20 November 2020: Review Paper
Infertility in Female and Male Solid Organ Recipients – From Diagnosis to Treatment: An Up-To-Date Review of the Literature
Iwona Szymusik1ABCDEF, Damian Warzecha1ABCDEFG*, Mirosław Wielgoś1DEG, Bronisława Pietrzak1ACDEFGDOI: 10.12659/AOT.923592
Ann Transplant 2020; 25:e923592
Abstract
ABSTRACT: Around 20% to 30% of patients after solid organ transplantation are of reproductive age. The estimated rate of infertility in this group is slightly higher than in the general population. Choosing the optimal moment for pregnancy in transplanted patients plays a pivotal role, regardless of the method of conception. The first part of this article presents an up-to-date review of the problem of infertility in female and male solid organ recipients, with special attention to the influence of specific immunosuppressive drugs on semen parameters. The second part discusses the current knowledge regarding infertility treatment and the results of assisted reproductive techniques in this specific group of patients. Despite restoring gonadal functions after transplantation, the patients should be informed about possible negative effects of medications on fertility, course of pregnancy, and the fetus. Interdisciplinary care should always be provided for infertile graft recipients, especially women, as it ensures safety both for the graft and for the potential gestation. The process of infertility diagnosis and tools used for that purpose are the same in transplanted patients as in the general population. The treatment with assisted reproductive techniques is acceptable and gives favorable results as long as patients are managed rationally, with special attention paid to prevention of iatrogenic complications.
Keywords: Hormones, Immunosuppression, Infertility, Kidney Transplantation, Semen, Fertility, Organ Transplantation, Pregnancy, Pregnancy Outcome, Reproductive Techniques, Assisted, transplant recipients
Background
According to the World Health Organization (WHO), infertility is diagnosed in couples who are not able to conceive within 1 year of regular intercourse without any kind of contraception [1]. However, there are some clinical conditions in which diagnostic and treatment methods may be introduced earlier (usually after 6 months), including woman’s age >35 years and chronic diseases with potentially detrimental effect on fertility. Women with chronic kidney or liver disease usually experience some kind of fertility disorders. The severity of the problem correlates with the stage of solid organ failure and results from the impairment of hypothalamus-pituitary-ovary axis functionality or other coexisting hormonal disturbances [2,3]. Apart from a higher rate of infertility, that specific group of patients also has a greater risk of various pregnancy complications, such as preterm delivery, gestational hypertension, anemia, or low birth weight.
Following a successful solid organ transplantation, the proper functionality of reproductive potential resolves after around 6 months [4]. Apart from ovulatory cycles, a significant improvement of sexual functions and libido are observed. Nevertheless, pregnancy planning should be deferred until a transplanted organ reaches stable functionality. The optimal time for conception, therefore, including any infertility treatment, is thought to be 18–24 months following successful transplantation. However, such a relatively long delay in treatment could be another problem in the group of patients older than 35 years, in whom fertility would be additionally impaired by age.
It is estimated that the rate of infertility among patients after solid organ transplantation (OTx) is slightly higher than in the general population [5]. For many women, motherhood is a priority; therefore, impaired conception may induce various psychosocial consequences, including depression or relationship problems. However, the available literature lacks recommendations dealing with infertility treatment in OTx patients. It seems obvious that the optimal time for conception should be determined individually, with regard to the initial condition of the patient and function of the transplanted organ. The situation is much more complicated in women receiving bone marrow transplantation (BMTx). The conditioning regimen administered prior to BMT is characterized by a highly gonadotoxic profile [6]. Secondary menstrual disorders and premature ovarian insufficiency are quite common symptoms in that specific group of patients. Nevertheless, there are few case reports of spontaneous resolution of menopausal symptoms and conception in BMTx women [7].
The number of living solid organ or bone marrow recipients has been constantly rising in recent years. Advances in treatment have resulted in life span extension and better prognosis; therefore, patients after OTx appear more frequently at gynecologists’ appointments. Women of reproductive age comprise about 20% of all transplanted females; therefore, specialists planning treatment of their primary disease should bear in mind their potential reproductive plans [8]. However, approximately 20% to 30% of men receiving a kidney transplant are below 50 years of age [9], and the choice of immunosuppressive drugs with the lowest possible teratogenic potential in patients planning to conceive is crucial. According to expert opinion, family planning counselling should be obligatory at the time of preparation for transplantation [10].
The Problem of Infertility in Female Graft Recipients
Any infertility treatment must be preceded with a thorough diagnostic workup, including both partners. Apart from a detailed medical history and physical examination, however, the management should always begin with the assessment of graft function because it has a direct influence on the reproductive system. Impaired function of kidneys or liver can lead to endocrinological disorders in the form of hypogonadotropic hypogonadism or hyperprolactinemia. These are a serious problem, especially during organ failure, but they regress after transplantation. In women undergoing kidney transplantation, the rate of ovulatory cycles is around 80% 1 year after the procedure if graft function is stable and creatinine concentration is below 1.4 mg/dl, similar to that of the general population. However, regular menstrual cycles with ovulation can occur as soon as 8 weeks after liver transplantation. After 7 months, up to 90% of women have regular cycles. Despite restoring gonadal functions after transplantation, the patients should be informed about possible negative effects of medications on the fetus and the course of pregnancy. Around 50–80% of kidney transplant recipients have hypertension [11]. All of the antihypertensive drugs cross the placenta. Several of them, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers should be withdrawn prior to conception due to possible teratogenic effects [12]. A recent report analyzed the influence of ACE inhibitors on the course of pregnancy and found significantly increased risk of major birth defects and spontaneous abortions compared to women without antihypertensive treatment [13].
The infertility workup in organ recipients is no different than in the general population. Nevertheless, the literature lacks epidemiological data regarding the distribution of particular disorders. The published material usually describes data based on case reports. The causes of fertility impairment in this specific group of patients appear to differ from those of the general population, among them ovarian (no ovulation), tubal (impaired patency), or male factor [14]. In around 25% of patients, a direct cause of infertility cannot be determined, which is called idiopathic infertility, and can be due to the imperfect diagnostic tools available in clinical practice. Nevertheless, the issue of so-called immunological factor of infertility is controversial in graft recipients. It is assumed that problems with conception are a result of improper reaction of the female immune system to paternal antigens. According to some theories, a high level of human leukocyte antigen (HLA) compatibility between partners can decrease pregnancy rates [15]. It is thought that immunological factor is largely responsible for idiopathic infertility. Immunomodulating and immunosuppressive therapies are sometimes used in such women to limit the improper reaction of their immune systems. Female graft recipients are continuously administered such therapies; therefore, immunological factor should be anticipated less frequently in them.
Lessan-Pezeshki et al. found that the rate of infertility among female kidney graft recipients was 10.4% [16]. They also assessed the contribution of various factors: 50% ovulatory disorders, 33% male factor, and 16.6% idiopathic. However, the described group of 126 kidney graft recipients included only 6 with fertility problems. The current literature lacks more precise data on transplanted patients. There is also insufficient information regarding hypothalamus-pituitary-ovary axis dysfunctions in women after lung, heart, or pancreas transplantation, and data are usually extrapolated from kidney recipients. The only difference is in patients with lungs transplanted due to cystic fibrosis, which can additionally impair fertility.
Diagnosing infertility problems in women should start with affirming ovulation by means of transvaginal ultrasound and/or progesterone concentration in the luteal phase of the cycle. Basic hormonal workup should also be similar to those performed in the general population, which includes thyroid gland function, prolactin, and gonadotropins concentrations in case of irregular menstrual cycles. It is suggested that 4–20% of female kidney recipients experience symptoms of premature ovarian insufficiency; therefore, the assessment of ovarian reserve is crucial. Age at transplantation and restoration of regular menstrual cycles after this procedure seem to be independent predictors of spontaneous pregnancy among renal graft recipients [17]. Mishra et al. found that menopause appeared on average 4.5 years earlier in graft recipients than in the general population [14]. The causes of the above are not well explained apart from situations in which cyclophosphamide, a highly gonadotoxic drug, was administered. Immunosuppressive therapy with glucocorticoids, azathioprine, and calcineurin inhibitors does not influence female fertility. However, anovulatory cycles were described in women treated with sirolimus, which can increase the concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) [18]. Because of the high risk of teratogenicity and pregnancy failure, mycophenolate mofetil (MMF) is contraindicated during pregnancy and should be discontinued if unexpected pregnancy occurs [19]. Moreover, its administration should be completed at least 6 weeks before planned pregnancy.
It is obvious that a male partner should be diagnosed in parallel with the woman. Further management and treatment options depend on semen results. Frequently, male factor is the only proven cause of infertility in couples. If the results of a spermiogram do not disqualify natural conception, verifying tubal patency should be the next step.
It is worth remembering that infertility management should include psychological counselling. Psychological research shows that female graft recipients report lower quality of life and decreased vitality in comparison to the general population. They also have more risk factors for postpartum depression. Therefore, psychological care should be an integral part of infertility treatment [20].
The Problem of Infertility in Male Graft Recipients
Prolonged survival of solid organ graft recipients makes fatherhood more likely in male patients. For example, uremia in end-stage kidney disease results in decreased testosterone and increased FSH and LH concentrations. Apart from an underlying disease, these patients usually have multiple comorbidities, such as diabetes mellitus, cardiovascular diseases, or hypertension, which frequently lead to poor sperm quality and impaired fertility [21,22]. Several immunosuppressants (e.g., glucocorticoids and calcineurin inhibitors) can lead to hypertension due to salt retention and vasoconstriction [11]. Successful organ transplantation usually restores most of these impairments. Moreover, recent data published by Reinhardt et al. showed a 50% rate of recovery from hypogonadism within 1 year following renal transplantation [23]. Nevertheless, recipients may be at increased risk of hypogonadism or erectile dysfunction [24]. In contrast to adults, semen quality does not improve after renal transplantation in patients subjected to uremia during adolescence, probably due to severe germ cell failure during the onset of spermatogenesis at puberty [25]. The most significant decrease was observed among renal failure patients 13 to 18 years of age. Immunosuppressive drugs, together with additional medications (for example antihypertensive agents, especially β-blockers), can affect fertility either by diminished functioning of the hypothalamic-pituitary-gonadal axis or by non-hormonal mechanisms [26]. Some male transplant recipients have altered testosterone and sperm production [27]. There is a dose-dependent correlation between immunosuppressants and plasma testosterone or gonadotropins concentrations [28]. Graft recipients usually require multidrug immunosuppression; therefore, it is difficult to assess the effect of a particular medication on fertility. Finally, these drugs can lead to decreased libido, sexual dysfunctions, and impaired spermatogenesis caused by germ cell toxicity.
The evaluation and management of male factor infertility after organ transplantation also does not differ from the general population. A detailed medical history and physical examination should always be the first step in clinical practice. Medical data should include the current and previous immunosuppressive therapy, as well as the duration and dosage of drug exposure. The physical examination should focus on findings suggestive of androgen deficiency. Obviously, it should also include the identification of reversible causes of possibly impaired fertility, such as clinically significant varicocele.
Semen analysis is the basic diagnostic tool of male factor infertility. According to the World Health Organization (WHO) manual, it should be performed after 2–7 days of sexual abstinence (preferably 3–5 days) [29]. In case of abnormal semen parameters, a repeated analysis is recommended due to existing variability in sperm concentrations and motility.
Male solid organ recipients might experience the detrimental effect of various immunosuppressive agents on their spermatogenesis. Some of these effects are presented below.
Corticosteroids
Steroids remain the main immunosuppressive therapy, and it is not contraindicated among patients who want to conceive. Glucocorticoids act via nuclear receptors located in different tissues. Possible negative influences of these drugs on reproductive functions result from downregulation of the hypothalamus-pituitary axis. This crosstalk leads to decreased synthesis and release of gonadotropins. Apart from iatrogenic hypogonadism, steroids affect receptors in Leydig cells, reducing gonadal synthesis of testosterone [30]. A study on 52 male heart transplant patients showed a transient but significant decline in mean serum testosterone concentration within the first month following transplantation [31]. These levels remained slightly, but not significantly, below the baseline concentrations 3 months after transplantation (489±29
Azathioprine
Azathioprine (AZA) is allowed during pregnancy in females and seems not to influence male fertility. Azathioprine can inhibit the synthesis of testosterone; however, it does not affect sperm parameters [33]. There are few studies in the literature investigating this dependency. Moreover, prolonged therapy with AZA seems not to affect semen parameters, nor cause any changes in semen quality [34]. A study on 23 males with irritable bowel syndrome treated with AZA for at least 3 months did not show any changes in semen parameters at the end of the observation period. It is hypothesized that azathioprine has a mutagenic and teratogenic effect. In this view, cryopreservation should be offered prior to the initiation of treatment. However, Leroy et al. analyzed the course of pregnancies fathered by men treated with AZA and showed that the rates of miscarriages and birth defects were similar to those in the general population [28].
Calcineurin Inhibitors (Cyclosporine A and Tacrolimus)
Most previous research found that gonadal hormones are not affected by cyclosporine (CsA) administration [35,36]. Moreover, gradual restoration of hormonal profile and sperm quality (density, motility, viability, and morphology) was observed within 4 months of successful transplantation in males treated with CsA [37]. However, higher doses (>4 mg/kg/1 day) are known to compromise spermatozoa motility and morphology [38]. Sperm count and motility are inversely related to CsA concentration in blood, and return to preexisting values following drug cessation. Therapy with tacrolimus seems to be safer than CsA in recovering most semen parameters in kidney transplant recipients (especially motility and morphology of the sperm) [39]. However, a recent study showed that antiepileptic drugs can enhance the testicular toxicity of therapeutic doses of CsA or tacrolimus, inducing morphological changes of the testicular tissue, mesenchymal cells, and testosterone secretion [40]. Therefore, further studies are needed to evaluate the safety of calcineurin inhibitors. Until then, it is recommended to use minimal doses of calcineurin inhibitors to prevent acute transplant rejection in patients of reproductive age.
Cyclophosphamide
Observational studies have shown severe negative effects of cyclophosphamide on semen quality. Cyclophosphamide is an alkylating agent with high potential for DNA impairment. Rivkees and Crawford evaluated testicular function after cyclophosphamide therapy in patients with inflammatory kidney disease and found that 58% had gonadal dysfunction [41]. Observational studies indicated a severe negative effect of the drug on semen quality, and this effect was dose-related. High-dose cyclophosphamide therapy can result in long-term gonadal damage [42]. Recent studies suggest that short-term cyclophosphamide administration to treat childhood leukemia results in long-term impairment of Leydig cell function, but not fertility or semen quality [43]. In rodents, it is widely used for laboratory-induced testicular toxicity.
mTOR Inhibitors (Sirolimus, Everolimus)
All mTOR (mammalian target of rapamycin) inhibitors cause a dose-dependent decrease of testosterone [45,46]. Sirolimus affects the hypothalamic-pituitary-gonadal axis, lowering free testosterone levels and increasing the gonadotropic hormones. Furthermore, it impairs the improvement of gonadal function after renal transplantation. It is hypothesized that tubular atrophy and reduction of testicular weight observed in rats results from suppression of protein kinase activity of mTOR involved in germ cell proliferation, meiosis, and apoptosis [47].
Graft recipients treated with sirolimus may suffer from reversible testicular atrophy, resulting in poor semen quality [48]. Male kidney recipients treated with sirolimus throughout the post-transplant period had significantly reduced sperm parameters (decreased count, motility, and morphology of spermatozoa). Moreover, in this group, a 15 fold lower fathered pregnancy rate were observed (5.9 per 1000 patient years
Mycophenolate
MMF, one of the most commonly used immunosuppressive drugs in graft recipients, seems not to affect male fertility, or sperm DNA fragmentation, and does not contribute to a mutagenic effect [33]. A previous study evaluated the course of 205 gestations fathered by 152 male transplant recipients exposed to mycophenolic acid [51]. The outcomes of pregnancies appeared similar to those in the general population, including prematurity rates (10.8%), spontaneous miscarriages (6.8%), and malformations (3.1%). Paternal exposure to MMF does not increase the risk of negative birth outcomes [52]. However, due to the hypothesized teratogenic effect, it is advised to cease treatment at least 3 months prior to conception [33].
New Immunosuppressants
Rituximab, anakinra, and abatacept are regarded as relatively new immunosuppressants utilized in rheumatic diseases. There is insufficient data on the safety of these new molecules in the context of male infertility. Rituximab, an anti CD20 monoclonal antibody, is considered safe for paternal use, but there are few reports in the literature [53]. There is one case series of male patients exposed to anakinra prior to conception, with no adverse effects [54]. According to the available data, there is no increased risk of congenital malformations or miscarriage related to peri-conception paternal exposure to abatacept [55].
Infertility Treatment
Family planning and infertility treatment should be provided by an interdisciplinary team including a nephrologist/hepatologist, transplantology specialist, and gynecologist. The treatment efficacy depends on various factors apart from graft function, among which the age of the woman and her ovarian reserve are the most important. Reproductive potential decreases with age due to diminishing quality of oocytes and their genetic material. Nevertheless, 70% of women who conceived after liver transplantation had live births, although this rate was lower than that of women in the general population [56].
As mentioned earlier, according to the WHO, infertility can be diagnosed after 1 year of regular intercourses with no contraception. However, female graft recipients can be treated differently. There are cases in which 12 months seems a long time due to lower ovarian reserve, more advanced age, or optimal timing for conception because of graft function.
Infertility treatment methods in graft recipients are no different than in the general population. The choices should be based on a diagnosed problem, and include ovulation induction in ovarian factor or more advanced assisted reproductive techniques (ART), if applicable. Indications for intrauterine insemination (IUI) or
Clomiphene citrate, letrozole, and gonadotropins are used to induce/stimulate ovulation. The available literature lacks information regarding the influence of these drugs on graft function. Experience in clinical practice suggests they do not interfere. However, clinicians should bear in mind the primary disease that caused organ failure in a patient – is it hereditary? does it favor thrombosis? Ovarian stimulation with gonadotropins induces higher estradiol concentrations, thus promoting clotting. Letrozole may be the preferred agent for ovulation induction in transplanted patients, as it more often stimulates a single follicle to grow, which results in fewer multiple gestations. Lack of registration of letrozole for this particular indication is not an obstacle, because evidence-based medicine and recommendations of societies prove its efficacy and safety [57].
Intrauterine inseminations are simple ART procedures. The literature once again lacks data regarding IUI use in female graft recipients. It therefore seems reasonable to choose IUI based on general indications and to expect comparable results. When IUIs are not effective, the patients should be qualified for more advanced and efficient procedures, such as IVF.
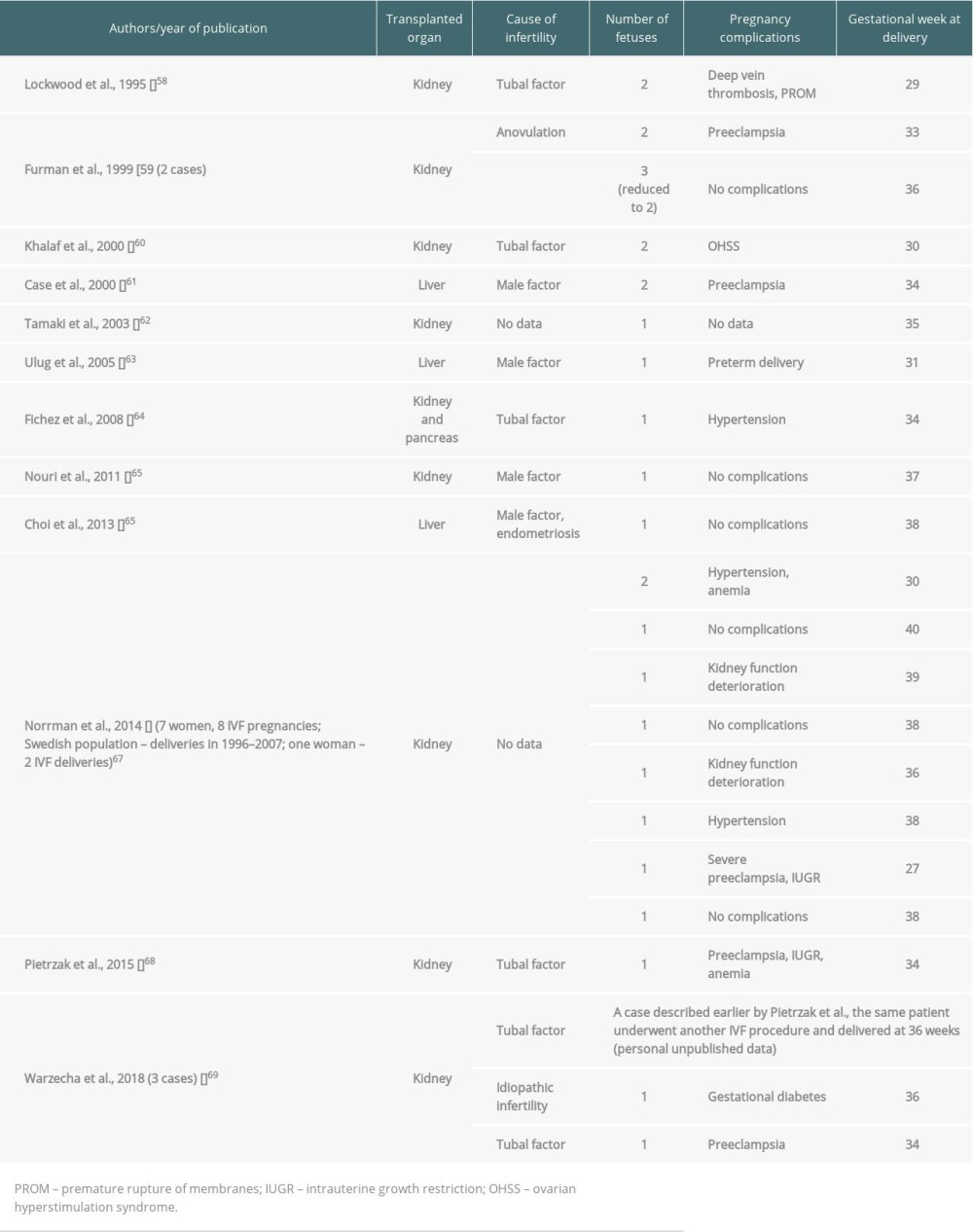
The first successful IVF in a kidney graft recipient was described by Lockwood et al. in 1995. It is difficult to assess how many women with transplanted organs were treated with
The application of ART, especially IVF, in graft recipients may raise controversies, as the medical situation is not typical, having an additional ethical issue. Nevertheless, such patients cannot be refused the right of parenthood, knowing that the proper interdisciplinary counselling and care may enable conception at an optimal moment.
One of the advantages of ART is that it provides the ability to become a parent, which is of great importance for the quality of life of transplanted patients. Moreover, such gestation would always be planned and therefore safer. Nevertheless, the complications of any ART therapy have to be analyzed individually and discussed with patients prior to procedures. The aforementioned complications (such as OHSS) may negatively affect graft function. In addition, infertility treatment favors inheritance of genetic causes of organ failure. Moreover, according to the literature, both IVF and organ transplantation are independent risk factors of prematurity. In case of IVF, the above is true only with regard to fresh embryo transfers. It therefore seems optimal to use milder stimulation and cycle segmentation (plan deferred embryo transfers after cryopreservation). Elective single-embryo transfer should also be the standard of choice in transplanted patients, as it almost eliminates the problem of multiples [70].
Conclusions
In conclusion, interdisciplinary care should always be provided for infertile graft recipients, especially women, as it ensures safety for the graft and for the potential gestation. Choosing the optimal moment for pregnancy plays a pivotal role in this population, regardless of the method of conception (spontaneous or as a result of ART). The process of infertility diagnosis and tools used for that purpose are the same in transplanted patients as in the general population. The treatment with assisted reproductive techniques is acceptable and gives favorable results, as long as patients are managed rationally, with special attention to prevent iatrogenic complications.
References
1. Practice Committee of American Society for Reproductive Medicine, Definitions of infertility and recurrent pregnancy loss: A committee opinion: Fertil Steril, 2013; 99(1); 63
2. Ahmed SB, Ramesh S, Sex hormones in women with kidney disease: Nephrol Dial Transplant, 2016; 31(11); 1787-95
3. Anantharaman P, Schmidt RJ, Sexual function in chronic kidney disease: Adv Chronic Kidney Dis, 2007; 14(2); 119-25
4. Delesalle AS, Robin G, Provôt FImpact of end-stage renal disease and kidney transplantation on the reproductive system: Gynecol Obstet Fertil, 2015; 43(1); 33-40 [in French]
5. Ghazizadeh S, Lessan-Pezeshki M, Khatami MR, Infertility among female renal transplant recipients: Saudi J Kidney Dis Transpl, 2007; 18(3); 387-90
6. Deeg HJ, Delayed complications and long-term effects after bone marrow transplantation: Hematol Oncol Clin North Am, 1990; 4(3); 641-57
7. Hershlag A, Schuster MW, Return of fertility after autologous stem cell transplantation: Fertil Steril, 2002; 77(2); 419-21
8. Deshpande NA, James NT, Kucirka LM, Pregnancy outcomes in kidney transplant recipients: A systematic review and meta-analysis: Am J Transplant, 2011; 11(11); 2388-404
9. Eckersten D, Giwercman A, Pihlsgård M, Impact of kidney transplantation on reproductive hormone levels in males: A longitudinal study: Nephron, 2018; 138(3); 192-201
10. Benagiano G, Brosens I, The multidisciplinary approach: Best Pract Res Clin Obstet Gynaecol, 2014; 28(8); 1114-22
11. Kasiske BL, Anjum S, Shah R, Hypertension after kidney transplantation: Am J Kidney Dis, 2004; 43(6); 1071-81
12. Cooper WO, Hernandez-Diaz S, Arbogast PG, Major congenital malformations after first-trimester exposure to ACE inhibitors: N Engl J Med, 2006; 354(23); 2443-51
13. Hoeltzenbein M, Tissen-Diabaté T, Fietz AK, Increased rate of birth defects after first trimester use of angiotensin converting enzyme inhibitors – treatment or hypertension related? An observational cohort study: Pregnancy Hypertens, 2018; 13; 65-71
14. Mishra VV, Nanda SS, Mistry K, An overview on fertility outcome in renal transplant recipients: J Obstet Gynaecol India, 2016; 66(Suppl 1); 330-34
15. Ober C, Hyslop T, Elias S, Human leukocyte antigen matching and fetal loss: Results of a 10 year prospective study: Hum Reprod, 1998; 13(1); 33-38
16. Lessan-Pezeshki M, Ghazizadeh S, Khatami MR, Fertility and contraceptive issues after kidney transplantation in women: Transplant Proc, 2004; 36(5); 1405-6
17. Yaprak M, Doğru V, Sanhal CY, Fertility outcome after renal transplantation: A single-center experience: Transplant Proc, 2019; 51(4); 1108-11
18. Boobes Y, Bernieh B, Saadi H, Gonadal dysfunction and infertility in kidney transplant patients receiving sirolimus: Int Urol Nephrol, 2010; 42(2); 493-98
19. Sifontis NM, Coscia LA, Constantinescu S, Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus: Transplantation, 2006; 82(12); 1698-702
20. Olbrisch ME, Benedict SM, Ashe K, Levenson JL, Psychological assessment and care of organ transplant patients: J Consult Clin Psychol, 2002; 70(3); 771-83
21. Pergialiotis V, Prodromidou A, Frountzas M, Diabetes mellitus and functional sperm characteristics: A meta-analysis of observational studies: J Diabetes Complications, 2016; 30(6); 1167-76
22. Xu L, Xu H, Zhu X, Effect of uremia on semen quality and reproductive function in humans: Cell Biochem Biophys, 2012; 62(1); 29-33
23. Reinhardt W, Kübber H, Dolff S, Rapid recovery of hypogonadism in male patients with end stage renal disease after renal transplantation: Endocrine, 2018; 60(1); 159-66
24. Prem AR, Punekar SV, Kalpana M, Male reproductive function in uraemia: Efficacy of haemodialysis and renal transplantation: Br J Urol, 1996; 78(4); 635-38
25. Inci K, Duzova A, Aki FT, Semen variables and hormone profiles after kidney transplantation during adolescence: Transplant Proc, 2006; 38(2); 541-42
26. Samplaski MK, Nangia AK, Adverse effects of common medications on male fertility: Nat Rev Urol, 2015; 12(7); 401-13
27. Drobnis EZ, Nangia AK, Immunosuppressants and male reproduction: Adv Exp Med Biol, 2017; 1034; 179-210
28. Leroy C, Rigot JM, Leroy M, Immunosuppressive drugs and fertility: Orphanet J Rare Dis, 2015; 10; 136
29. Cooper TG, Noonan E, von Eckardstein S, World Health Organization reference values for human semen characteristics: Hum Reprod Update, 2010; 16(3); 231-45
30. Brandenburg VM, Ketteler M, Heussen N, Lumbar bone mineral density in very long-term renal transplant recipients: impact of circulating sex hormones: Osteoporos Int, 2005; 16(12); 1611-20
31. Shane E, Rivas M, McMahon DJ, Bone loss and turnover after cardiac transplantation: J Clin Endocrinol Metab, 1997; 82(5); 1497-506
32. Mack-Shipman LR, Ratanasuwan T, Leone JP, Reproductive hormones after pancreas transplantation: Transplantation, 2000; 70(8); 1180-83
33. Semet M, Paci M, Saïas-Magnan J, The impact of drugs on male fertility: A review: Andrology, 2017; 5(4); 640-63
34. Dejaco C, Mittermaier C, Reinisch W, Azathioprine treatment and male fertility in inflammatory bowel disease: Gastroenterology, 2001; 121(5); 1048-53
35. Georgiou GK, Dounousi E, Harissis HV, Calcineurin inhibitors and male fertility after renal transplantation – a review: Andrologia, 2016; 48(5); 483-90
36. Fleischer J, McMahon DJ, Hembree W, Serum testosterone levels after cardiac transplantation: Transplantation, 2008; 85(6); 834-39
37. Wang GC, Zheng JH, Xu LG, Measurements of serum pituitary-gonadal hormones and investigation of sexual and reproductive functions in kidney transplant recipients: Int J Nephrol, 2010; 2010 612126
38. Xu LG, Zhu XF, Jin LMImpregnate occasion for male renal transplant recipients: Zhonghua Nan Ke Xue, 2008; 14(5); 448-50 [in Chinese]
39. Cao ZG, Liu JH, Zhu YPEffects of different immunodepressants on the sperm parameters of kidney transplant recipients: Zhonghua Nan Ke Xue, 2006; 12(5); 405-7 [in Chinese]
40. Lin Y, Zhang J, Lei W, Diltiazem aggravates testicular function impairment induced by cyclosporine A or tacrolimus in unilateral nephrectomised rats: Andrologia, 2019; 51(5); e13251
41. Rivkees SA, Crawford JD, The relationship of gonadal activity and chemotherapy-induced gonadal damage: JAMA, 1988; 259(14); 2123-25
42. Kenney LB, Laufer MR, Grant FD, High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood: Cancer, 2001; 91(3); 613-21
43. Jahnukainen K, Heikkinen R, Henriksson M, Semen quality and fertility in adult long-term survivors of childhood acute lymphoblastic leukemia: Fertil Steril, 2011; 96(4); 837-42
44. Onaolapo AY, Oladipo BP, Onaolapo OJ, Cyclophosphamide-induced male subfertility in mice: An assessment of the potential benefits of Maca supplement: Andrologia, 2018; 50(3); 12911
45. Huyghe E, Zairi A, Nohra J, Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: An overview: Transpl Int, 2007; 20(4); 305-11
46. Kaczmarek I, Groetzner J, Adamidis I, Sirolimus impairs gonadal function in heart transplant recipients: Am J Transplant, 2004; 4(7); 1084-88
47. Tondolo V, Citterio F, Panocchia N, Gonadal function and immunosuppressive therapy after renal transplantation: Transplant Proc, 2005; 37(4); 1915-17
48. Deutsch MA, Kaczmarek I, Huber S, Sirolimus-associated infertility: Case report and literature review of possible mechanisms [published correction appears in Am J Transplant, 2008; 8(2): 472–76]: Am J Transplant, 2007; 7(10); 2414-21
49. Zuber J, Anglicheau D, Elie C, Sirolimus may reduce fertility in male renal transplant recipients: Am J Transplant, 2008; 8(7); 1471-79
50. Wald K, Cakmak H, Noel M: J Assist Reprod Genet, 2019; 36(5); 947-50
51. Jones A, Clary MJ, McDermott E, Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products: Prog Transplant, 2013; 23(2); 153-57
52. Midtvedt K, Bergan S, Reisæter AV, Exposure to mycophenolate and fatherhood: Transplantation, 2017; 101(7); e214-17
53. Mouyis M, Flint JD, Giles IP, Safety of anti-rheumatic drugs in men trying to conceive: A systematic review and analysis of published evidence: Semin Arthritis Rheum, 2019; 48(5); 911-20
54. Youngstein T, Hoffmann P, Gül A, International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors: Rheumatology (Oxford), 2017; 56(12); 2102-8
55. Kumar M, Ray L, Vemuri S, Simon TA, Pregnancy outcomes following exposure to abatacept during pregnancy: Semin Arthritis Rheum, 2015; 45(3); 351-56
56. Kamarajah SK, Arntdz K, Bundred J, Outcomes of pregnancy in recipients of liver transplants: Clin Gastroenterol Hepatol, 2019; 17(7); 1398-404.e1
57. Legro RS, Brzyski RG, Diamond MP, Letrozole versus clomiphene for infertility in the polycystic ovary syndrome [published correction appears in N Engl J Med, 2014; 317(15): 1465]: N Engl J Med, 2014; 371(2); 119-29
58. Lockwood GM, Ledger WL, Barlow DH: Hum Reprod, 1995; 10(6); 1528-30
59. Furman B, Wiznitzer A, Hackmon R, Multiple pregnancies in women after renal transplantation. Case report that rises a management dilemma: Eur J Obstet Gynecol Reprod Biol, 1999; 84(1); 107-10
60. Khalaf Y, Elkington N, Anderson H, Ovarian hyperstimulation syndrome and its effect on renal function in a renal transplant patient undergoing IVF treatment: Case report: Hum Reprod, 2000; 15(6); 1275-77
61. Case AM, Weissman A, Sermer M, Greenblatt EM: Hum Reprod, 2000; 15(3); 626-28
62. Tamaki M, Ami M, Kimata N: Transplantation, 2003; 75(7); 1082-83
63. Ulug U, Mesut A, Jozwiak EA, Bahceci M, Successful pregnancy in a liver transplant recipient following controlled ovarian hyperstimulation and intracytoplasmic sperm injection: J Assist Reprod Genet, 2005; 22(7–8); 311-13
64. Fichez A, Labrousse C, Fromajoux C: Fertil Steril, 2008; 90(3); 849.e1-3
65. Nouri K, Bader Y, Helmy S: J Assist Reprod Genet, 2011; 28(4); 351-53
66. Choi JM, Mahany EB, Sauer MV: Reprod Med Biol, 2012; 12(2); 69-70
67. Norrman E, Bergh C, Wennerholm UB: Hum Reprod, 2015; 30(1); 205-13
68. Pietrzak B, Mazanowska N, Kociszewska-Najman B: Ann Transplant, 2015; 20; 338-41
69. Warzecha D, Szymusik I, Grzechocińska B: Transplant Proc, 2018; 50(6); 1892-95
70. Douglas NC, Shah M, Sauer MV, Fertility and reproductive disorders in female solid organ transplant recipients [published correction appears in Semin Perinatol, 2008; 32(1): 67]: Semin Perinatol, 2007; 31(6); 332-38
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860