we were aware of the advantages of ASV, it was not our first choice because length-adjusted insertion of an MHV conduit was more difficult than expected [9]. Separate reconstruction of an MHV conduit is often simpler than ASV. This was one of the reasons why only a small number of patients at our institution underwent ASV. ASV has been sporadically reported in the literature [6,7,9].
The patency rate of the SHV was higher after ASV than after separate reconstruction or QUV. The 5-year patency rate of the SHV was 93.8% in the present study, compared with 70–80% in our previous study [10]. This higher patency rate was likely due to the SHVs being implanted at the IVC substitute patch at the back table, thus minimizing the risks of anastomotic stenosis and torsion. By contrast, the patency of the MHV was not higher after ASV than after separate MHV reconstruction. The causes underlying MHV conduit occlusion are different from those underlying SHV occlusion. SHV anastomosis is protected within the IVC, whereas the MHV conduits are exposed to extrinsic compression and other mechanical and liver hemodynamic factors affected their luminal patency.
None of the patients in the present study experienced RHV stenosis or required endovascular stenting, which may have contributed to the absence of graft failure and patient death. These findings suggest that ASV provides highly reliable and secure graft outflow vein reconstruction for grafts with complex hepatic vein anatomy or for patients with IVCs in poor condition.
We have maintained an institutional tissue bank to supply various vascular homografts. All human tissues stored at the tissue bank were donated after obtaining informed consent from the donors’ family members. All the procedures for vascular tissue procurement and processing comply with Korean legislation and conform to the ethical and safety concerns for therapeutic use [13,14]. Currently, cryopreserved homografts of the femoral vein and artery and greater saphenous vein are available through the Korea Public Tissue Bank. Any sizable vessel homograft can be effectively used for graft venoplasty.
The present study has several limitations, including its retrospective design and small sample size from a single center. Therefore, multi-center studies including a larger number of patients are necessary to validate its results. Another limitation of the present study was absence of control groups for comparison of patency rates.
Conclusions
The results of the present study suggest that ASV using various vascular patches can be a useful technical option enabling secure reconstruction of an RLG in situations of complex graft hepatic vein anatomy or poor recipient IVC condition.
Figures
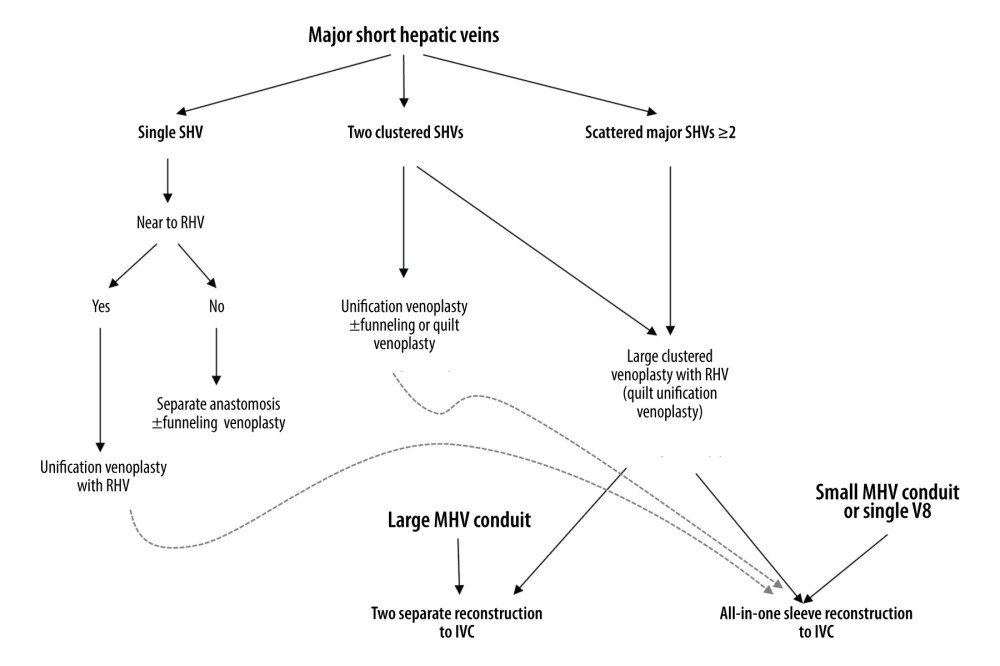 Figure 1. Institutional guidelines for reconstructing 1 or multiple major short hepatic veins (SHVs) with the right hepatic vein (RHV) and middle hepatic vein (MHV). V8 and IVC indicate the segment VIII hepatic vein and the inferior vena cava, respectively.
Figure 1. Institutional guidelines for reconstructing 1 or multiple major short hepatic veins (SHVs) with the right hepatic vein (RHV) and middle hepatic vein (MHV). V8 and IVC indicate the segment VIII hepatic vein and the inferior vena cava, respectively. 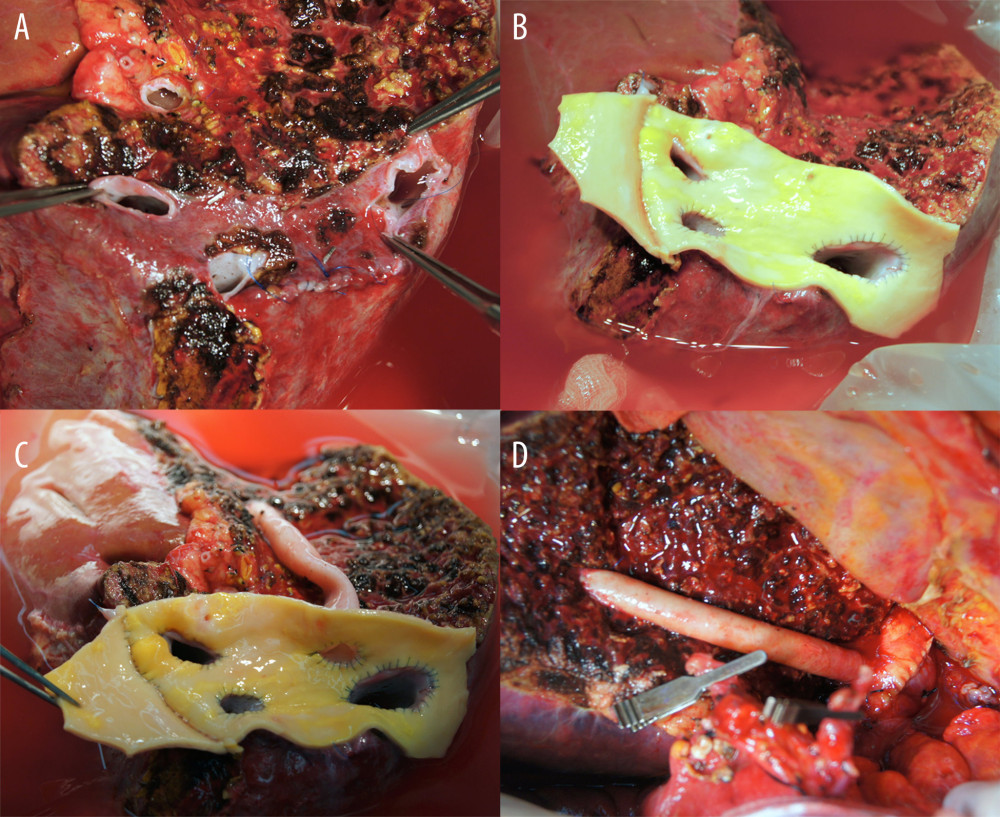 Figure 2. Intraoperative photographs showing all-in-one sleeve venoplasty. (A) A right liver graft has 1 right hepatic vein opening, 2 inferior right hepatic vein openings, and 1 middle hepatic vein openings. (B) The orthodox and inferior right hepatic vein openings are unified with an aorta homograft patch. (C) An iliac artery homograft conduit is connected between the segment V vein and the aorta patch. (D) The aorta patch is anastomosed to the recipient’s inferior vena cava in a side-to-side fashion.
Figure 2. Intraoperative photographs showing all-in-one sleeve venoplasty. (A) A right liver graft has 1 right hepatic vein opening, 2 inferior right hepatic vein openings, and 1 middle hepatic vein openings. (B) The orthodox and inferior right hepatic vein openings are unified with an aorta homograft patch. (C) An iliac artery homograft conduit is connected between the segment V vein and the aorta patch. (D) The aorta patch is anastomosed to the recipient’s inferior vena cava in a side-to-side fashion. 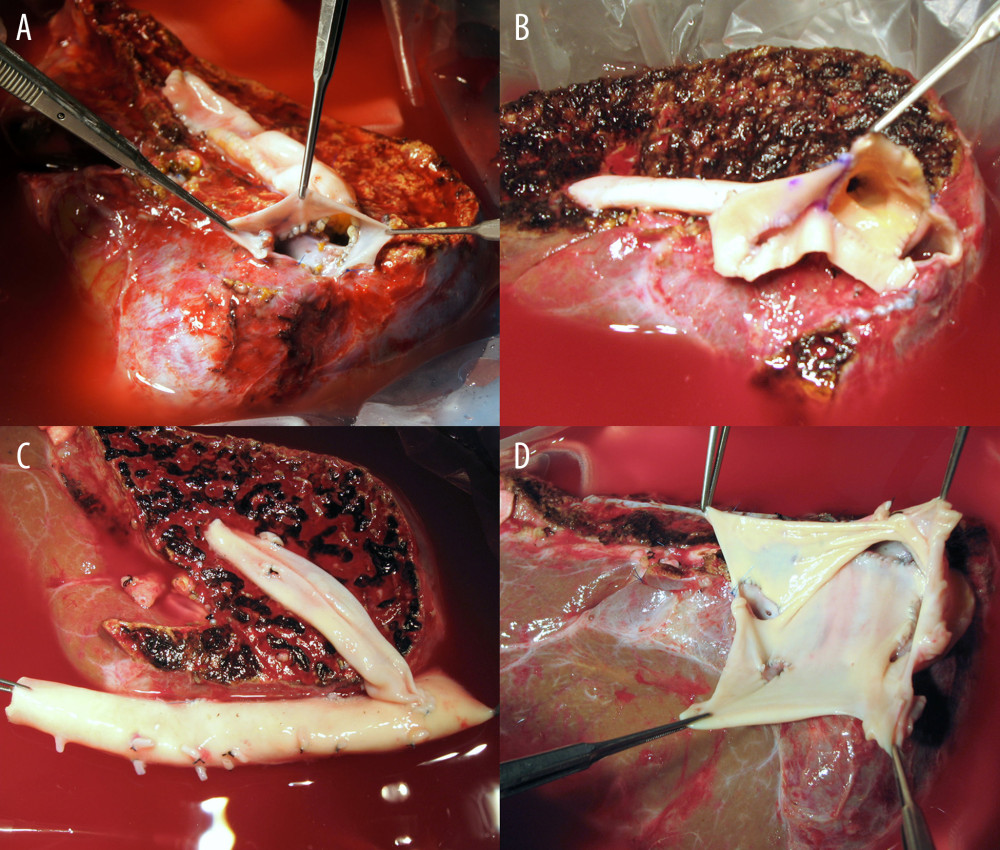 Figure 3. Configurations of all-in-one sleeve venoplasty. (A) A right liver graft (RLG) has 1 right hepatic vein (RHV) opening, 1 adjacent inferior right hepatic vein (IRHV) opening, 1 segment V vein (V5) opening, and 1 segment VIII vein (V8) opening. The RHV and IRHV openings were directly unified. An iliac vein conduit was attached to the V5. V8 was located close to the RHV opening, so they were unified. A semicircular fence with the autologous greater saphenous vein patch was attached (type 1). (B) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An iliac artery conduit was attached to the V5 and V8, and then unified with the RHV opening. Arterial patches were attached to the IRHV (type 1). (C) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An abdominal aorta was attached to the RHV and IRHV. An iliac vein conduit was attached to the V5 and V8, and then connected to the aorta graft (type 1). (D) An RLG has 1 RHV opening, 2 distant IRHV openings, and 1 V8 opening. They were unified with a large-sized common iliac vein patch (type 2).
Figure 3. Configurations of all-in-one sleeve venoplasty. (A) A right liver graft (RLG) has 1 right hepatic vein (RHV) opening, 1 adjacent inferior right hepatic vein (IRHV) opening, 1 segment V vein (V5) opening, and 1 segment VIII vein (V8) opening. The RHV and IRHV openings were directly unified. An iliac vein conduit was attached to the V5. V8 was located close to the RHV opening, so they were unified. A semicircular fence with the autologous greater saphenous vein patch was attached (type 1). (B) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An iliac artery conduit was attached to the V5 and V8, and then unified with the RHV opening. Arterial patches were attached to the IRHV (type 1). (C) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An abdominal aorta was attached to the RHV and IRHV. An iliac vein conduit was attached to the V5 and V8, and then connected to the aorta graft (type 1). (D) An RLG has 1 RHV opening, 2 distant IRHV openings, and 1 V8 opening. They were unified with a large-sized common iliac vein patch (type 2). 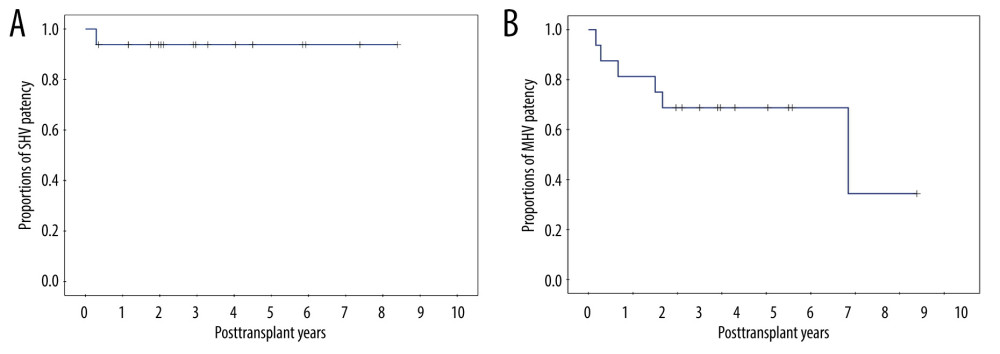 Figure 4. Kaplan-Meier patency curves of the (A) short hepatic vein (SHV) and (B) middle hepatic vein (MHV).
Figure 4. Kaplan-Meier patency curves of the (A) short hepatic vein (SHV) and (B) middle hepatic vein (MHV). References
1. Uchida K, Taniguchi M, Shimamura T, Three-dimensional computed tomography scan analysis of hepatic vasculatures in the donor liver for living donor liver transplantation: Liver Transpl, 2010; 16; 1062-68
2. Varotti G, Gondolesi GE, Goldman J, Anatomic variations in right liver living donors: J Am Coll Surg, 2004; 198; 577-82
3. Hwang S, Lee SG, Park KM, Hepatic venous congestion in living donor liver transplantation: Preoperative quantitative prediction and follow-up of its sequences using computed tomogram: Liver Transpl, 2004; 10; 763-70
4. Lee SG, Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft: J Hepatobiliary Pancreat Surg, 2006; 13; 131-38
5. Sugawara Y, Makuuchi M, Akamatsu N, Refinement of venous reconstruction using cryopreserved veins in right liver grafts: Liver Transpl, 2004; 10; 541-47
6. Chen CL, Yap AQ, Concejero AM, Liu CY, All-in-one sleeve patch graft venoplasty for multiple hepatic vein reconstruction in living donor liver transplantation: HPB (Oxford), 2012; 14; 274-78
7. Shimizu S, Onoe T, Ishiyama K, Multiple hepatic vein reconstruction using an all-in-one sleeve patch graft technique in living donor liver transplantation: A case report: Transplant Proc, 2014; 46; 982-85
8. Hwang S, Lee SG, Park KM, Quilt venoplasty using recipient saphenous vein graft for reconstruction of multiple short hepatic veins in right liver grafts: Liver Transpl, 2005; 11; 104-7
9. Jung DH, Hwang S, Ahn CS, Quilt unification venoplasty of the right hepatic veins enabling double inferior vena cava anastomosis in living donor liver transplantation using a right liver graft: Ann Liver Transplant, 2021; 1; 86-94
10. Hwang S, Ha TY, Ahn CS, Reconstruction of inferior right hepatic veins in living donor liver transplantation using right liver grafts: Liver Transpl, 2012; 18; 238-47
11. Hwang S, Ha TY, Ahn CS, Standardized surgical techniques for adult living donor liver transplantation using a modified right lobe graft: A video presentation from bench to reperfusion: Korean J Hepatobiliary Pancreat Surg, 2016; 20; 97-101
12. Moon DB, Hwang S, Ahn CS, Living donor liver transplantation-associated retransplantation in adult patients: Ann Liver Transplant, 2021; 1; 48-57
13. Hwang S, Bae JH, Kim IO, Hong JJ, Current vascular allograft procurement, cryopreservation and transplantation techniques in the Asan Medical Center Tissue Bank: Ann Liver Transplant, 2021; 1; 79-85
14. Jung DH, Hwang S, Bae JH, Kim IO, Usability of cryopreserved homologous great saphenous vein for hepatobiliary-pancreatic surgery and living donor liver transplantation: Ann Liver Transplant, 2022; 2; 48-55
Figures
 Figure 1. Institutional guidelines for reconstructing 1 or multiple major short hepatic veins (SHVs) with the right hepatic vein (RHV) and middle hepatic vein (MHV). V8 and IVC indicate the segment VIII hepatic vein and the inferior vena cava, respectively.
Figure 1. Institutional guidelines for reconstructing 1 or multiple major short hepatic veins (SHVs) with the right hepatic vein (RHV) and middle hepatic vein (MHV). V8 and IVC indicate the segment VIII hepatic vein and the inferior vena cava, respectively. Figure 2. Intraoperative photographs showing all-in-one sleeve venoplasty. (A) A right liver graft has 1 right hepatic vein opening, 2 inferior right hepatic vein openings, and 1 middle hepatic vein openings. (B) The orthodox and inferior right hepatic vein openings are unified with an aorta homograft patch. (C) An iliac artery homograft conduit is connected between the segment V vein and the aorta patch. (D) The aorta patch is anastomosed to the recipient’s inferior vena cava in a side-to-side fashion.
Figure 2. Intraoperative photographs showing all-in-one sleeve venoplasty. (A) A right liver graft has 1 right hepatic vein opening, 2 inferior right hepatic vein openings, and 1 middle hepatic vein openings. (B) The orthodox and inferior right hepatic vein openings are unified with an aorta homograft patch. (C) An iliac artery homograft conduit is connected between the segment V vein and the aorta patch. (D) The aorta patch is anastomosed to the recipient’s inferior vena cava in a side-to-side fashion. Figure 3. Configurations of all-in-one sleeve venoplasty. (A) A right liver graft (RLG) has 1 right hepatic vein (RHV) opening, 1 adjacent inferior right hepatic vein (IRHV) opening, 1 segment V vein (V5) opening, and 1 segment VIII vein (V8) opening. The RHV and IRHV openings were directly unified. An iliac vein conduit was attached to the V5. V8 was located close to the RHV opening, so they were unified. A semicircular fence with the autologous greater saphenous vein patch was attached (type 1). (B) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An iliac artery conduit was attached to the V5 and V8, and then unified with the RHV opening. Arterial patches were attached to the IRHV (type 1). (C) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An abdominal aorta was attached to the RHV and IRHV. An iliac vein conduit was attached to the V5 and V8, and then connected to the aorta graft (type 1). (D) An RLG has 1 RHV opening, 2 distant IRHV openings, and 1 V8 opening. They were unified with a large-sized common iliac vein patch (type 2).
Figure 3. Configurations of all-in-one sleeve venoplasty. (A) A right liver graft (RLG) has 1 right hepatic vein (RHV) opening, 1 adjacent inferior right hepatic vein (IRHV) opening, 1 segment V vein (V5) opening, and 1 segment VIII vein (V8) opening. The RHV and IRHV openings were directly unified. An iliac vein conduit was attached to the V5. V8 was located close to the RHV opening, so they were unified. A semicircular fence with the autologous greater saphenous vein patch was attached (type 1). (B) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An iliac artery conduit was attached to the V5 and V8, and then unified with the RHV opening. Arterial patches were attached to the IRHV (type 1). (C) An RLG has 1 RHV opening, 1 distant IRHV opening, 1 V5 opening, and 1 V8 opening. An abdominal aorta was attached to the RHV and IRHV. An iliac vein conduit was attached to the V5 and V8, and then connected to the aorta graft (type 1). (D) An RLG has 1 RHV opening, 2 distant IRHV openings, and 1 V8 opening. They were unified with a large-sized common iliac vein patch (type 2). Figure 4. Kaplan-Meier patency curves of the (A) short hepatic vein (SHV) and (B) middle hepatic vein (MHV).
Figure 4. Kaplan-Meier patency curves of the (A) short hepatic vein (SHV) and (B) middle hepatic vein (MHV). Tables
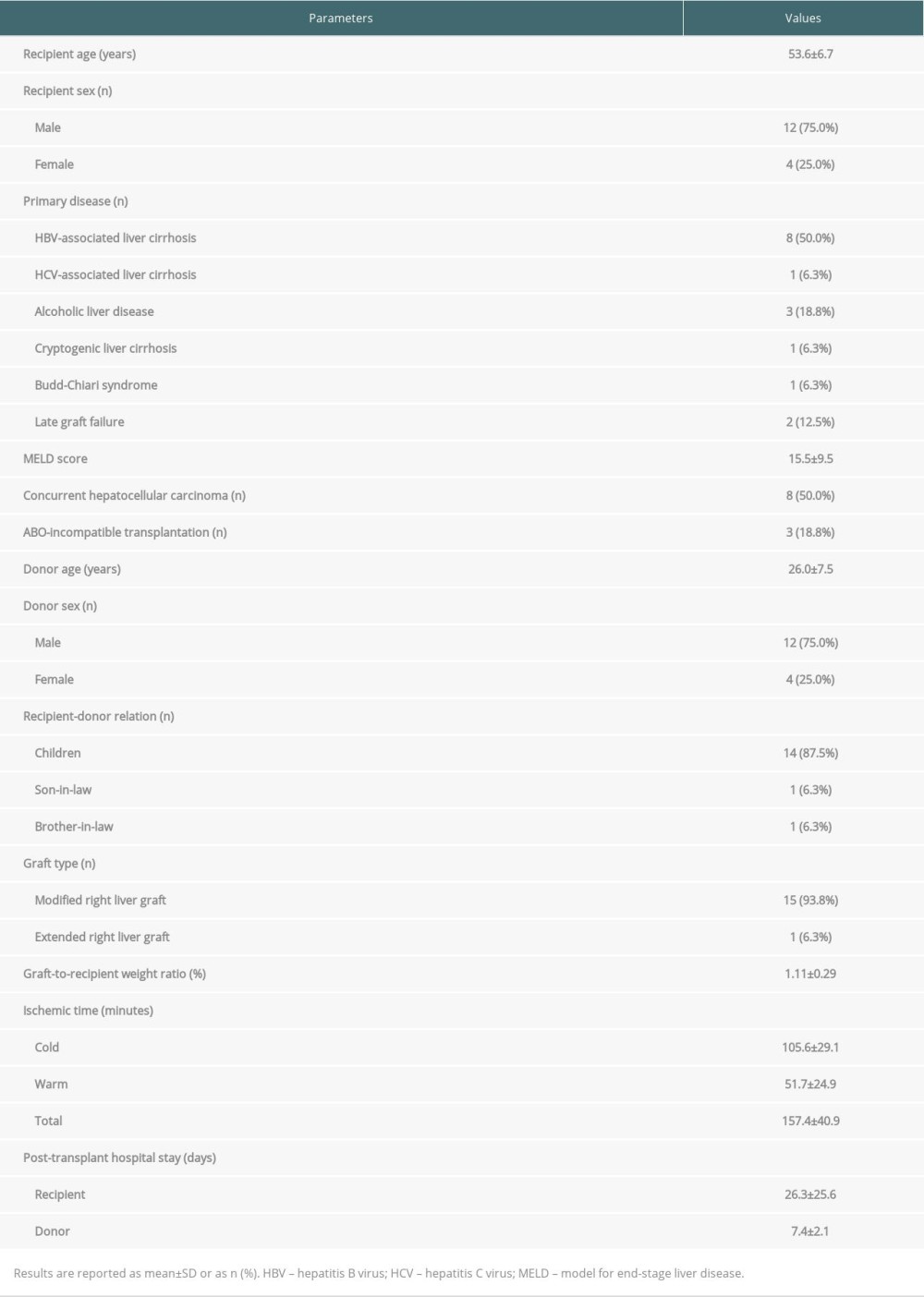 Table 1. Profiles of the 16 patients who underwent living donor liver transplantation using a right liver graft with outflow vein reconstruction through all-in-one sleeve venoplasty.
Table 1. Profiles of the 16 patients who underwent living donor liver transplantation using a right liver graft with outflow vein reconstruction through all-in-one sleeve venoplasty. Table 1. Profiles of the 16 patients who underwent living donor liver transplantation using a right liver graft with outflow vein reconstruction through all-in-one sleeve venoplasty.
Table 1. Profiles of the 16 patients who underwent living donor liver transplantation using a right liver graft with outflow vein reconstruction through all-in-one sleeve venoplasty. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








