26 February 2021: Original Paper
Pharmacodynamic Drug–Drug Interaction on Human Peripheral Blood Mononuclear Cells Between Everolimus and Tacrolimus at the Therapeutic Concentration Range in Renal Transplantation
Masaaki Okihara1BDEF, Hironori Takeuchi2ABCD, Shinichi Akiyama3BCD, Reichi Yoshinaga3BCD, Sayuri Osato3BCD, Isao Akashi1B, Yu Kihara1B, Osamu Konno1B, Hitoshi Iwamoto4EF, Takashi Oda4EF, Sachiko Tanaka5AE, Sakae Unezaki3AE, Toshihiko Hirano5ADEF*DOI: 10.12659/AOT.928817
Ann Transplant 2021; 26:e928817
Abstract
BACKGROUND: Everolimus (EVL) plus tacrolimus (TAC) therapy is effective and safe in renal transplantation. However, the pharmacokinetic and pharmacodynamic information for EVL combined with TAC is limited. We investigated the pharmacodynamic drug–drug interaction between EVL and TAC at their therapeutic concentration range.
MATERIAL AND METHODS: Isolated peripheral blood mononuclear cells (PBMCs) from 22 healthy participants aged 22 to 24 years were cultured with concanavalin A (Con A) in the presence of EVL and/or TAC for 4 days, and the proliferation rate of the PBMCs was calculated.
RESULTS: TAC promoted the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs at the EVL therapeutic concentration range. When 0.175 ng/mL or more of TAC was combined with 30 ng/mL or more of EVL, the antagonistic effect of TAC on the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs was observed. Conversely, when 0.4 ng/mL TAC and 10 ng/mL or more of EVL were combined, the antagonistic effect of EVL on the inhibitory efficacy of TAC against the mitogen-activated proliferation of PBMCs was observed.
CONCLUSIONS: The pharmacodynamic synergistic efficacy of EVL and TAC in combination on mitogen-activated PBMCs was evident at the therapeutic concentration range, which is used in renal transplantation. However, these drugs antagonize each other to suppress the proliferation of activated PBMCs at concentrations higher than those clinically used.
Keywords: Drug Antagonism, Drug Interactions, Drug Synergism, Kidney Transplantation, Tacrolimus, Drug Therapy, Combination, Everolimus, Immunosuppressive Agents, Leukocytes, Mononuclear
Background
In renal transplantation, immunosuppressive drugs and the therapies based on these drugs have markedly improved graft survival and function, enabling successful transplantation. Factors that influence long-term graft survival include nephrotoxicity due to the use of calcineurin inhibitors (CNIs) [1] and infections and malignant tumors, which occur due to the long-term immunosuppression from the drugs. The mTOR inhibitor everolimus (EVL) has been used as an efficient immunosuppressive drug for renal transplantation since December 2011, under the health insurance of Japan. EVL is currently used in combination with CNIs, prednisolone, mycophenolate mofetil, and basiliximab. Combining EVL with the other drugs is expected to reduce the renal toxicity of CNIs, prevent viral infections such as cytomegalovirus and polyomavirus BK, [2], suppress tumors [3–5], and reduce cardiovascular events by suppressing intimal thickening [6] and improving left ventricular systolic function [7], while maintaining an immunosuppressive effect.
EVL forms a complex with the intracellular tacrolimus (TAC) binding protein FKBP12, and the EVL-FKBP12 complex inhibits cell proliferation through binding with mTOR, which associates with the G1/S1 cell cycle [8]. Accordingly, EVL inhibits the proliferation of T cells [9], B cells [8], and vascular smooth muscle cells [9], and suppresses the development of neoatherosclerosis [6] and renal interstitial fibrotic lesions [10]. Both TAC and EVL bind with FKBP12 [11,12]. However, TAC represents a different pharmacological action from that of EVL, inhibiting calcineurin in the T-cell signaling pathway, which subsequently suppresses the production of cytokines such as IL-2 [13].
Van Rossum et al reported that the combination regimen of EVL and TAC enhances the immunosuppressive effect of TAC in vitro, whereas the combination gives an antagonistic effect when the concentration of EVL and TAC are high enough to saturate the FKBP12 molecule in immune cells [14]. In Japan, we have carefully considered the combination regimen of EVL and TAC in renal transplantation because of the antagonistic effect between these drugs, and we gradually began using the combination after the health insurance approval of EVL. However, the pharmacodynamic drug–drug interaction between EVL and TAC at a clinically therapeutic concentration has not been clarified, which is the reason we planned this study.
In the present study, we investigated the effect of TAC on the inhibitory efficacy of EVL against the T-cell mitogen-activated proliferation of peripheral blood mononuclear cells (PBMCs) obtained from healthy participants. We also investigated the antagonistic effects of EVL and TAC on the mitogen-activated proliferation of PBMCs. Taken together with these points, we discuss the pharmacodynamic drug–drug interaction between EVL and TAC at their therapeutic concentration range.
Material and Methods
REAGENTS:
Roswell Park Memorial Institute (RPMI)-1640 culture medium and fetal bovine serum were purchased from Gibco BRL (Grand Island, NY, USA). Concanavalin A (Con A) was purchased from Seikaguku Kogyo Co (Tokyo, Japan). EVL and TAC were purchased from Fujifilm Wako Pure Chemical Co (Osaka, Japan). The EVL and TAC were dissolved in ethanol, and the working concentrations were prepared after dilution. All other reagents were of the best available grade.
PARTICIPANTS:
Blood samples were obtained from 22 healthy participants (10 men and 12 women) aged 22 to 24 years. These participants had no history of immunological disorders or of taking immunosuppressive drugs. This study was approved by the institutional ethics committee for studies in humans at the Tokyo University of Pharmacy and Life Sciences (Tokyo, Japan; approval No. 17–7).
PBMC ISOLATION AND CULTURE:
For each participant, a sample of 10 mL of venous blood was loaded onto 4 mL of Ficoll-Hypaque (Nakarai Co, Japan) lymphocyte separation medium, and centrifuged at 1300×g for 20 min. The PBMC layer was transferred to another tube, and 5 mL of RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 000 IU/L penicillin, and 100 mg/L streptomycin were added and mixed well. Then, the cells were centrifuged at 1300×g for 20 min. After removing the supernatant, fresh RPMI-1640 medium was added and the cell suspension was mixed and centrifuged. Finally, the PBMCs were diluted to 1×106 cells/mL with the medium. An amount of 195 μL of the PBMC suspension and 1 μL of 1 mg/mL Con A solution were added to each well of a 96-well plate. Next, 4 μL of ethanol as a control or 4 μL of immunosuppressive drug was added, making a total volume of 200 μL. Thus, the final concentrations were 10 to 1000 ng/mL of EVL and 0.05 to 0.25 ng/mL of TAC. In the combination of EVL and TAC, 2 μL of each drug solution was added to the culture well. After mixing, the plate was cultured at 37°C with 5% CO2 for 72 h. Subsequently, 0.5 μL of [3H] thymidine solution (18.5 KBq/well) was added to each well, and the plate was cultured for an additional 20 h.
EVALUATION OF THE EFFECT OF IMMUNOSUPPRESSIVE DRUGS ON PBMC PROLIFERATION RATE:
After culturing, the cells were harvested and the radioactivity of [3H] thymidine incorporated into the PBMCs was measured using a liquid scintillation counter. The proliferation rate of PBMCs stimulated by Con A was calculated using the following formula:
in which E0, E1, and E2 represent the radioactivity incorporated into unstimulated PBMCs without drug (disintegrations per minute [dpm]), the radioactivity incorporated into PBMCs stimulated by Con A in the absence of drug (dpm), and the radioactivity incorporated into PBMCs stimulated by Con A in the presence of drug (dpm), respectively.
THE EFFECT OF TAC ON THE INHIBITORY EFFICACY OF EVL AGAINST THE MITOGEN-ACTIVATED PROLIFERATION OF PBMCS:
The concentration-proliferation curves of EVL and TAC against the Con A-activated PBMCs were obtained according to the procedures described above. Then, the theoretical curve that was obtained by simply subtracting the inhibition rates of TAC on PBMC proliferation from the EVL curve was defined as the “simulated combination curve”. The difference between the proliferation rates of the PBMCs treated with EVL and those of the PBMCs treated with the drug combination or those of PBMCs under the simulated combination was evaluated at the EVL therapeutic concentration range. The effect of TAC on the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs was also evaluated at the same concentration range. We demonstrated the synergistic effect between CNI and steroids with same method used in a previous study that analyzed PBMC sensitivity to steroids [15].
THE ANTAGONISTIC EFFECT OF TAC ON THE INHIBITORY EFFICACY OF EVL AGAINST THE MITOGEN-ACTIVATED PROLIFERATION OF PBMCS:
The proliferation rate of PBMCs treated with 10 ng/mL of EVL, in which the antagonistic effect on TAC action was not observed, was used as a reference value, and the differences between the PBMC proliferation rates in the presence of 10 ng/mL of EVL and those in the presence of 30, 50, 80, 100, and 1000 ng/mL of EVL were analyzed by Dunnet tests. We evaluated the concentration at which the antagonistic effect of TAC on the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs was observed.
THE ANTAGONISTIC EFFECT OF EVL ON THE INHIBITORY EFFICACY OF TAC AGAINST MITOGEN-ACTIVATED PROLIFERATION OF PBMCS:
Using a method similar to that described above, the concentration-proliferation curves for EVL and TAC were obtained. Then, the theoretical curve that was obtained by simply subtracting the inhibition rates of EVL on the PBMC proliferation from the TAC curve was defined as the “simulated combination curve”.
The differences among the proliferation rates of PBMCs treated with TAC, those treated with the combination, and those under the simulated combination were evaluated at the TAC therapeutic concentration range. The effect of EVL on the inhibitory efficacy of TAC against mitogen-activated proliferation of PBMCs was evaluated at the same concentration range.
CONVERSION FROM THE WHOLE-BLOOD MASS CONCENTRATION TO THE PROTEIN-FREE MOLAR CONCENTRATION:
The purpose of the present study was to investigate whether saturation of FKBP12 results in an antagonistic effect of EVL and TAC at their therapeutic concentration ranges on the mitogen-activated proliferation of PBMCs. Therefore, we converted the concentrations relevant to the therapeutic range to the corresponding concentrations used in this experiment, as shown in Table 1. EVL and TAC concentrations relevant to the therapeutic range are suggested by the whole-blood mass concentration (ng/mL), while only protein-free drugs show clinical efficacy in vivo. Therefore, EVL and TAC concentrations were converted from the whole-blood mass concentration (ng/mL) to the protein-free blood concentration (ng/mL) in consideration of their clinical distribution in blood; specifically, 26% of EVL and 1% of TAC exist as protein-free drugs in blood, as shown in the second row and column of Table 1. In addition, we converted the protein-free blood concentration (ng/mL) to the protein-free molar concentration (nmol/mL) to compare with the experimental results, as shown in the third row and column of Table 1.
TROUGH AND PEAK CONCENTRATIONS OF EVL AND TAC:
We defined the whole-blood mass concentrations of EVL at trough as 3 to 8 ng/mL and at peak as 20 to 30 ng/mL, and those of TAC at trough as 4 to 7 ng/mL and peak as 20 to 40 ng/mL. These values were defined based on various facility standards, existing literature, and guidelines in Japan [16–18].
STATISTICAL ANALYSIS:
Statistical analyses were conducted using JMP® 11 (SAS Institute Inc, Cary, NC, USA). The differences between PBMC proliferation rates in the presence of 10 ng/mL EVL and those in the presence of other concentrations of ECL were analyzed by Dunnett tests. The Wilcoxon signed-rank test was used to analyze differences in the interaction indices between 2 treatment groups. Differences were considered to be statistically significant at values of
Results
EFFECT OF TAC ON THE INHIBITORY EFFICACY OF EVL AGAINST THE MITOGEN-ACTIVATED PROLIFERATION OF PBMCS:
Concentration-proliferation curves for EVL alone or in combination with TAC against the Con A-activated proliferation of PBMCs are shown in Figure 1. EVL suppressed the proliferation rates of activated PBMCs in a concentration-dependent manner in the presence or absence of 0.05 ng/mL TAC (Figure 1A). On the other hand, when 0.1 ng/mL or more of TAC was combined with 100 to 1000 ng/mL EVL, the proliferation of PBMCs conversely increased, and thus the antagonistic effect of TAC was observed at these concentrations (Figure 1B, 1C). These actual concentration-proliferation curves for EVL in combination with TAC (Figure 1, solid lines) were quite different from those of the simulated concentration-proliferation curves for EVL (Figure 1, dashed lines) when 0.1 ng/mL or more of TAC was combined (Figure 1B, 1C).
Thus, the data showed that TAC promoted the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs at the protein-free blood EVL therapeutic concentration range (0.8–7.8 ng/mL) [15], whereas higher concentrations (>0.1 ng/mL) of TAC conversely disturbed the inhibitory efficacy of EVL.
ANTAGONISTIC EFFECT OF TAC ON THE INHIBITORY EFFICACY OF EVL AGAINST THE MITOGEN-ACTIVATED PROLIFERATION OF PBMCS:
Based on the results shown in Figure 1, we examined more precisely the concentration-proliferation curves for EVL in combination with TAC against the Con A-activated proliferation of PBMCs to determine the concentration at which the inhibitory effect of TAC against EVL pharmacodynamics was observed (Figure 2). As mentioned above, TAC in combination with EVL markedly decreased the mitogen-activated proliferation of PBMCs, compared with the effect of EVL alone. Of note, TAC at concentrations over 0.175 ng/mL attenuated the suppressive effects of EVL in an EVL-concentration-dependent manner. The mean PBMC proliferation rate in the presence of 0.175 ng/mL TAC combined with 30 ng/mL EVL was significantly higher than the mean PBMC proliferation rate in the presence of 0.175 ng/mL TAC combined with 10 ng/mL EVL (P<0.05). In addition, significant differences were observed in all cases when 0.175 ng/mL or more of TAC was combined with 30 ng/mL or more of EVL (P<0.05).
Thus, when 0.175 ng/mL or more of TAC was combined with 30 ng/mL or more of EVL, the antagonistic effect of TAC on the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs was observed.
ANTAGONISTIC EFFECT OF EVL ON THE INHIBITORY EFFICACY OF TAC AGAINST THE MITOGEN-ACTIVATED PROLIFERATION OF PBMCS:
We also examined the concentration-proliferation curves for TAC alone or in combination with EVL against the Con A-activated proliferation of PBMCs, as shown in Figure 3. TAC suppressed the PBMC proliferation concentration dependently, and the addition of EVL to TAC efficiently decreased PBMC proliferation. These additional effects of EVL were more obvious when higher concentrations of EVL were used (Figure 3C–3E). However, the additional suppressive efficacies of EVL combined with relatively high concentrations of TAC (10–100 ng/mL) were weakened, and the additional efficacy even disappeared when 0.01 and 1 ng/mL of EVL were combined (Figure 3A, 3C). When 0.1 to 1 ng/mL EVL (Figure 3B, 3C) and 0.001 to 1 ng/mL TAC were combined, the PBMC suppressive effects (Figure 3, solid lines) were almost equal with those of the simulated combination curves (Figure 3, dashed lines). Conversely, the antagonistic effect of EVL was observed when it was combined with 10 to 100 ng/mL TAC. The combination of 10 ng/mL or more of EVL and 0.1 ng/mL or more of TAC also exhibited antagonistic effects, and the PBMC proliferation rates increased over those of the simulated combination curves (Figure 3D, 3E). In addition, we compared the actual combination and simulated combination at each TAC and EVL concentration. Significant differences between the 2 groups were observed when 10 ng/mL of EVL was combined with 10 ng/mL of TAC (P=0.028) and 100 ng/mL of EVL was combined with 10 ng/mL of TAC (P=0.043), and we found that the antagonistic effect of TAC on the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs was observed at high concentrations.
The interaction indices calculated by the ratio of the “PBMC proliferation rates in the actual combination effects” to “those in the simulated combination effects” at the protein-free blood TAC therapeutic concentration range (0.04–0.4 ng/mL) [6] are shown in Figure 4. The interaction indices were approximately 1 at 0.04 ng/mL TAC combined with all EVL concentrations examined. Conversely, the indices were noticeably higher than 1 when 10 ng/mL or more of EVL was combined with 0.4 ng/mL of TAC. Therefore, the antagonistic effect of EVL on the inhibitory efficacy of TAC against the mitogen-activated proliferation of PBMCs was observed at these concentrations (P<0.05).
Discussion
The ATHENA study [19] and the TRANSFORM study [2] demonstrated that EVL and a reduced dose of TAC in combination are effective and safe for the achievement of long-term graft survival and functioning in renal transplantation, while providing additional benefits of lower rates of viral infections such as cytomegalovirus and polyomavirus BK and lower rates of de novo DSA and overall mortality. However, pharmacokinetic and pharmacodynamic information for EVL combined with TAC is limited, even though this combination therapy seems promising.
In the present study, we found that TAC promoted the inhibitory efficacy of EVL against the T-cell mitogen-activated proliferation of PBMCs of healthy participants in vitro. Our present findings and those of von Rossum et al [14] demonstrated that EVL and TAC exhibit a pharmacodynamic interaction to promote the inhibitory efficacy of each other against the mitogen-activated proliferation of PBMCs. On the other hand, these drugs antagonize when higher concentrations were combined, probably because of a competitive interaction at their binding protein, FKBP12. Comparing the simulated combination curve and the actual combination curve at the protein-free mass concentration of EVL and TAC at trough (0.03–0.05 and 0.78–1.3 ng/mL, respectively) (Figures 1A, 3C), TAC weakly promoted the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs, and EVL scarcely promoted the inhibitory efficacy of TAC against PBMC proliferation. Similarly, EVL and TAC strongly promoted the inhibitory efficacy of each other against the mitogen-activated proliferation of PBMCs at the protein-free mass concentration of EVL and TAC at peak (0.2–0.4 and 5.2–7.8 ng/mL, respectively) (Figures 1C, 3D), when compared with their effects at trough concentrations.
We then used the CompusSyn 1.0.1 computer program to quantitate the synergism and the antagonism of these drug combinations [20] (
It was considered that the antagonistic effect was mediated via the saturation of FKBP12. We precisely investigated the drug concentration by which the antagonistic effect was observed using the information shown in Table 1. The whole-blood concentration of EVL and TAC at peak are 20 to 30 ng/mL and 20 to 40 ng/mL, respectively, and the protein-free molar concentrations of those are 5.43 to 8.14×10−3 and 0.24 to 0.49×10−3 nmol/mL, respectively. Thus, the maximum sum of the protein-free molar concentration of EVL and TAC at peak was 8.63×10−3 nmol/mL. When 0.4 ng/mL or more of TAC was combined with 10 ng/mL or more of EVL, the antagonistic effect of EVL on the inhibitory efficacy of TAC against the mitogen-activated proliferation of PBMCs was observed, and the sum of the protein-free molar concentration of EVL and TAC at those concentrations, 10.93×10−3 nmol/mL, was higher than 8.63×10−3 nmol/mL. Moreover, when 30 ng/mL or more of EVL was combined with 0.175 ng/mL or more of TAC, the antagonistic effect of TAC on the inhibitory efficacy of EVL against the mitogen-activated proliferation of PBMCs was observed, and the sum of the protein-free molar concentration of EVL and TAC at those concentrations, 31.53×10−3 nmol/mL, was higher than 8.63×10−3 nmol/mL. Accordingly, it can be concluded that the antagonistic effect of EVL and TAC will not be observed at the EVL and TAC therapeutic concentration range in vitro, as shown in Table 1. When the sum of protein-free molar concentration of EVL and TAC exceeded 10.93×10−3 nmol/mL, the antagonistic effect of EVL on the inhibitory efficacy of TAC against the mitogen-activated proliferation of PBMCs was firstly observed. Then, when the sum of the protein-free molar concentration of EVL and TAC exceeded 31.53×10−3 nmol/mL, the antagonistic effect of EVL and TAC on the mitogen-activated proliferation of PBMCs was subsequently observed. However, the information for the drug concentrations shown in Table 1 had a limitation that should be kept in mind. Even though the affinity of TAC for FKBP12 is about twice that of EVL [8], the data shown in Table 1 were simply evaluated from the number of the drug-receptor bindings and were not based on the affinity and reactivity of the receptors.
In the present study, we used PBMCs obtained from healthy participants instead of those obtained from patients with chronic renal failure. In our previous studies, we investigated the PBMC sensitivity to cyclosporine A (CYA), steroids, and TAC between patients with chronic renal failure and that of healthy participants [21,22]. No significant differences in the average IC50 values of CYA and TAC against the PBMC proliferation between these groups were observed, while the patients with chronic renal failure exhibited a significantly low response to steroids. It is known that the peripheral lymphocytes of patients on dialysis exhibit a low response to mitogens or other lymphocyte stimulants, as compared with those of healthy participants. However, PBMC responses to the suppressive efficacy of CNI are suggested to be unchanged, as was described above.
Since similar studies for EVL have not been conducted, the difference of PBMC sensitivity to EVL between patients with chronic renal failure and healthy participants is unclear, and the PBMC sensitivity to EVL in vivo may differ from that found in the present study. Furthermore, the data shown in the present study were average values, and we need to pay attention to individual differences of PBMC sensitivity to drugs. The data actually obtained in the present study had great individual differences, which corresponded with the findings of our previous studies [21–23]. In addition, the evidence regarding a possible effect of EVL on TAC bioavailability should be considered in renal transplant recipients [24–26]. Therefore, further investigations concerning the pharmacodynamic drug–drug interaction between EVL and TAC in renal transplant recipients would be valuable for the improvement of the long-term clinical outcomes of renal transplantation.
Conclusions
The pharmacological synergistic effects of EVL and TAC combined on the mitogen-activated PBMCs in vitro were evident at their therapeutic concentration range in renal transplantation. On the other hand, these drugs antagonized when higher concentrations were combined. Thus, when the clinical pharmacokinetic parameters of these drugs are considered, safe and reliable immunosuppressive therapy with EVL and TAC in combination could be achieved in renal transplant patients.
Figures
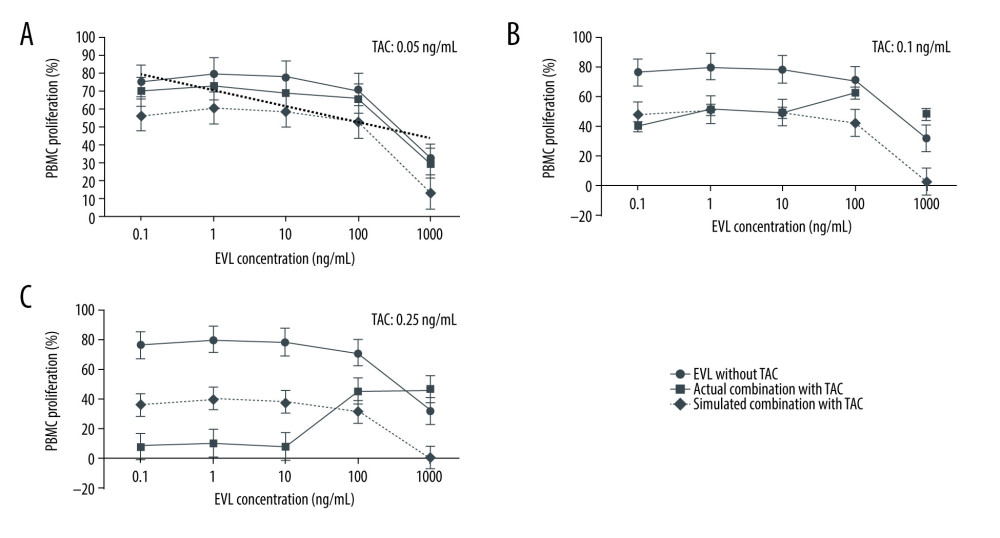 Figure 1. Concentration-proliferation curves for EVL alone or in combination with TAC against Con A-activated proliferation of PBMCs.
Figure 1. Concentration-proliferation curves for EVL alone or in combination with TAC against Con A-activated proliferation of PBMCs. 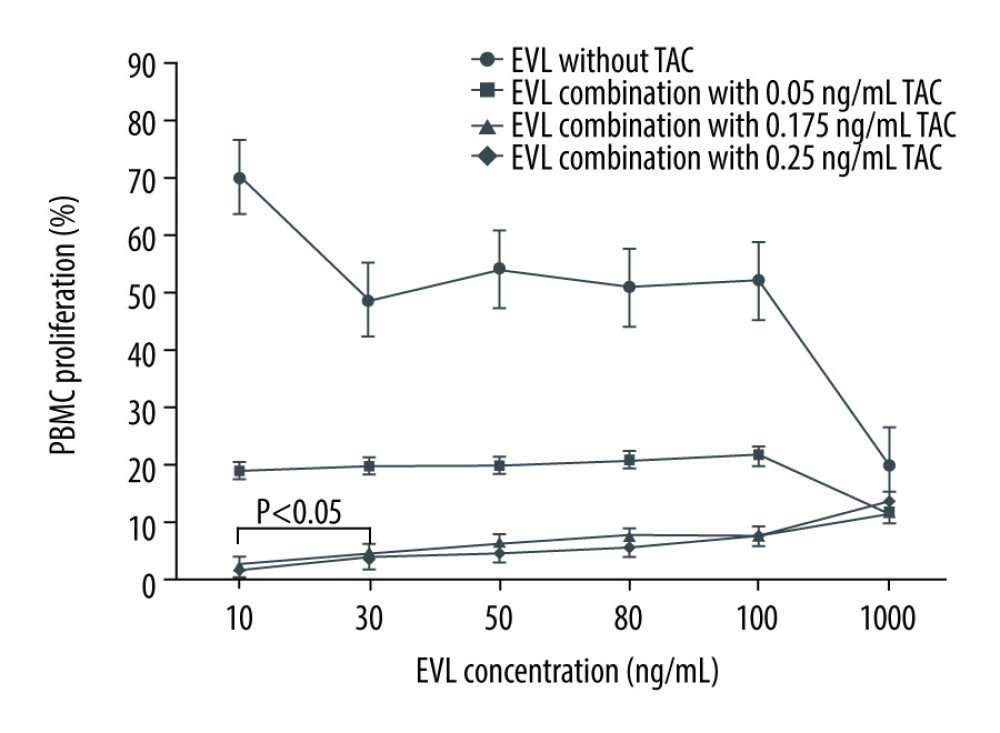 Figure 2. Actual drug–drug interaction of EVL and TAC against the proliferation of Con A-activated PBMCs.
Figure 2. Actual drug–drug interaction of EVL and TAC against the proliferation of Con A-activated PBMCs. 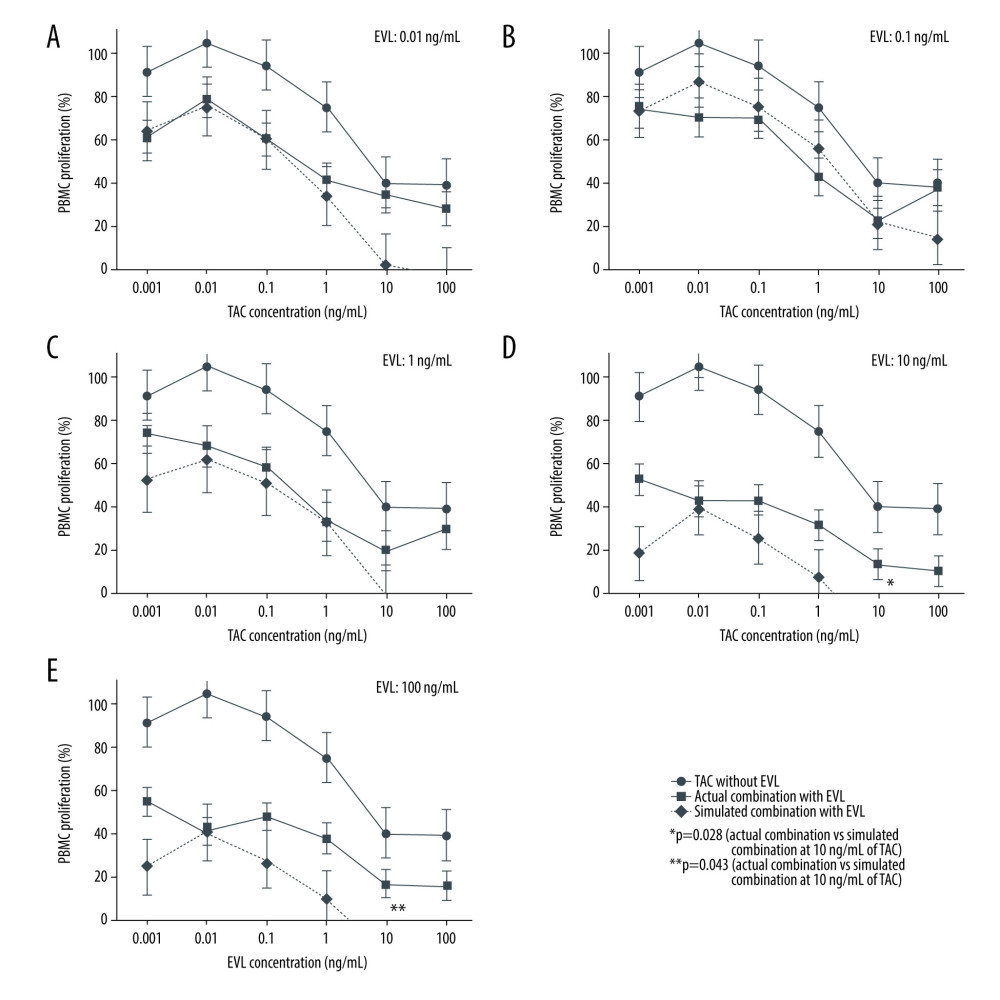 Figure 3. Concentration-proliferation curves for TAC alone or in combination with EVL against Con A-activated proliferation of PBMCs.
Figure 3. Concentration-proliferation curves for TAC alone or in combination with EVL against Con A-activated proliferation of PBMCs. 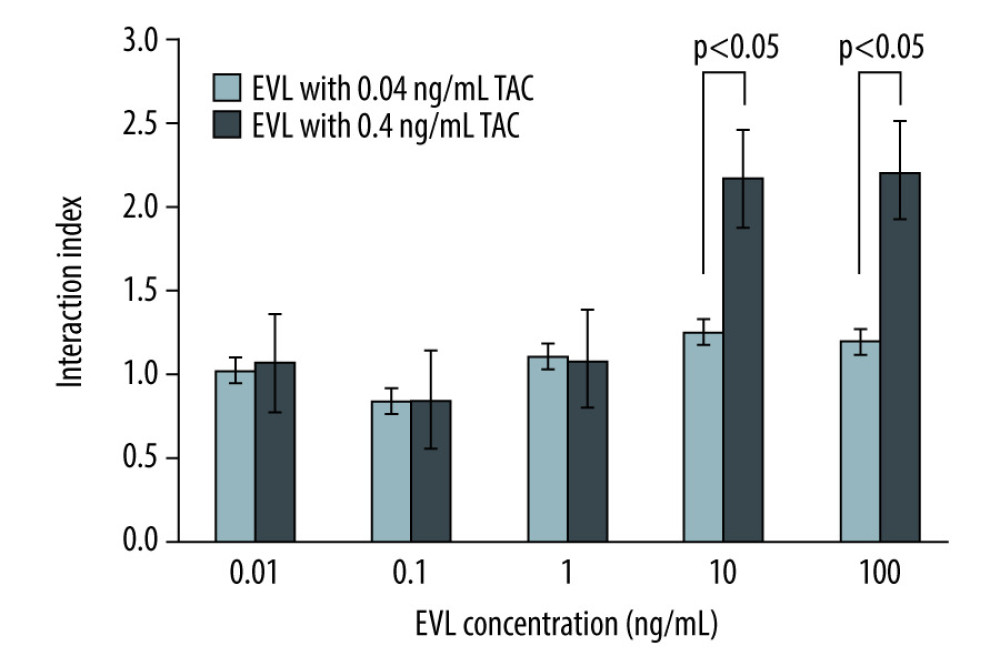 Figure 4. The interaction indices of TAC and EVL against Con A-activated proliferation of PBMCs.
Figure 4. The interaction indices of TAC and EVL against Con A-activated proliferation of PBMCs. References
1. Naesens M, Kuypers DR, Sarwal M, Calcineurin inhibitor nephrotoxicity: Clin J Am Soc Nephrol, 2009; 4(2); 481-508
2. Berger SP, Sommerer C, Witzke O, Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study: Am J Transplant, 2019; 19(11); 3018-34
3. Motzer RJ, Escudier B, Oudard S, Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomized, placebo-controlled phase III trial: Lancet, 2008; 372(9637); 449-56
4. Yao JC, Shah MH, Ito T, Everolimus for advanced pancreatic neuroendocrine tumors: N Engl J Med, 2011; 364(6); 514-23
5. Baselga J, Campone M, Piccart M, Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer: N Engl J Med, 2012; 366(6); 520-29
6. Zhao HQ, Nikanorov A, Virmani R, Schwartz LB, Inhibition of experimental neointimal hyperplasia and neoatherosclerosis by local, stent-mediated delivery of everolimus: J Vasc Surg, 2012; 56(6); 1680-88
7. Tsujimura K, Ota M, Chinen K, supplementary administration of everolimus reduces cardiac systolic function in kidney transplant recipients: Ann Transplant, 2017; 22; 315-22
8. Schuler W, Sedrani R, Cottens S, SDZ RAD, a new rapamycin derivative: Pharmacological properties in vitro and in vivo: Transplantation, 1997; 64(1); 36-42
9. Cottens S, Kallen J, Schuler W, Sedrani R, Derivation of rapamycin: Adventures in natural product chemistry: Chimia, 2019; 73(7); 581-90
10. Ishida H, Ogura G, Uehara S, Preventive effect of early introduction of everolimus and reduced exposure tacrolimus on renal interstitial fibrosis in de novo living donor renal transplant recipients: Clin Exp Nephrol, 2020; 24(3); 268-76
11. Perry I, Neuberger J, Immunosuppression: Towards a logical approach in liver transplantation: Clin Exp Immunol, 2005; 139(1); 2-10
12. Diem VM, Shijie Q, Dasheng X, Tacrolimus (FK506) and sirolimus (rapamycin) in combination are not antagonistic but produce extended graft survival in cardiac transplantation in the rat: Transplantation, 1997; 64(12); 1853-56
13. Lee RA, Gabardi S, Current trends in immunosuppressive therapies for renal transplant recipients: Am J Health Syst Pharm, 2012; 69(22); 1961-75
14. van Rossum HH, Romijn FP, Smit NP, Everolimus and sirolimus antagonize tacrolimus based calcineurin inhibition via competition for FK-binding protein 12: Biochem Pharmacol, 2009; 77(7); 1206-20
15. Takeuchi H, Iwamoto H, Nakamura Y, Synergistic effects of calcineurin inhibitors and steroids on steroid sensitivity of peripheral blood mononuclear cells: Cell Med, 2015; 7(2); 51-57
16. Niioka T, Kagaya H, Inoue M, Influence of everolimus on the pharmacokinetics of tacrolimus in Japanese renal transplant patients: Int J Urol, 2016; 23(6); 484-90
17. Ekberg H, Tedesco-Silva H, Demirbas A, Reduced exposure to calcineurin inhibitors in renal transplantation: N Engl J Med, 2007; 357(25); 2562-75
18. Masuda S, Sato S, Tanigawara Y, Guidelines on TDM of immunosuppressive drugs in organ transplantation: Transplantation, 2014; 49(6); 384-92
19. Sommerer C, Suwelack B, Dragun D, An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients: Kidney Int, 2019; 96(1); 231-44
20. Foucquier J, Guedj M, Analysis of drug combination: Current methodological landscape: Pharmacol Res Perspect, 2015; 3(3); e00149
21. Hirano T, Oka K, Takeuchi H, Immunosuppresant pharmacodynamics on lymphocytes from healthy participants ant patients with chronic renal failure, nephrosis and psoriasis: Possible implications for individual therapeutic efficacy: Clin Pharmacol Ther, 1997; 62(6); 652-64
22. Takeuchi H, Hirano T, Oka K, Lymphocyte sensitivity to cyclosporine and tacrolimus in chronic renal failure patients and clinical significance in renal transplantation: Transplant Proc, 1998; 30(1); 36-39
23. Kihara Y, Matsuno N, Mijiti A, Comparative study of the cellular pharmacodynamics of calcinurin inhibitors between patients with chronic renal failure awaiting renal transplantation and cirrhosis patients awaiting liver transplantation: Cell Transplant, 2009; 18(5); 639-46
24. Kovarik JM, Curtis JJ, Hricik DE, Differential pharmacokinetic interaction of tacrolimus and cyclosporine on everolimus: Transplant Proc, 2006; 38(10); 3456-58
25. Brandhorst G, Tenderich G, Zittermann A, Everolimus exposure in cardiac transplant recipients is influenced by concomitant calcineurin inhibitor: Ther Drug Monit, 2008; 30(1); 113-16
26. Pascual J, Castillo D, Cabello M, Interaction between everolimus and tacrolimus in renal transplant recipients: A pharmacokinetic controlled trial: Transplantation, 2010; 89(9); 994-1000
Figures
 Figure 1. Concentration-proliferation curves for EVL alone or in combination with TAC against Con A-activated proliferation of PBMCs.
Figure 1. Concentration-proliferation curves for EVL alone or in combination with TAC against Con A-activated proliferation of PBMCs. Figure 2. Actual drug–drug interaction of EVL and TAC against the proliferation of Con A-activated PBMCs.
Figure 2. Actual drug–drug interaction of EVL and TAC against the proliferation of Con A-activated PBMCs. Figure 3. Concentration-proliferation curves for TAC alone or in combination with EVL against Con A-activated proliferation of PBMCs.
Figure 3. Concentration-proliferation curves for TAC alone or in combination with EVL against Con A-activated proliferation of PBMCs. Figure 4. The interaction indices of TAC and EVL against Con A-activated proliferation of PBMCs.
Figure 4. The interaction indices of TAC and EVL against Con A-activated proliferation of PBMCs. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860









