11 December 2020: Original Paper
Outcomes of Non-Cryopreserved Versus Cryopreserved Peripheral Blood Stem Cells for Autologous Stem Cell Transplantation in Multiple Myeloma
Pokpong Piriyakhuntorn1ABCDE*, Adisak Tantiworawit1AE, Thanawat Rattanathammethee1E, Sasinee Hantrakool1E, Chatree Chai-Adisaksopha1E, Ekarat Rattarittamrong1E, Lalita Norasetthada1EDOI: 10.12659/AOT.927084
Ann Transplant 2020; 25:e927084
Abstract
BACKGROUND: Autologous stem cell transplantation (ASCT) has become a standard procedure in multiple myeloma (MM) patients. Cryopreservation (CRYO) of stem cells may be associated with adverse reactions of dimethyl sulfoxide. Previous studies showed that stem cell storage at 4°C (non-cryopreserved [NC] method) may have some advantages. This analysis focused on comparing the transplant-related outcomes of the 2 preservation methods.
MATERIAL AND METHODS: This was a cohort study of consecutive MM patients who underwent ASCT at Chiang Mai University from 2014 to 2019. Primary outcomes were time to neutrophil and platelet engraftment. Key secondary outcomes were the incidence of infusion reactions, duration of hospitalization, cost, and survival.
RESULTS: A total of 42 MM patients underwent ASCT. Of these, 26 patients and 16 patients underwent NC and CRYO stem cell collections, respectively. There was no difference in time to neutrophil engraftment (median 12 vs. 10.5 days, P=0.203) or platelet engraftment (median 14 vs. 12 days, P=0.809) between groups. The incidence of infusion reactions and duration of hospitalization were similar in both groups. The average cost of ASCT was 10% lower in the NC group. There was no difference in progression-free survival (median 16 vs. 22 months, P=0.701) or overall survival between NC and CRYO groups.
CONCLUSIONS: ASCT in MM using the NC preservation method is effective and safe compared to the CRYO method in both short-term and survival outcomes.
Keywords: Cryopreservation, Multiple Myeloma, Transplantation, Autologous, Peripheral Blood Stem Cell Transplantation, peripheral blood stem cells
Background
Autologous stem cell transplantation (ASCT) is beneficial and has become the current standard procedure in young multiple myeloma (MM) patients who responded to treatment [1]. Cryopreservation (CRYO) of stem cells using dimethyl sulfoxide (DMSO) is a conventional standard method but can be associated with adverse reactions of DMSO and relatively higher cost of the procedure [2]. The non-cryopreserved (NC) method is less expensive and less time consuming since stem cell collection can be performed before transplantation in the same admission. An
Material and Methods
STUDY DESIGN AND PARTICIPANTS:
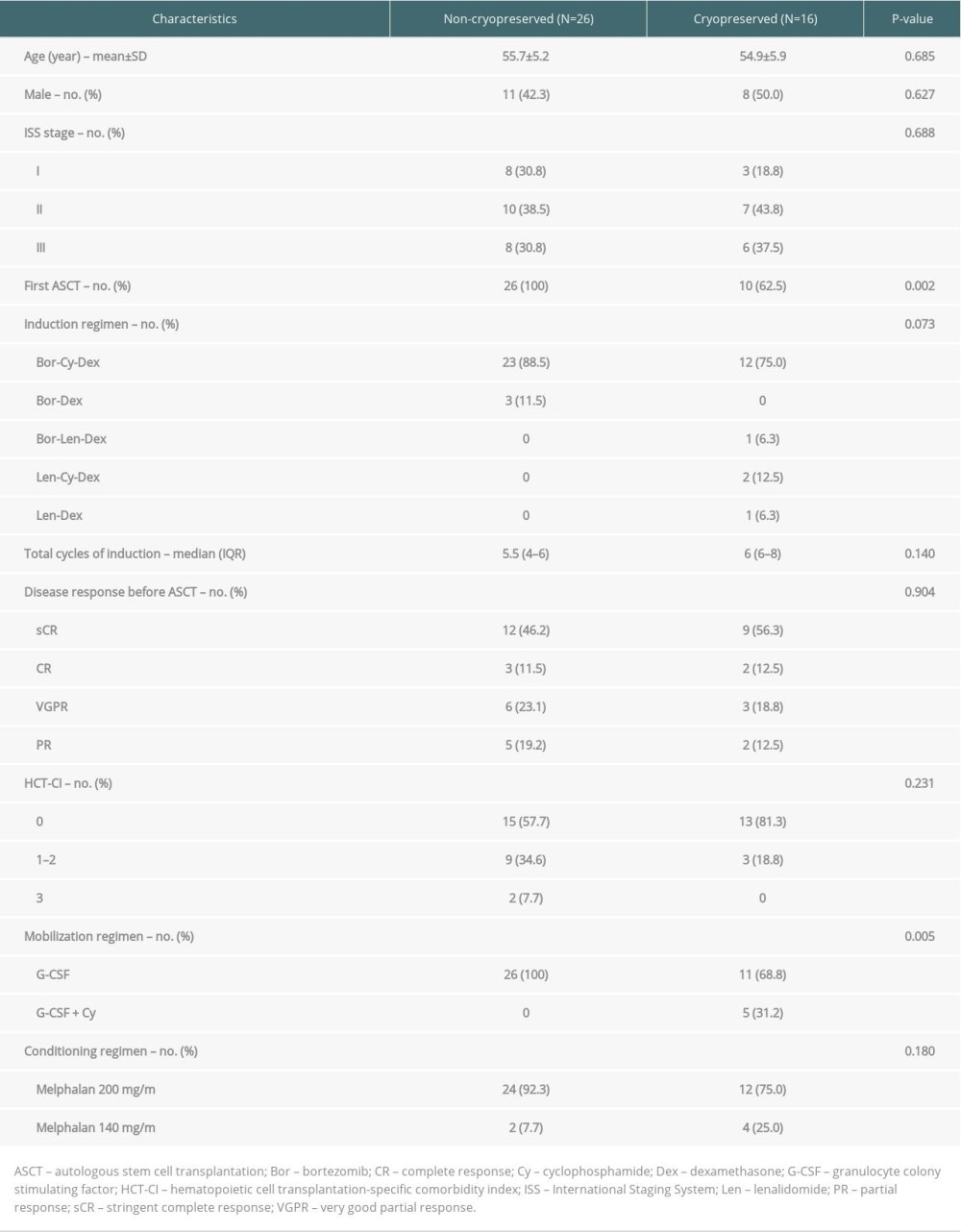
This was a retrospective cohort study. Consecutive MM patients who underwent ASCT from March 2014 to October 2019 at the Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University were enrolled. Collected data were age, sex, disease stage according to the International Staging System (ISS) [9], first or second ASCT, time from diagnosis or relapse to ASCT, disease response before ASCT according to the International Myeloma Working Group (IMWG) consensus criteria [10], hematopoietic cell transplantation-specific comorbidity index (HCT-CI) [11], induction regimen, the dose of melphalan, mobilization regimen, number of CD34+ cells collected, and stem cells preservation methods (CRYO or NC).
STEM CELL MOBILIZATION, PROCESSING, AND TRANSPLANTATION PROTOCOL:
All newly diagnosed transplant-eligible MM patients received bortezomib (Bor)-containing regimen induction of at least 4 cycles. Patients with the relapsed disease received induction regimen by physicians’ decisions based on previous treatments and duration of response [12]. Patients who achieved up to partial response (PR) or more proceeded to stem cell collection using granulocyte colony-stimulating factor (G-CSF) mobilization alone, or in combination with cyclophosphamide (Cy) depending on the attending physicians’ decision. For the NC method, patients received subcutaneous G-CSF 10 μg/kg/d (rounded up to 600 or 900 μg) from day −8 to day −4. Peripheral blood stem cell collection was performed on day −3. Conditioning regimen with intravenous melphalan 200 mg/m2 fractionally was given on day −2 and −1, or 140 mg/m2 on day −1, according to kidney function. Stem cells were infused on day 0. All patients received premedication with chlorpheniramine, hydrocortisone, and acetaminophen. G-CSF 300 μg/d was given on day +1 until the engraftment of neutrophils. For patients receiving the CRYO method, stem cell collection was performed in 2 separate admissions. Patients underwent similar mobilization protocol, conditioning regimen, and G-CSF after the infusion of stem cells. In G-CSF+Cy mobilization, intravenous Cy 2.5 g/m2 was given, then G-CSF 10 μg/kg/d was given for 10–11 days before apheresis. All patients received prophylactic antiviral and antifungal agents. Antibacterial prophylaxis was not given during transplantation.
The peripheral blood CD34+ cell count was measured by flow cytometry on the morning of the planned apheresis day. The acceptable CD34+ cell count was at least 5×109/L. Spectra Optia® Apheresis System (Terumo Corporation, Tokyo, Japan) was used for apheresis procedures. Patients with a total of CD34+ cells harvested of fewer than 2.0×106 cells/kg underwent another apheresis session on the following day. All patients achieved the target total CD34+ cells without using plerixafor. In the NC method, stem cell bags were stored in a stem cell laboratory refrigerator at 4oC. In the CRYO method, cryopreservation was prepared by adding DMSO to the final concentration at 10% before being cooled in a controlled-rate freezer (CryoMed™, Thermo Fisher Scientific, Inc.), then stored in liquid nitrogen storage at −190°C. The bags were thawed in a 37°C water bath just before the infusion procedure. Trypan blue exclusion test was used for cell viability analysis on the day of infusion [13].
OUTCOMES:
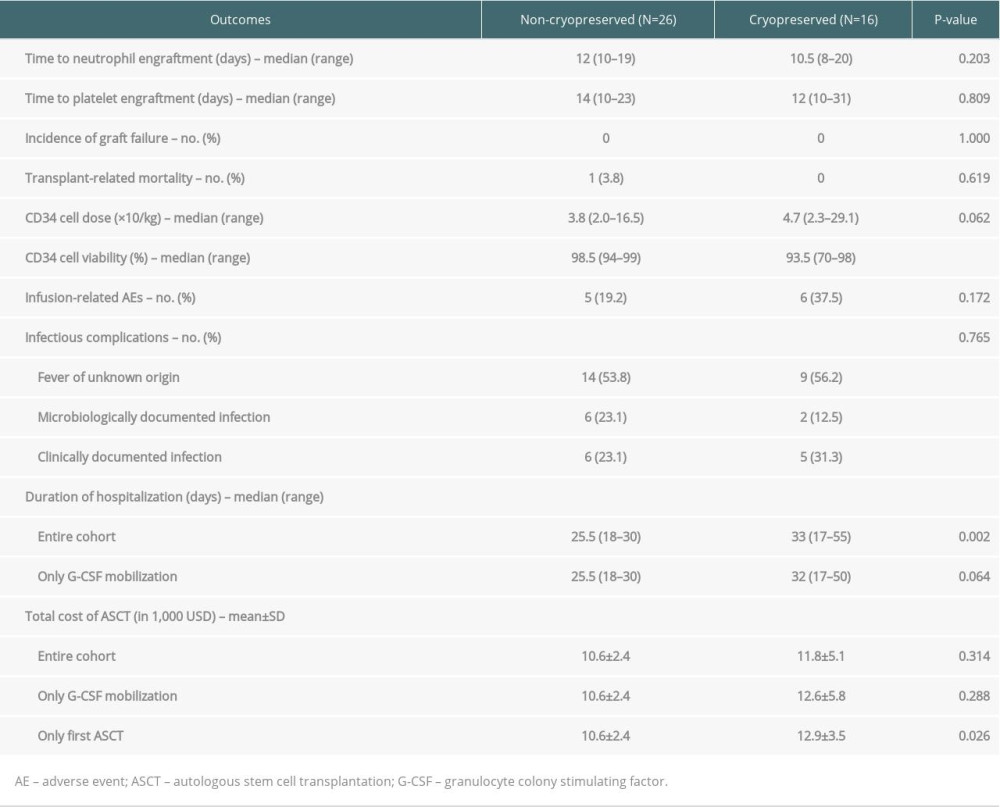
Primary outcomes were time to neutrophil engraftment and time to platelet engraftment, defined as the time from the day of stem cell infusion to the first of 3 consecutive days with absolute neutrophil counts more than 0.5×109/L or platelet counts more than 20×109/L without transfusion, respectively [14,15]. Key secondary outcomes were cell viability, graft failure rate, infusion-related reactions, the incidence of hematological and non-hematological complications according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) v4.0, early transplant-related mortality (TRM), duration of hospital stay, and total cost of ASCT in United States dollars (USD). Graft failure was defined as either failure to have neutrophil engraftment by day +28 of transplantation (primary graft failure), or loss of initial neutrophil engraftment not related to relapse, infection, or toxicity (secondary graft failure) [16]. The duration of hospitalization was the total number of days that the patient was admitted to the hospital for stem cell mobilization, collection, and ASCT. Early TRM was defined as death due to any transplantation-related cause on the first 30 days of ASCT. Other secondary outcomes included disease response at day +100 after ASCT, progression-free survival (PFS), and overall survival (OS).
STATISTICAL ANALYSIS:
Data were compared between the NC group and CRYO group using Fisher’s exact test for categorical variables, and the independent
The study was conducted with approval from the Institutional Research Ethics Committee at the Faculty of Medicine, Chiang Mai University (reference no. 351/2019).
Results
BASELINE CHARACTERISTICS:
Forty-two ASCT were included, with 26 (61.9%) utilizing the NC method. Clinical characteristics are shown in Table 1. There were no significant differences between groups in demographic data and disease characteristics. Of 16 ASCT in CRYO group, 6 (37.5%) were second-time ASCT after disease relapsed and 5 (31.2%) used G-CSF+Cy chemomobilization. Thirteen NC and 9 CRYO ASCT were administered from 2014–2016, and 13 NC and 7 CRYO were administered after the beginning of 2017.
TRANSPLANTATION OUTCOMES:
The median total CD34+ cell dose of the entire cohort was 4.3×106/kg (range 2.0–29.1). The collected CD34+ in the NC group had significantly higher cell viability than the CRYO group (98.5% vs. 93.5%, P<0.001). However, infused doses of total CD34+ cells were not significantly different in both groups (3.8 vs. 4.7×106/kg, P=0.062). The median time to neutrophil engraftment of the entire cohort was 11 days (IQR 10–14). The median time to platelet engraftment was 13 days (IQR 11–18). The neutrophil and platelet engraftments were not significantly different between the 2 groups (12 vs. 10.5 days, P=0.203 and 14 vs. 12 days, P=0.809, respectively). One patient without a history of cardiac disease developed sudden cardiac arrest and died on day +12; this event was considered as TRM. The other 41 patients achieved engraftment without any secondary graft failure. Summarized key transplant-related outcomes are shown in Table 2. The NC method was associated with a shorter median duration of hospitalization (25.5 vs. 33 days, P=0.002). However, when excluding 5 patients using G-CSF+Cy mobilization, which required 10 days longer duration of hospitalization, the difference was not statistically significant (25.5 vs. 32 days, P=0.064). The NC group had a lower average cost, but the difference was not statistically significant (10 600±2400 vs. 11 800±5100 USD, P=0.314). We additionally performed cost comparison with excluding six-second ASCT in the CRYO group, which used existing stem cells from their first ASCT, and the difference in cost was more obvious (10 600±2400 vs. 12 900±3500 USD, P=0.026) (Table 2).
Infusion-related adverse events (IREs) were not statistically different among the 2 groups (Table 2). There were 13 IREs in a total of 11 patients. All IREs were considered Grade 1, including 3 nausea, 7 vomiting, 1 rash, and 2 chest pain, and all recovered after symptomatic treatment such as oxygenation, antiemetic, and antihistamine. All patients developed febrile neutropenia during ASCT, in which the majority (54.7%) had a fever of unknown origin (FUO). Pathogens were detected in 8 of 42 patients (19%). The incidence of Grade 3–4 adverse events was similar in the NC and CRYO groups (88.5% vs. 93.8%, P=0.505). Most Grade 3–4 adverse events were affected by diarrhea, which was, as expected, caused by the conditioning regimen. Engraftment syndrome occurred in 4 patients (2 (7.7%) in the NC group and 2 (12.5%) in the CRYO group, P=0.495), which responded well to short-course systemic corticosteroids.
Univariable Cox proportional hazards models revealed that time to neutrophil engraftment was not influenced by stem cell preservation method, age, sex, total CD34+ dose, melphalan dose, first or second ASCT, or mobilization regimen.
LONG-TERM OUTCOMES:
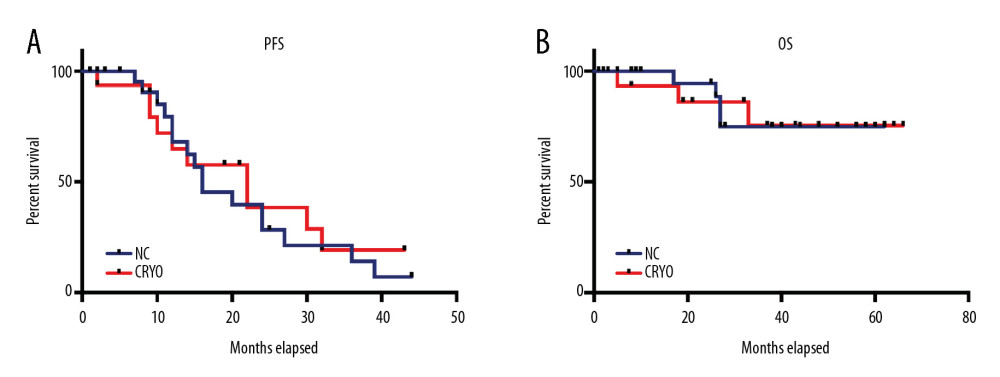
Disease response at the time of ASCT and after ASCT at day +100 were similar in both groups (data not shown). Consolidation and maintenance treatment was given to 6 patients in the NC group (4 received Bor-Cy-Dex then bortezomib maintenance, 2 received Bor-Dex then lenalidomide maintenance), and 5 patients in the CRYO group (all received Bor-Cy-Dex, then bortezomib maintenance) (26.1% vs. 33.3%, P=0.722). At the median follow-up time of 37.5 months, there was no difference in PFS between the 2 groups (median PFS 16 vs. 22 months, P=0.701 by log-rank test) (Figure 1) or overall survival (median OS not reached in both groups; P=0.942) (Figure 1).
Discussion
Previous data showed that adequate engraftment of ASCT was observed when using stem cell storage at 4°C in the refrigerator for 1 to 6 days [2,4–6,8,17–19]. In the present study, all patients in the NC group achieved engraftment before day +30. This ensures that NC is a feasible stem cell preservation method in MM.
Two recent studies revealed that the NC method led to faster engraftment compared with CRYO [2,8]. However, one study compared 2 strategies in 2 different centers [8], while another was a single-center study but had a different time between groups, with only 25% of the CRYO undergoing ASCT in the same period of the NC group [2]. Thus, the faster engraftment effect of the NC method remains to be further investigated. Our center has started an ASCT program since March 2014 and used both the NC and CRYO methods from the beginning. The primary results of our study showed a comparable engraftment duration between NC and CRYO groups. However, there were some unbalanced characteristics between groups, such as total CD34+ dose, melphalan dose, time of ASCT, and mobilization regimen, which may affect the outcomes. We further performed univariable analyses to evaluate whether each factor affected time to neutrophil engraftment, and found that none of these influenced the outcome. Thus, further multivariable analysis was not performed. In previous reports using the NC method for ASCT in MM, the graft failure rate and TRM were consistently low [2,4–6,8,17–19]. A 3.8% TRM in our cohort was due to 1 sudden cardiac death during febrile neutropenia on day +12, which is a rate similar to that of other reports.
The NC group had significantly higher cell viability than the CRYO group. The lowest cell viability in the CRYO group was the first ASCT case at our center. The 70% cell viability may be partly due to our inexperience in stem cell processing in our first case. The cryopreserved product was used 50 days after the collection. The later cases of the CRYO group had cell viability ranging from 85% to 99% and the difference between groups was still significant. The adequate engraftment with lower stem cell dose (less than 2.0×106/kg) in ASCT using NC preservation was demonstrated by a small number of patients in several studies [4,17,19]. This may indicate better the quality of stem cells using the NC method and possibly explain the faster engraftment effects, which need to be further investigated.
The incidence of IREs in our entire cohort was 26.2%, which was lower in the NC group but the difference was not statistically significant. All events were Grade 1 and most were gastrointestinal symptoms, without any fever. However, Bittencourt et al. found lower IREs in the NC group, in which most events in the NC group were fever within 24 hours from their infusion [2]. This probably was because we gave premedication given before the stem cell infusion, while they did not.
Mucositis is a well-known adverse effect of high-dose melphalan conditioning. There are many interventions to prevent chemotherapy-associated mucositis, including ice chips [20]. We observed a low incidence of severe oropharyngeal mucositis, which could be due to our protocol of using ice chips before melphalan infusion in every case. However, up to 85% of Grade 3–4 gastrointestinal mucositis were found, which were similar between groups. The results are consistent with other reports using the NC method for ASCT in MM, which reported 63–79% Grade 3–4 mucositis [4,5,19]. Bittencourt et al. demonstrated similar rates of fever and infection between the NC and CRYO groups [2]. All patients in our cohort and also most of the patients in another cohort from India [5] had neutropenic fever or infection during ASCT in both the NC and CRYO groups. The high incidence of febrile neutropenia in the present study is possibly due to not routinely prescribing antibiotic prophylaxis in our center. Conversely, Sarmiento et al. recently reported significantly lower incidence of severe mucositis, including both oropharyngeal and gastrointestinal, in NC compared with CRYO [8]. Also, the incidence of febrile neutropenia in the same study was lower in the NC group [8]. The results should be carefully interpreted as 30% of the population had lymphoma using a different conditioning regimen in different transplantation centers. Therefore, the benefits of NC in avoiding mucositis and infectious complications are still equivocal.
Our cohort study revealed a similar duration of hospital stay in both groups, while Sarmiento et al. demonstrated a shorter inpatient hospital stay in the NC group compared with CRYO [8]. However, they found more Grade 3–4 adverse events and febrile neutropenia and higher total parenteral nutrition (TPN) requirements in the CRYO group, which could lengthen the hospital stay, whereas rates of severe adverse events, infections, and TPN requirements in our cohort were not different between groups (data not shown).
The average cost of ASCT using the NC method in our study was around 10% reduction comparing with the CRYO method, and it can be up to 15% when considered only in the first ASCT population. The cost of cryopreservation is approximately 900 USD in our center. Although the stem cell collection process cannot be used in an outpatient setting due to our safety policy, patients in the NC group also underwent stem cell collection in the hospital. After excluding 5 patients using Cy mobilization, which led to a prolonged hospital stay, the total average cost was still lower in the NC group. Therefore, the comparison of total cost was not biased by the cost of the first admission, and the expense of stem cell cryopreservation can be avoided when using the NC method.
There are several limitations to our study. First, this was a retrospective study. However, we enrolled every patient undergoing ASCT at our center and there was little missing data. Second, the 6-year duration of the study was rather long, which may lead to some bias from a time effect. Lastly, the unbalanced clinical characteristics between groups influenced the interpretation of the results. Even though we performed a regression analysis, which revealed that the outcome was unaffected by these factors, the results should be carefully interpreted due to the relatively small sample size of the study.
Conclusions
ASCT in MM using the NC preservation method is effective and safe compared to the CRYO method in both short-term and survival outcomes.
References
1. Al Hamed R, Bazarbachi AH, Malard F, Current status of autologous stem cell transplantation for multiple myeloma: Blood Cancer J, 2019; 9(4); 44
2. Bittencourt MCB, Mariano L, Moreira F, Cryopreserved versus non-cryopreserved peripheral blood stem cells for autologous transplantation after high-dose Melphalan in multiple myeloma: comparative analysis: Bone Marrow Transplant, 2019; 54(1); 138-41
3. Hechler G, Weide R, Heymanns J, Storage of noncryopreserved periphered blood stem cells for transplantation: Ann Hematol, 1996; 72(5); 303-6
4. Kulkarni U, Devasia AJ, Korula A, Use of non-cryopreserved peripheral blood stem cells is associated with adequate engraftment in patients with multiple myeloma undergoing an autologous transplant: Biol Blood Marrow Transplant, 2018; 24(12); e31-35
5. Kayal S, Sharma A, Iqbal S, High-dose chemotherapy and autologous stem cell transplantation in multiple myeloma: A single institution experience at All India Institute of Medical Sciences, New Delhi, using non-cryopreserved peripheral blood stem cells: Clin Lymphoma Myeloma Leuk, 2014; 14(2); 140-47
6. Ramzi M, Zakerinia M, Nourani H, Non-cryopreserved hematopoietic stem cell transplantation in multiple myeloma, a single center experience: Clin Transplant, 2012; 26(1); 117-22
7. Ruiz-Arguelles GJ, Gomez-Rangel D, Ruiz-Delgado GJ, Results of an autologous noncryopreserved, unmanipulated peripheral blood hematopoietic stem cell transplant program: A single-institution, 10-year experience: Acta Haematol, 2003; 110(4); 179-83
8. Sarmiento M, Ramirez P, Parody R, Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy: Bone Marrow Transplant, 2018; 53(8); 960-66
9. Greipp PR, San Miguel J, Durie BG, International staging system for multiple myeloma: J Clin Oncol, 2005; 23(15); 3412-20
10. Kumar S, Paiva B, Anderson KC, International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma: Lancet Oncol, 2016; 17(8); e328-46
11. Sorror ML, Maris MB, Storb R, Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT: Blood, 2005; 106(8); 2912-19
12. Moreau P, San Miguel J, Sonneveld P, Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: Ann Oncol, 2017; 28(Suppl 4); iv52-61
13. Strober W, Trypan blue exclusion test of cell viability: Curr Protoc Immunol, 2001 Appendix 3: Appendix 3B
14. CIBMTR: Transplant Essential Data (TED) Manuals Q14-16: Initial ANC recovery, 2019 https://www.cibmtr.org/manuals/fim/1/en/topic/q8-11-initial-anc-recovery
15. CIBMTR: Transplant Essential Data (TED) Manuals Q17-18: Initial Platelet Recovery, 2019 https://www.cibmtr.org/manuals/fim/1/en/topic/q12-14-initial-platelet-recovery
16. Valcarcel D, Sureda A, Graft failure: The EBMT Handbook: Hematopoietic stem cell transplantation and cellular therapies, 2019; 307-13, Cham (CH)
17. Jeyaraman P, Borah P, Dayal N, Adequate engraftment with lower hematopoietic stem cell dose: Clin Lymphoma Myeloma Leuk, 2020; 20(4); 260-63
18. Kardduss-Urueta A, Gale RP, Gutierrez-Aguirre CH, Freezing the graft is not necessary for autotransplants for plasma cell myeloma and lymphomas: Bone Marrow Transplant, 2018; 53(4); 457-60
19. Naithani R, Dayal N, Pathak S, Rai R, Hematopoietic stem cell transplantation using non-cryopreserved peripheral blood stem cells graft is effective in multiple myeloma and lymphoma: Bone Marrow Transplant, 2018; 53(9); 1198-200
20. Worthington HV, Clarkson JE, Eden OB, Interventions for preventing oral mucositis for patients with cancer receiving treatment: Cochrane Database Syst Rev, 2007(4); CD000978
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860