22 December 2021: Original Paper
Comparison of Coronary Artery Calcium Scoring with Dobutamine Stress Echo for Detection of Coronary Artery Disease Before Liver Transplantation
Cerise Kleb1ABDEF, Vardhmaan Jain1CE, Chirag Sheth2ABD, Kathy Wolski3CE, Samir Kapadia3EG, Richard Grimm3AB, Milind Desai3A, Amar Krishnaswamy3A, Nicholas Kassis1E, Calvin Sheng3E, Huili ZhengDOI: 10.12659/AOT.934163
Ann Transplant 2021; 26:e934163
Abstract
BACKGROUND: Dobutamine stress echocardiography (DSE) is commonly used for cardiovascular assessment before orthotopic liver transplantation (OLT). The coronary artery calcium score (CACS) is a useful screening tool for coronary artery disease (CAD). We aimed to compare the sensitivity and specificity of DSE and CACS for CAD in OLT candidates.
MATERIAL AND METHODS: A total of 265 of the 1589 patients who underwent OLT at our center between 2008 and 2019 had preoperative coronary angiography (CAG). Of these, 173 had DSE and 133 had a CT scan suitable for CACS calculation within 1 year of OLT. Patients with a nondiagnostic DSE were excluded (n=100). Two reviewers evaluated CACS on CT scans. The sensitivity/specificity of DSE and CACS for detection of angiographically significant CAD were calculated for patients with both tests (n=36). A separate analysis compared the sensitivity/specificity of a diagnostic DSE (n=73) and CACS (n=133) against CAG for all patients with either test.
RESULTS: Sensitivity and specificity were 57.1% and 89.7%, respectively, for DSE, compared with 71.4% and 62.1% for CACS at ≥100 Agatston score. For the analysis of all patients with either test, the sensitivity/specificity of DSE for detection of CAD and CACS were 30.8% and 85.0% and 80.0% and 62.8%, respectively. On ROC analysis, CACS was a satisfactory predictor of obstructive CAD (AUC, 0.76±0.06, 95% CI, 0.66-0.87; P<0.001).
CONCLUSIONS: CACS may be an important tool for cardiovascular assessment in patients undergoing OLT. DSE was nondiagnostic in a large percentage of OLT candidates, limiting its use in this population.
Keywords: Coronary Artery Disease, Coronary Vessels, Echocardiography, Stress, Liver Transplantation, dobutamine, Humans, Sensitivity and Specificity
Background
Major adverse cardiovascular events have a significant impact on survival after orthotopic liver transplantation (OLT). While cardiovascular mortality after liver transplantation has declined, it remains a leading cause of early and late mortality after transplantation [1]. The presence of pre-existing coronary artery disease (CAD) is one such predictor of early cardiovascular mortality [1]. Therefore, it is essential to stratify the risk for ischemic CAD in patients listed for OLT. The current American Association for the Study of Liver Disease guidelines recommend that patients with traditional risk factors for CAD, including diabetes, smoking, and obesity [2], should undergo noninvasive testing with stress echocardiography, and subsequently with coronary angiography (CAG) if CAD cannot be definitively excluded (class IB recommendation) [3]. Dobutamine stress echocardiography (DSE) is a widely used screening test for preoperative risk assessment before OLT. Existing literature suggests that the sensitivity of DSE for CAD is low in liver transplant patients, ranging from 13% to 41% [4–6]. Furthermore, patients with cirrhosis have hyperdynamic ventricles with poor chronotropic response as well as vasodilated circulatory systems with a low systemic vascular resistance [7]. Hence, they may not meet the target heart rate or generate an appropriate heart rate response to dobutamine required for the pharmacologic stress test to be considered diagnostic. As a result, many patients with cirrhosis have a nondiagnostic DSE test and subsequently need invasive CAG for risk stratification, which has its own associated risks of bleeding and contrast-induced nephropathy [8].
In patients with end-stage liver disease, the coronary artery calcium score (CACS) has been shown to have a positive correlation with many cardiovascular risk factors, such as family history of cardiovascular disease, systolic/diastolic blood pressure, and metabolic syndrome [9]. Additionally, some studies, including a study by Agatston et al, which defined a “high” CACS as ≥400 AS, found that that a high CACS is associated with higher rates of early postoperative cardiac complications after liver transplantation, including nonfatal myocardial infarction, arrhythmia, heart block, and cardiac death [10]. Patients with higher CACSs are also more likely to have significant CAD (defined as > 50% occlusive disease) [11].
We postulated that the CACS may be a useful tool for cardiovascular risk stratification prior to OLT and be a good alternative test in many patients when DSE is nondiagnostic or inconclusive. The CACS offers several advantages, including that it is noninvasive, does not require contrast administration, and does not require patients to reach a target heart rate [12]. While the sensitivity and specificity of CACS and DSE have been assessed independently in OLT candidates, they have not been directly compared against each other in the same sample of patients for their ability to detect CAD against the criterion standard test, CAG. The goal of this study was to compare the diagnostic accuracy of DSE with CACS for the detection of significant CAD in patients undergoing OLT.
Material and Methods
STATISTICAL ANALYSIS:
Continuous data were reported as medians (interquartile range) and means (standard deviation) based on the distribution of the data. Categorical data were reported as a count (proportion). We used the Wilcoxon rank-sum test and
Results
DSE VS CACS: HEAD-TO-HEAD COMPARISON:
We compared the sensitivity, specificity, positive predictive value, and negative predictive values of DSE and CACS for CAD in patients who had both studies (n=36). A cutoff CACS of ≥100 was used for comparison. The CACS has a sensitivity of 71.4% for the detection of obstructive CAD, while DSE had a sensitivity of 57.1%. The specificity of DSE was significantly higher than that of the CACS when a cutoff of ≥100 AS in the CACS was used (89.7% and 62.1%, respectively). However, when the cutoff for the CACS was increased to ≥400 AS, the specificity increased to 86.2%, while the sensitivity decreased to 57.1% (Table 2).
DSE AND CACS SUBSETS (ALL PATIENTS WITH VALID RESULT FOR EITHER TEST):
The sensitivity and specificity of DSE and the CACS were calculated separately for all patients in the sample with a valid result for either test. In the DSE subset (n=73), the sensitivity and specificity for detecting obstructive CAD were 30.8% and 85%, respectively. In the CACS subset (n=133), the sensitivity was 80.0% at a cutoff of ≥100 AS and 55.0% at a cutoff of ≥400 AS. The specificity of the CACS was 62.8% at ≥100 AS, which improved to 79.6% at the higher cutoff of ≥400 AS (Table 3). Because the sensitivity and specificity were not calculated on the same patients for each test in a head-to-head fashion, it was not possible to determine whether this difference was statistically significant.
The DSE and CACS subsets were also used to calculate the positive and negative predictive values for each of the tests. Using a threshold of ≥100 AS, the positive predictive value of the CACS was low at 28.2%, but the specificity was high at 93.4%. The positive predictive value of DSE was low at 28.2%, but the negative predictive value was high at 83.9%. However, the assessment of the predictive value of DSE did not include all of the patients owing to the high number of nondiagnostic studies.
ROC analysis showed that a CACS ≥100 AS was a robust predictor of the presence of obstructive CAD (AUC 0.76±0.06; 95% CI 0.66–0.87, P<0.001) (Figure 3).
Discussion
LIMITATIONS:
A major limitation of our study is the potential bias introduced when considering that patients referred for CAG may have been at a high risk for CAD. It is important to note that the inclusion criteria for the study was all patients undergoing OLT evaluation who had a CAG prior to the transplant. This included patients who either had a nondiagnostic DSE or other indications for CAG as listed above. These patients could have had a higher risk of obstructive CAD than a general cohort of patients undergoing preoperative cardiac evaluation for OLT. The physicians who referred these patients to either CAG or DSE likely had a reasonable clinical suspicion that these patients had CAD, which may have confounded our results. This is a possible explanation for why in the head-to-head comparison, we found a higher sensitivity of DSE for obstructive CAD (57.1%) than other previous studies (Table 4). The disparity between the sensitivity of DSE of our study in the head-to-head comparison and that described in previous literature may have also been due to random error related to the small sample size, since our subset analysis of all patients who had either study found a sensitivity of 30.8%. Furthermore, our comparison of DSE and CACS subsets (all patients with a valid result for either test) compared test results in different patients. This analysis may have been affected by unknown confounders since the test subjects were different patients with different baseline demographics and comorbidities.
Another limitation of this study is that the CT scans used for the calcium scoring were not originally intended for calcium scoring. Hence, the calcium scores may have been overestimated due to motion artifact. Additionally, the Agatston method was originally developed using electrocardiography-gated CT scans [12]. Our CT scans were non-gated. However, more recent research has shown that the CACS on non-gated CT scans correlated well with the Agatson score on gated CT scans [22].
Furthermore, there were still many patients with obstructive CAD who had calcium scores less than the various thresholds for what would be considered a “positive” result. Although the average CACS for patients without CAD (69.4 AS) and with obstructive CAD (769.8) were mostly divergent, there were still outliers in each category. For example, even some patients with obstructive CAD had calcium scores of 0. These patients with clinically significant CAD would be missed if calcium scoring alone was used to risk stratify patients and determine which patients should go on to invasive CAG. Because of this, it is important to consider the presence of clinical risk factors such as age, diabetes, hypertension, obesity, and family history of CAD when interpreting the CACS and using it to guide clinical decision making. For instance, patients with high risk based on clinical risk stratification may be preferentially evaluated using CAG, while patients with low to moderate risk could be further screened with the CACS (with DSE/CAG as the next step).
Conclusions
In conclusion, the CACS has comparable sensitivity for the detection of obstructive CAD compared with DSE at all AS thresholds investigated in this study. ROC analysis showed that the CACS was a satisfactory test for detecting the presence of underlying obstructive CAD. DSE was nondiagnostic in an unacceptably high number of patients, restricting its utility as a screening test for CAD. Therefore, we conclude that calcium scoring may be a suitable alternative method for preoperative cardiac risk stratification prior to liver transplantation, at least in patients with low to moderate risk or when DSE is nondiagnostic. Until more prospective research is available, this retrospective study provides some insight into the role of the CACS as an alternative to DSE for preoperative cardiac risk assessment prior to liver transplantation.
Figures
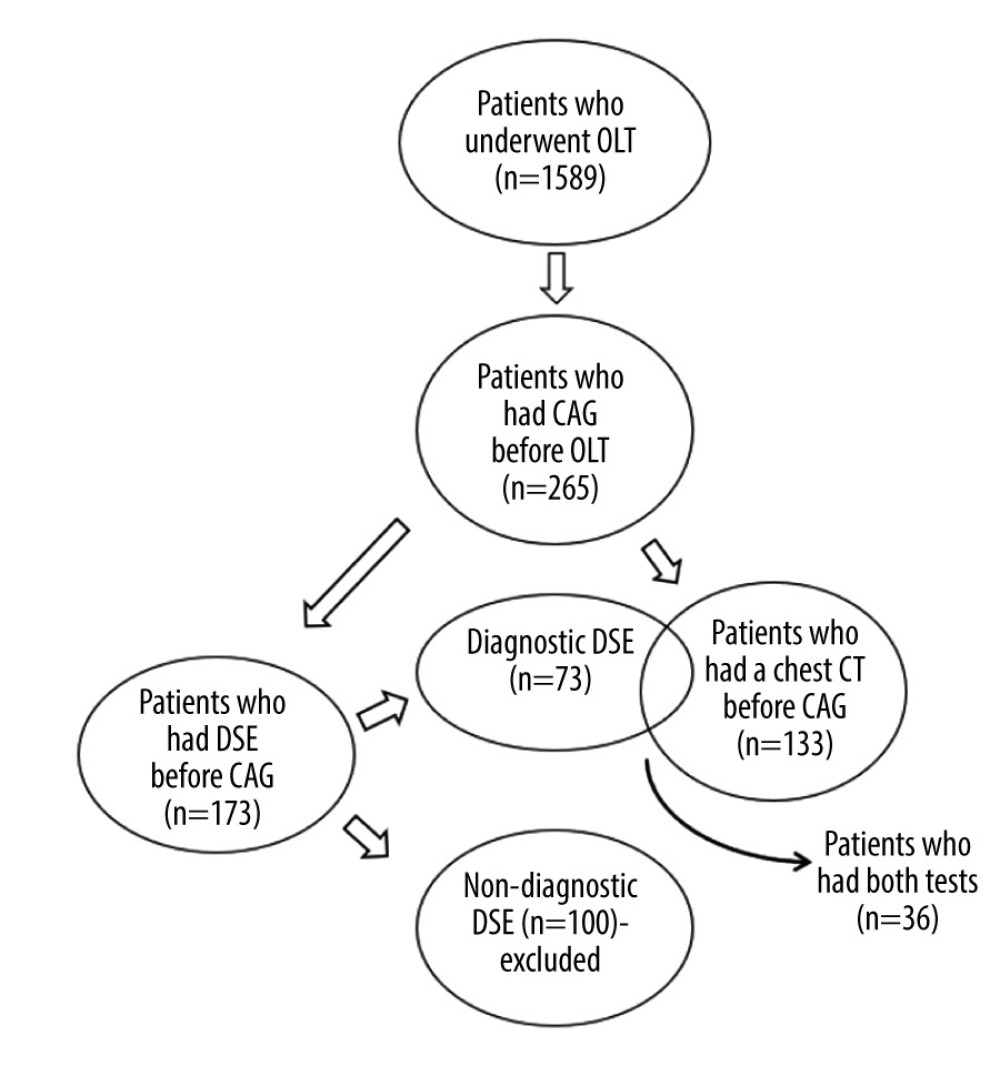 Figure 1. Flow diagram of study population selection. A total of 265 patients underwent coronary angiography prior to orthotopic liver transplantation. Of this sample, 173 patients had dobutamine stress echocardiography (DSE). Only patients with a diagnostic DSE were used for data analysis (n=73). A total of 133 patients had a chest computed tomography scan suitable for calcium scoring. A total of 36 patients had a diagnostic DSE and could be used for the head-to-head comparison of the 2 tests. Figure was created using Microsoft Word version 2010.
Figure 1. Flow diagram of study population selection. A total of 265 patients underwent coronary angiography prior to orthotopic liver transplantation. Of this sample, 173 patients had dobutamine stress echocardiography (DSE). Only patients with a diagnostic DSE were used for data analysis (n=73). A total of 133 patients had a chest computed tomography scan suitable for calcium scoring. A total of 36 patients had a diagnostic DSE and could be used for the head-to-head comparison of the 2 tests. Figure was created using Microsoft Word version 2010. 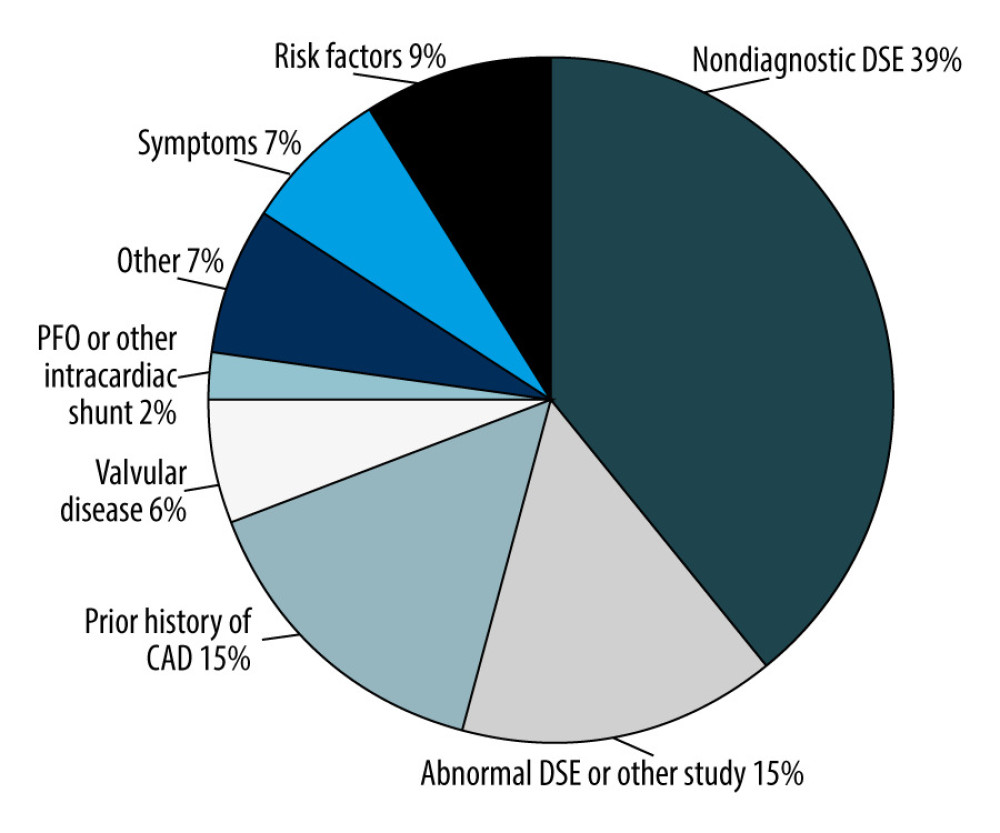 Figure 2. Distribution of various indications for angiography. Of the 265 patients who had coronary angiography (CAG), manual chart review was performed to determine why the patients were referred for CAG. The most common reason for referral for CAG was nondiagnostic dobutamine stress echocardiography (DSE) (39%), followed by an abnormal DSE (15%), and prior history of coronary artery disease (15%). Figure was created using Microsoft Word version 2010.
Figure 2. Distribution of various indications for angiography. Of the 265 patients who had coronary angiography (CAG), manual chart review was performed to determine why the patients were referred for CAG. The most common reason for referral for CAG was nondiagnostic dobutamine stress echocardiography (DSE) (39%), followed by an abnormal DSE (15%), and prior history of coronary artery disease (15%). Figure was created using Microsoft Word version 2010. 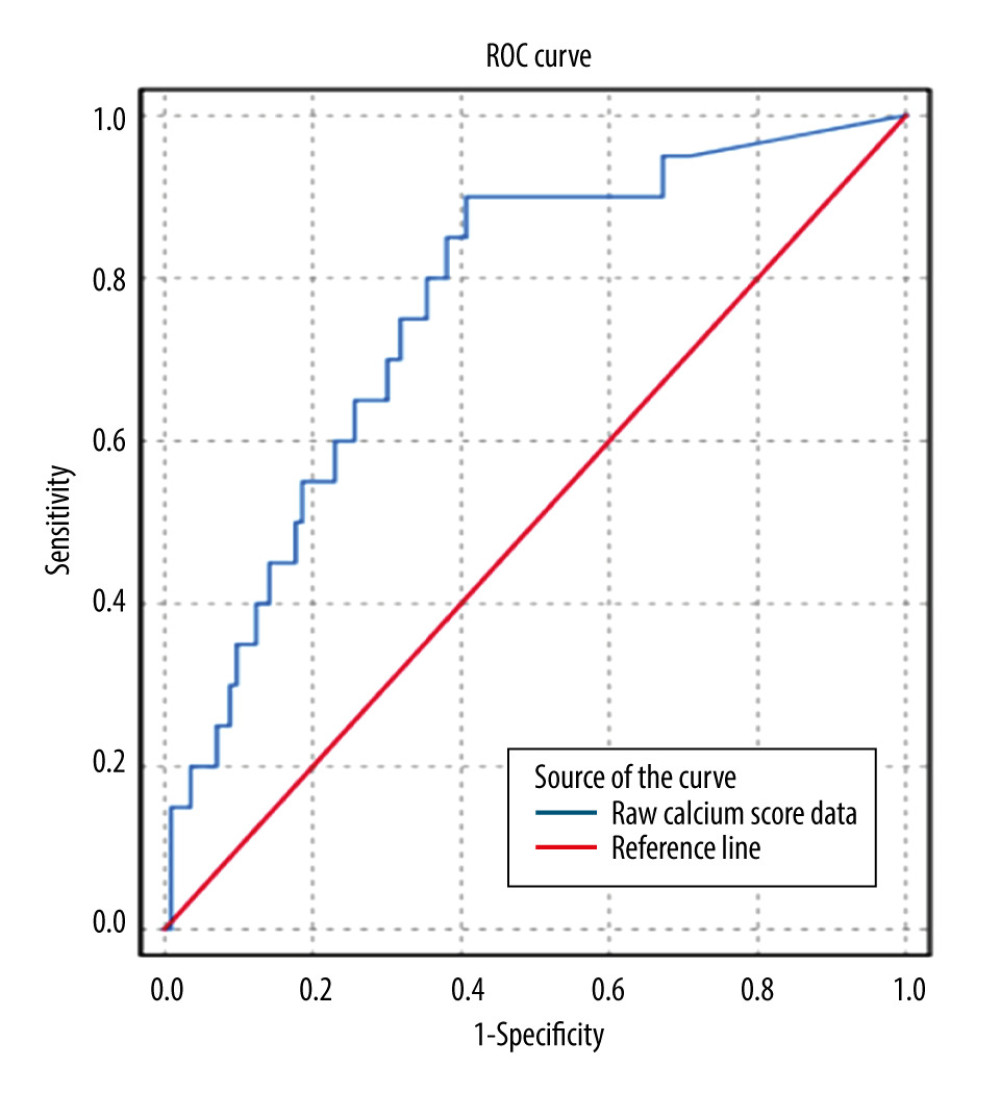 Figure 3. Receiver operating characteristic (ROC) curve for coronary artery calcium score (CACS) to predict obstructive coronary artery disease (CAD). Using the raw calcium score values, ROC analysis was performed to pictorially represent how the sensitivity and specificity of the CACS varied at different Agatston score (AS) values. CACS was a satisfactory predictor of the presence of obstructive CAD at a cutoff of ≥100 AS (AUC 0.76±0.06; 95% CI 0.66–0.87, P<0.001). Figure was created using SPSS v25.
Figure 3. Receiver operating characteristic (ROC) curve for coronary artery calcium score (CACS) to predict obstructive coronary artery disease (CAD). Using the raw calcium score values, ROC analysis was performed to pictorially represent how the sensitivity and specificity of the CACS varied at different Agatston score (AS) values. CACS was a satisfactory predictor of the presence of obstructive CAD at a cutoff of ≥100 AS (AUC 0.76±0.06; 95% CI 0.66–0.87, P<0.001). Figure was created using SPSS v25. Tables
Table 1. Baseline characteristics of patients.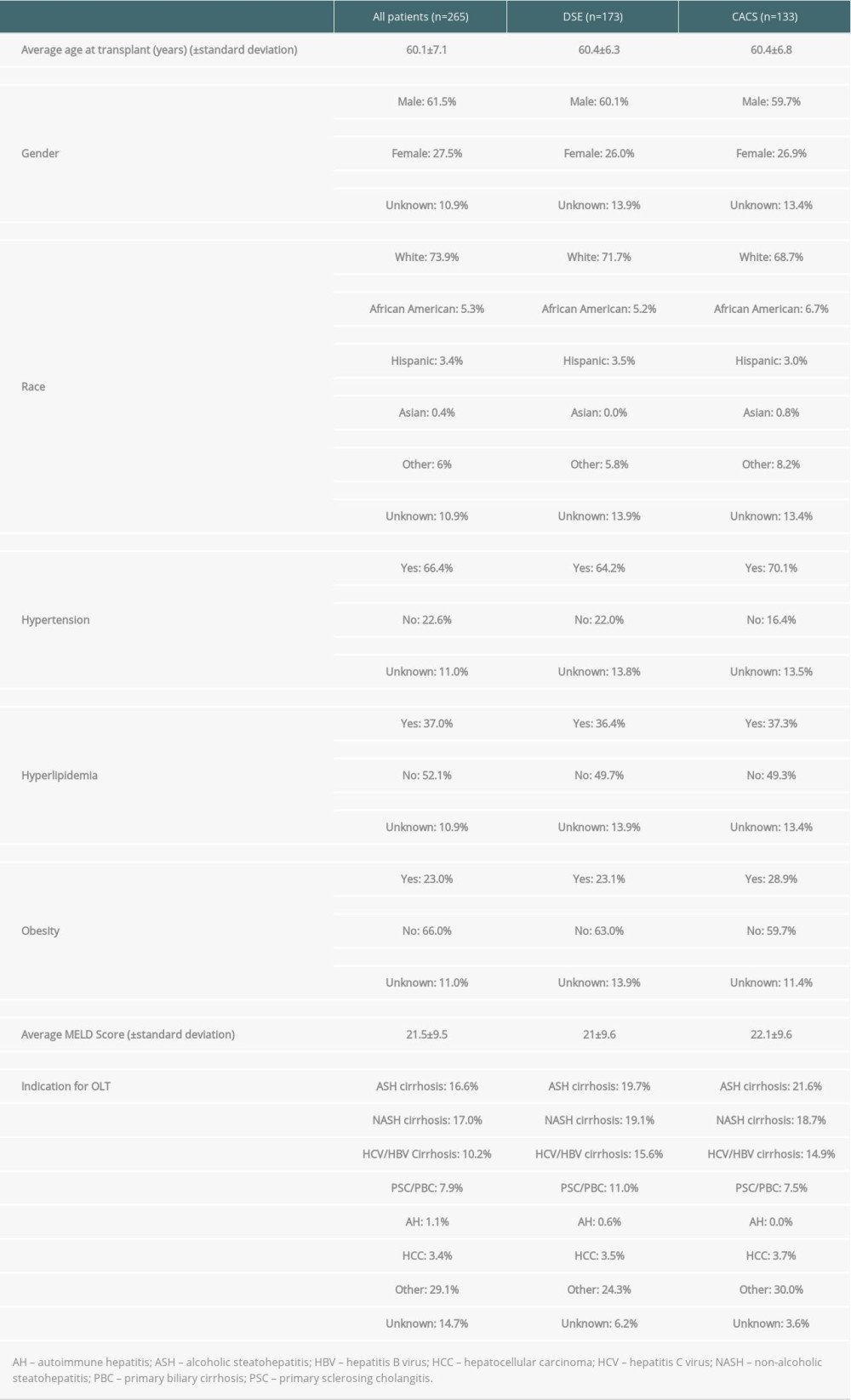 Table 2. Sensitivity, specificity, and positive and negative predictive value of dobutamine stress echocardiography vs coronary artery calcium score (head-to-head-comparison).
Table 2. Sensitivity, specificity, and positive and negative predictive value of dobutamine stress echocardiography vs coronary artery calcium score (head-to-head-comparison).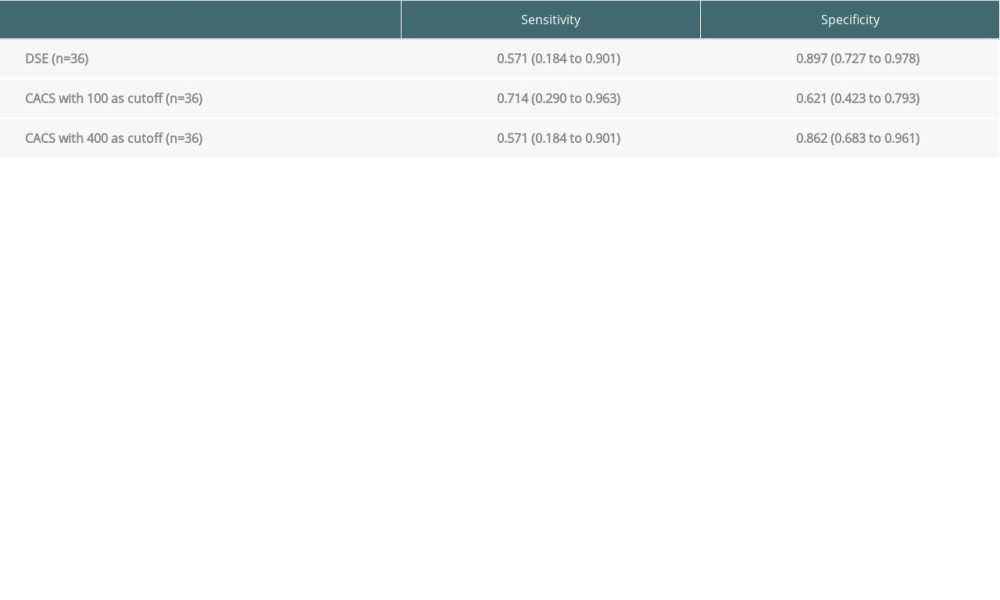 Table 3. Sensitivity, specificity, and positive and negative predictive value of coronary artery calcium score compared with dobutamine stress echocardiography for detection of coronary artery disease prior to liver transplantation (all patients with valid result for either test).
Table 3. Sensitivity, specificity, and positive and negative predictive value of coronary artery calcium score compared with dobutamine stress echocardiography for detection of coronary artery disease prior to liver transplantation (all patients with valid result for either test).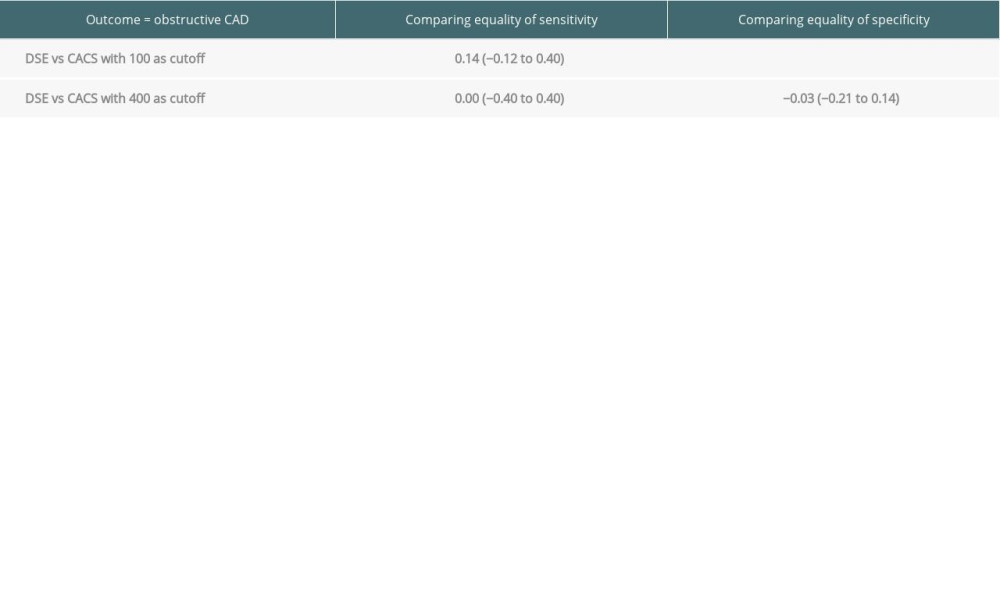 Table 4. Prior studies that have evaluated the sensitivity and specificity of dobutamine stress echocardiography for coronary artery disease in liver transplant candidates.
Table 4. Prior studies that have evaluated the sensitivity and specificity of dobutamine stress echocardiography for coronary artery disease in liver transplant candidates.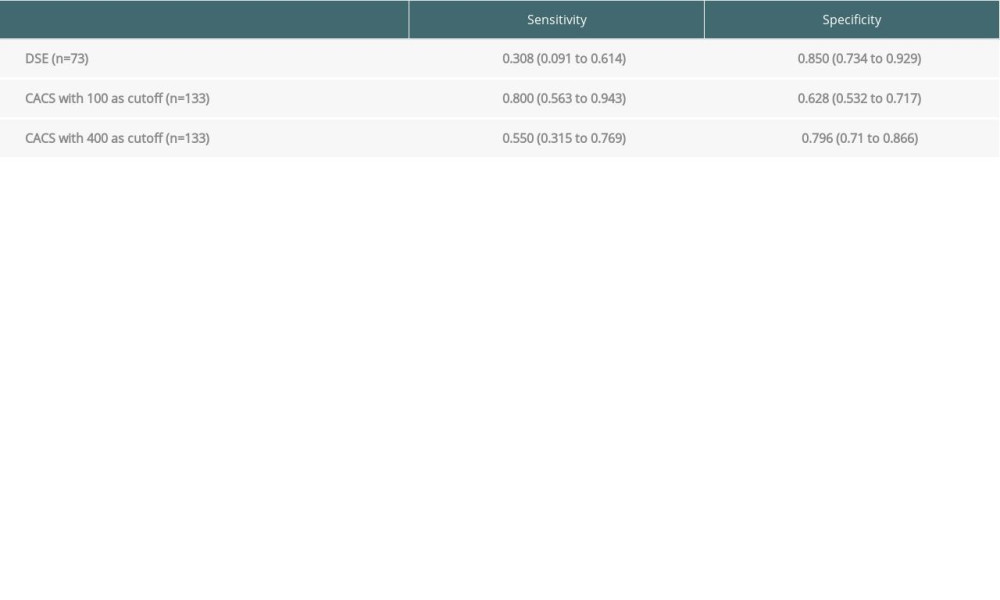 Table 5. Literature review: sensitivity/specificity of the coronary artery calcium score for coronary artery disease.
Table 5. Literature review: sensitivity/specificity of the coronary artery calcium score for coronary artery disease.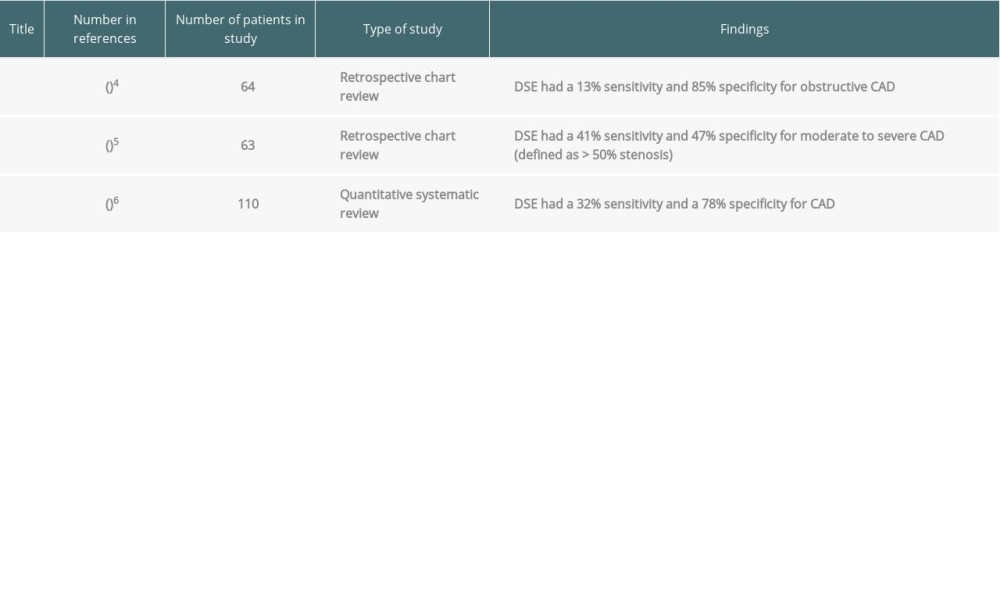
References
1. Koshy AN, Gow PJ, Han H-C, Cardiovascular mortality following liver transplantation: Predictors and temporal trends over 30 years: Eur Hear J, 2020; 6(4); 243-53
2. Arnett DK, Blumenthal RS, Albert MA, 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: Circulation, 2019; 140(11); e595-646
3. Martin P, Dimartini A, Feng S, Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation: Hepatology, 2014; 59(3); 1144-65
4. Harinstein ME, Flaherty JD, Ansari AH, Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates: Am J Transplant, 2008; 8(7); 1523-28
5. Snipelisky D, Levy M, Shapiro B, Utility of dobutamine stress echocardiography as part of the pre-liver transplant evaluation: An evaluation of its efficacy: Clin Cardiol, 2014; 37(8); 468-72
6. Nguyen P, Plotkin J, Fishbein TM, Dobutamine stress echocardiography in patients undergoing orthotopic liver transplantation: A pooled analysis of accuracy, perioperative and long term cardiovascular prognosis: Int J Cardiovasc Imaging, 2013; 29(8); 1741-48
7. Moller S, Cirrhotic cardiomyopathy: A pathophysiological review of circulatory dysfunction in liver disease: Heart, 2002; 87(1); 9-15
8. Sharma M, Yong C, Majure D, Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation: Am J Cardiol, 2009; 103(5); 742-46
9. McAvoy NC, Kochar N, McKillop G, Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation: Liver Transplant, 2008; 14(12); 1725-31
10. Kong YG, Kang JW, Kim YK, Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients: Br J Anaesth, 2015; 114(3); 437-43
11. Cheng VY, Lepor NE, Madyoon H, Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium: Am J Cardiol, 2007; 99(9); 1183-86
12. Agatston AS, Janowitz WR, Hildner FJ, Quantification of coronary artery calcium using ultrafast computed tomography: J Am Coll Cardiol, 1990; 15(4); 827-32
13. Doytchinova AT, Feigenbaum TD, Pondicherry-Harish RC, Diagnostic performance of dobutamine stress echocardiography in end-stage liver disease: JACC Cardiovasc Imaging, 2019; 12(11P1); 2115-22
14. Tiwari N, Margapuri J, Katamreddy A, Diagnostic accuracy of cardiac testing for coronary artery disease in potential liver transplant recipients: A systematic review and meta-analysis: IJC Heart Cardiol Heart Vasc, 2021; 32; 100714
15. Budoff MJ, Jollis JG, Dowe D, Min J, Diagnostic accuracy of coronary artery calcium for obstructive disease: Results from the ACCURACY trial: Int J Cardiol, 2013; 166(2); 505-8
16. Bielak LF, Rumberger JA, Sheedy PF, Probabilistic model for prediction of angiographically defined obstructive coronary artery disease using electron beam computed tomography calcium score strata: Circulation, 2000; 102(4); 380-85
17. Budoff MJ, Diamond GA, Raggi P, Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography: Circulation, 2002; 105(15); 1791-96
18. Lu DY, Steitieh D, Feldman DN, Impact of cirrhosis on 90-day outcomes after percutaneous coronary intervention (from A Nationwide Database): Am J Cardiol, 2020; 125(9); 1295-304
19. Arad Y, Goodman KJ, Roth M, Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: The St. Francis Heart Study: J Am Coll Cardiol, 2005; 46(1); 158-65
20. Blaha M, Budoff MJ, Shaw LJ, Absence of coronary artery calcification and all-cause mortality: JACC Cardiovasc Imaging, 2009; 2(6); 692-700
21. West BH, Low CG, Bista BB, Significance of coronary artery calcium found on non-electrocardiogram-gated computed tomography during preoperative evaluation for liver transplant: Am J Cardiol, 2019; 124(2); 278-84
22. Azour L, Kadoch MA, Ward TJ, Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges: J Cardiovasc Comput Tomogr, 2017; 11(1); 8-15
23. Mittal TK, Pottle A, Nicol E, Prevalence of obstructive coronary artery disease and prognosis in patients with stable symptoms and a zero-coronary calcium score: Eur Heart J Cardiovasc Imaging, 2017; 18(8); 922-29
24. Kemmer N, Case J, Chandna S, Neff GW, The role of coronary calcium score in the risk assessment of liver transplant candidates: Transplant Proc, 2014; 46(1); 230-33
Figures
 Figure 1. Flow diagram of study population selection. A total of 265 patients underwent coronary angiography prior to orthotopic liver transplantation. Of this sample, 173 patients had dobutamine stress echocardiography (DSE). Only patients with a diagnostic DSE were used for data analysis (n=73). A total of 133 patients had a chest computed tomography scan suitable for calcium scoring. A total of 36 patients had a diagnostic DSE and could be used for the head-to-head comparison of the 2 tests. Figure was created using Microsoft Word version 2010.
Figure 1. Flow diagram of study population selection. A total of 265 patients underwent coronary angiography prior to orthotopic liver transplantation. Of this sample, 173 patients had dobutamine stress echocardiography (DSE). Only patients with a diagnostic DSE were used for data analysis (n=73). A total of 133 patients had a chest computed tomography scan suitable for calcium scoring. A total of 36 patients had a diagnostic DSE and could be used for the head-to-head comparison of the 2 tests. Figure was created using Microsoft Word version 2010. Figure 2. Distribution of various indications for angiography. Of the 265 patients who had coronary angiography (CAG), manual chart review was performed to determine why the patients were referred for CAG. The most common reason for referral for CAG was nondiagnostic dobutamine stress echocardiography (DSE) (39%), followed by an abnormal DSE (15%), and prior history of coronary artery disease (15%). Figure was created using Microsoft Word version 2010.
Figure 2. Distribution of various indications for angiography. Of the 265 patients who had coronary angiography (CAG), manual chart review was performed to determine why the patients were referred for CAG. The most common reason for referral for CAG was nondiagnostic dobutamine stress echocardiography (DSE) (39%), followed by an abnormal DSE (15%), and prior history of coronary artery disease (15%). Figure was created using Microsoft Word version 2010. Figure 3. Receiver operating characteristic (ROC) curve for coronary artery calcium score (CACS) to predict obstructive coronary artery disease (CAD). Using the raw calcium score values, ROC analysis was performed to pictorially represent how the sensitivity and specificity of the CACS varied at different Agatston score (AS) values. CACS was a satisfactory predictor of the presence of obstructive CAD at a cutoff of ≥100 AS (AUC 0.76±0.06; 95% CI 0.66–0.87, P<0.001). Figure was created using SPSS v25.
Figure 3. Receiver operating characteristic (ROC) curve for coronary artery calcium score (CACS) to predict obstructive coronary artery disease (CAD). Using the raw calcium score values, ROC analysis was performed to pictorially represent how the sensitivity and specificity of the CACS varied at different Agatston score (AS) values. CACS was a satisfactory predictor of the presence of obstructive CAD at a cutoff of ≥100 AS (AUC 0.76±0.06; 95% CI 0.66–0.87, P<0.001). Figure was created using SPSS v25. Tables
 Table 1. Baseline characteristics of patients.
Table 1. Baseline characteristics of patients. Table 2. Sensitivity, specificity, and positive and negative predictive value of dobutamine stress echocardiography vs coronary artery calcium score (head-to-head-comparison).
Table 2. Sensitivity, specificity, and positive and negative predictive value of dobutamine stress echocardiography vs coronary artery calcium score (head-to-head-comparison). Table 3. Sensitivity, specificity, and positive and negative predictive value of coronary artery calcium score compared with dobutamine stress echocardiography for detection of coronary artery disease prior to liver transplantation (all patients with valid result for either test).
Table 3. Sensitivity, specificity, and positive and negative predictive value of coronary artery calcium score compared with dobutamine stress echocardiography for detection of coronary artery disease prior to liver transplantation (all patients with valid result for either test). Table 4. Prior studies that have evaluated the sensitivity and specificity of dobutamine stress echocardiography for coronary artery disease in liver transplant candidates.
Table 4. Prior studies that have evaluated the sensitivity and specificity of dobutamine stress echocardiography for coronary artery disease in liver transplant candidates. Table 5. Literature review: sensitivity/specificity of the coronary artery calcium score for coronary artery disease.
Table 5. Literature review: sensitivity/specificity of the coronary artery calcium score for coronary artery disease. Table 1. Baseline characteristics of patients.
Table 1. Baseline characteristics of patients. Table 2. Sensitivity, specificity, and positive and negative predictive value of dobutamine stress echocardiography vs coronary artery calcium score (head-to-head-comparison).
Table 2. Sensitivity, specificity, and positive and negative predictive value of dobutamine stress echocardiography vs coronary artery calcium score (head-to-head-comparison). Table 3. Sensitivity, specificity, and positive and negative predictive value of coronary artery calcium score compared with dobutamine stress echocardiography for detection of coronary artery disease prior to liver transplantation (all patients with valid result for either test).
Table 3. Sensitivity, specificity, and positive and negative predictive value of coronary artery calcium score compared with dobutamine stress echocardiography for detection of coronary artery disease prior to liver transplantation (all patients with valid result for either test). Table 4. Prior studies that have evaluated the sensitivity and specificity of dobutamine stress echocardiography for coronary artery disease in liver transplant candidates.
Table 4. Prior studies that have evaluated the sensitivity and specificity of dobutamine stress echocardiography for coronary artery disease in liver transplant candidates. Table 5. Literature review: sensitivity/specificity of the coronary artery calcium score for coronary artery disease.
Table 5. Literature review: sensitivity/specificity of the coronary artery calcium score for coronary artery disease. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








