01 March 2022: Review Paper
Techniques for Closing the Abdominal Wall in Intestinal and Multivisceral Transplantation: A Systematic Review
Allana C. Fortunato1ABCEF*, Rafael S. Pinheiro1ABCEG, Cal S. MatsumotoDOI: 10.12659/AOT.934595
Ann Transplant 2022; 27:e934595
Abstract
ABSTRACT: Short bowel syndrome is the most common etiology of intestinal failure, resulting from either resections of different intestinal segments or a congenital condition. Due to the absence or considerable reduction of intestinal loops in the abdominal cavity, patients with short bowel syndrome present with atrophy and muscle retraction of the abdominal wall, which leads to loss of abdominal domain and elasticity. This complication is an aggravating factor of intestinal transplantation since it can prevent the primary closure of the abdominal wall. A vast array of surgical techniques to overcome the challenges of the complexity of the abdominal wall have been described in the literature. The aim of our study was to review the modalities of abdominal wall closure in intestinal/multivisceral transplantation. Our study consisted of a systematic review following the methodological instructions described in the PRISMA guidelines. Duplicate studies and studies that did not meet the criteria for the systematic review were excluded, especially those without relevance and an explicit relationship with the investigated theme. After this step, 63 articles were included in our study. The results obtained with these techniques have been encouraging, but a high incidence of wound complications in some reports has raised concerns. There is no consensus among transplantation centers regarding which technique would be ideal and with higher success rates and lower rates of complications.
Keywords: Open Abdomen Techniques, Transplantation, Abdominal Wall, Organ Transplantation, Short Bowel Syndrome, Humans, Incidence, Intestines, Plastic Surgery Procedures
Background
Intestinal failure is defined as the inability to maintain homeostasis through enteral absorption of nutrients, electrolytes, and water [1–3]. Short bowel syndrome is the most common etiology of intestinal failure and results from either resections of different intestinal segments or a congenital condition. Parenteral nutrition is the first line therapy for intestinal failure; however, when life-threatening complications of parenteral nutrition occur, intestinal transplantation is the only curative treatment possible for these patients.
Owing to the absence or considerable reduction of intestinal loops in the abdominal cavity, these patients present with atrophy and muscle retraction of the abdominal wall, which leads to the loss of abdominal domain and elasticity. Furthermore, patients with short bowel syndrome often undergo multiple surgical procedures, leading to extensive abdominal scarring or even a frozen abdomen (Figure 1). This complication is an aggravating factor of intestinal transplantation since it can prevent the primary closure of the abdominal wall. Even in patients without short bowel syndrome, other situations commonly faced by potential intestinal transplantation recipients, such as abdominal adhesions resulting from previous surgical procedures, fistulas, ostomies, or extensive abdominal fibrosis that result from second intention healing of peritoneostomies/laparostomies [4], can also pose challenges for abdominal wall closure. In addition, edema after revascularization of the intestinal graft tends to exacerbate the difficulty of tension-free primary closure [5]. A forced closure attempt can result in intestinal ischemia, wound dehiscence, infection, abdominal compartment syndrome, respiratory complications, vascular thrombosis, and consequent graft necrosis [6,7].
Successful closure in this chronically ill and highly immunosuppressed population has been proven to be crucial, not only to decrease the risk of infections, fistulas, and mycotic aneurysms, but also to improve the survival of the graft and the patient [8,9]. The rate of primary abdominal wall closure after intestinal transplantation or multivisceral transplantation varies from 40% to 85% [10]. A vast array of surgical techniques to overcome the challenges of the complex abdominal wall have been described in the literature. The aim of our study was to review the modalities of abdominal wall closure in intestinal/multivisceral transplantation.
Our study consisted of a systematic review following the methodological instructions described in the PRISMA guidelines [11].
Initially, a search for data was performed in the Medline-PubMed database in English. Data collection was completed in November 2019. The interventions analyzed in our study consisted of the techniques used for abdominal wall closure during intestinal or multivisceral transplantation. The search on Medline was performed in the PubMed database (www.ncbi.nlm.nih.gov/pubmed) and was adapted using MeSH terms in English (intestinal OR small bowel OR multivisceral) AND (transplantation). After this initial search, we made other selections with the more specific terms as follows: (abdominal OR wall) AND (technique) AND (humans).
The sample consisted of articles published in indexed journals and selected by 2 independent researchers (ACF and RSP) who read and analyzed the abstracts according to the following inclusion criteria: 1) publication vehicle: indexed journals, since they present greater dissemination and access to researchers; 2) publication language
Our study was approved by the Research Ethics Committee of the institution.
The bibliographic search encompassed articles from national and international literature regarding techniques to correct complex abdominal defects in the setting of intestinal transplantation. In total, 250 abstracts of scientific articles were found in the databases. Studies that did not meet the criteria for the systematic review were excluded, including duplicate studies and especially studies not meeting the parameters of relevance and explicit relationship with the investigated theme. After this step, 63 articles were included in our study, as shown in Figure 2.
Discussion
REDUCED-SIZE GRAFTS:
Incompatibility in size makes the primary closure of the recipient’s abdominal wall one of the most important technical challenges related to intestinal transplantation. The use of pediatric donors for adult recipients or anatomical reduction of the graft are alternatives. Since the 1990s, small donors have been predominantly used for intestinal transplantation. The donor-to-recipient body weight ratio should be ideally between 1.1 and 0.76 [13], demonstrating that recipients with intestinal failure commonly have reduced weight in relation to their size. However, transplantation centers have increasingly accepted organs with considerable size incompatibility owing to the scarcity of donors that meet the ideal characteristics. The discrepancy between donors and recipients is a critical issue that significantly limits the availability of organs. In the United States, after 3 years on the intestinal transplantation waiting list, 17.6% of patients were still waiting for a donor [14]. Organ donation from larger donors would increase the availability of organs, potentially reducing waiting time. Pediatric recipients are the most affected because of the scarcity of pediatric donors. Thus, an option to prevent high mortality rates of patients on the waiting list is to consider the anatomical reduction of the intestinal graft.
The development of reduced-size intestinal grafts was aimed to increase the pool of availability of donors or isolated intestinal grafts; it is possible to overcome size differences greater than 10: 1 (body weight) between donors and recipients with this technique [15]. For liver and intestine reduced-size grafts, the use of reduction techniques [16] resulted in the use of organs from donors that were up to 5 times larger than recipients [17].
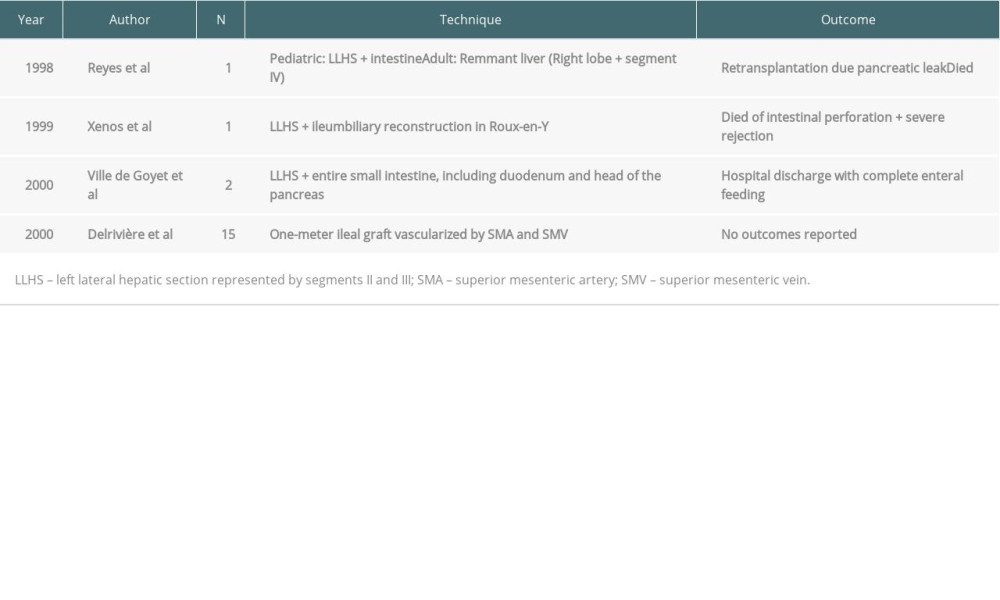
The first reduced-size graft was reported by Reyes et al [16] in 1998 (Table 1). The recipients were a 3-year-old boy with hepatic-intestinal insufficiency and a 63-year-old man with hepatitis C and hepatocarcinoma, both transplanted with the same adult deceased donor liver. The pediatric recipient received the left lateral hepatic section (segments II and III) in continuity with the intestine, with no need of biliary reconstruction (Figure 3). The remnant liver of the donor was transplanted to the adult recipient. In 1999, Xenos et al [18] described their technique for graft reduction. The technique consists of dividing the liver (left lateral section represented by segments II and III) and the small intestine (ileum) during a combined transplantation; biliary reconstruction was performed by Roux-en-Y hepaticojejunostomy.
In 2000, de Ville de Goyet et al [17] performed transplants in 2 children with chronic intestinal insufficiency, weighing 7.6 and 9.8 kg, respectively, with a multivisceral graft from 2 donors weighing 35 kg each. Multivisceral grafts had their size reduced during bench surgery (leaving the hepatic hilum untouched) and consisted of segments II and III of the liver and entire small intestine, including the duodenum and head of the pancreas. In these cases, there was also no need for biliary reconstruction owing to the preservation of the donors’ duodenum in continuity with the intestinal graft.
Also in the 2000s, 15 isolated small intestines were successfully reduced by Delrivière et al [15] by obtaining a 100-cm ileal graft vascularized by the superior mesenteric artery and vein. The technique consisted of dissecting the vascular pedicle through mesenteric transillumination. The dissection was initiated on the right side of the mesenteric pedicle, following the path of the superior mesenteric artery and superior mesenteric vein, and the branches on the right were connected and sectioned (ileocecal, right colic and medium colic arteries), except for the ileal branch, which was dissected all the way. The lymphatics of the ileal mesentery were preserved to guarantee the maintenance of lymphatic drainage. The use of the distal ileum effectively reduced the graft size due to the reduction in the size of the mesentery. During the dissection of the pedicle’s left side, all venous and arterial branches that did not supply the potential graft were connected and sectioned. All adipose tissue and lymph nodes around the mesenteric pedicle were removed to reduce mesenteric volume.
Graft reduction techniques had their “golden age” until the end of the 1990s, becoming obsolete due to their potential complications, complex techniques, and unsatisfactory results. Moreover, there is a significant risk of bleeding, fistula, or stenosis of the stapled portion, and, therefore, other techniques have been preferred to facilitate abdominal wall closure. The need for finding a standard and reproducible technique favored the emergence of alternative techniques, such as components separation, tissue expanders, synthetic and biologic meshes, aponeurosis transplantation with the rectus muscle fascia [7], and even total abdominal wall transplantation [4].
TISSUE EXPANDERS:
The first description of tissue expanders was made by Byrd and Hobar in 1989 [19]. Currently, they have been successfully used in general, pediatric, and plastic surgery to close large defects of the skin and abdominal wall (Figure 4).
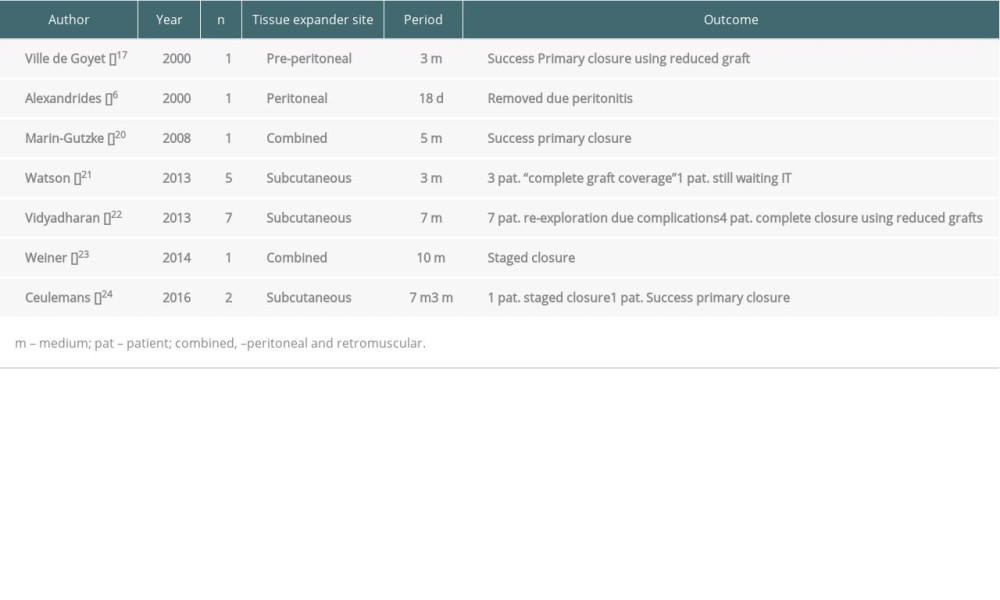
The use of tissue expanders before intestinal transplantation was first reported by Ville de Goyet et al in 2000 [17], following other authors (Table 2).
As in other abdominal closure techniques, tissue expanders have several potential disadvantages, such as the impossibility of predicting the date for performing the intestinal transplantation, additional exposure to anesthesia, difficulty in the healing process of malnourished patients, and complications related to gradual injection in the inflatable tissue expander [20].
Despite their versatility, tissue expanders have been associated with significant complications since the start of their use. An overall complication rate of 13% to 20% has been reported, with infection, prosthesis migration, and hematoma being most frequent [21]. The use of intraperitoneal tissue expanders can result in potentially worse complications, such as peritonitis and compression of intra-abdominal organs. The experience of using tissue expanders in patients awaiting intestinal transplantation is limited because of concerns related to the use of foreign material in critically ill patients as well as to space limitations caused by fistulas, scar tissue, and often stomata.
COMPONENT SEPARATION:
One possibility to facilitate abdominal closure after intestinal transplantation is abdominal wall component separation (CS). The first attempt to increase the capacity of closure in an abdominal defect was described in 1920 by Gibson [21] while performing relaxation incisions in the anterior fascia of the rectus abdominis muscle. A few decades later, Albanese [22] reported the fascial incision of the external oblique muscle to aid the repair of a large eventration. This surgery was repeatedly reproduced by Young [23], who modified it and described the performance of fascial incisions to relieve tension, separating the anterior and posterior sheath of the rectus abdominis muscle and then incising the lateral margin of the anterior fascia to repair epigastric hernias.
In 1990, the term “component separation” was defined by Ramirez et al [24], and the technique was widely popularized through the report of the correction of abdominal defects in 11 patients. The epigastric region was closed by approaching the left rectus abdominis, with its transverse abdominal and internal oblique muscles attached to the flaps of both the transverse abdominal and right internal oblique muscles. The left rectus abdominis was separated from its insertion in the lower rib cage, creating a rectus-pectoral flap to facilitate closure. The defect in the middle and lower abdominal wall was closed by rejoining the rectus abdominis, internal oblique, and transverse abdominal muscles to the remaining portion of the rectus abdominis, internal oblique, and transverse abdominal muscles. The external oblique muscles were independently advanced, as were the overlying skin flaps. After 4 months, the patient was reassessed, and the surgical wound was fully healed. The other 10 patients also had successful correction of the abdominal defect and none of them presented postoperative complications.
Surgical dissection and CS in the avascular plane fully preserves the innervation of the rectus abdominis muscles since the intercostal nerves that innervate this muscle run deeply to the fascia of the internal oblique muscle. According to Nguyen and Shestak [25], this innervated muscle complex can be advanced up to approximately 4 cm in the upper abdomen, 8 cm in the waist region, and 3 cm in the lower abdomen on each side, allowing the surgeon to reconstruct defects up to 16 cm wide at the waistline level. A small addition of 2 cm can be obtained by separating the anterior and posterior fascia of the rectus abdominis muscle above the arcuate line [25,26]. Access to the external oblique aponeurosis usually requires significant release of the skin and subcutaneous tissue. However, the extensive release of the subcutaneous tissue sharply increases complications related to the wound, reaching rates higher than 60% [27]. In response to this problem, several variations of CS have been described, including the release of the rectus abdominis sheath alone or in combination with the release of the transverse abdominal [28] and the use of botulinum toxin injection into the oblique muscles to perform a temporary relaxation of the components [29].
Some studies, such as the one by Black et al [30], reported the experience of using CS associated with the use of prosthetic meshes to correct abdominal wall defects in the transplanted population. Of the 19 patients who underwent defect correction with CS + biologic or synthetic mesh, 31.6% presented complications, such as seroma, hematoma, abscess, and dehiscence. Scheuerlein et al [31] identified greater recurrence of the abdominal defect using CS alone than with the combination of CS and biologic mesh. They also observed a lower incidence of postoperative complications in transplanted patients when using CS in association with biologic mesh, to the detriment of using synthetic mesh. Brewer et al [32] also demonstrated an advantage in the use of biologic mesh in transplanted patients after repairing abdominal hernias in 34 patients with biologic mesh and 26 with synthetic mesh. They observed that the patients who received the biologic mesh had a lower infection rate (65.4% vs 14.7%, P<0.05), fewer recurrences (76.9% vs 23.5%, P<0.05), and less need for mesh removal (69.2% vs 11.8%, P<0.05) in comparison with patients who received synthetic mesh.
A study at MedStar Georgetown University Hospital by Zolper et al [33] demonstrated that the use of CS associated with the use of biological meshes can be a successful strategy in the transplant population in an immunosuppressive regimen, despite concerns about the compromised abdominal vascularization of anterior abdominals surgeries. Soft tissue intervention is crucial for abdominal wall reconstruction in that population with previous scars; in addition, achieving stable skin coverage is equally important for strength to fascial repair, as wound healing compromise leads to a cycle of recurrent hernia [34].
A relevant factor for good results of the CS technique is the need for the wall components to be intact, well vascularized, innervated, and with complacency; that is, without fibrosis from previous scars [26]. However, this is a condition often lacking in intestinal transplantation candidates, owing to multiple previous interventions. Another complication factor is the presence of stomata, since the closure of the aponeurotic defect during the colostomy/ileostomy reversal surgery provides some tension on the ipsilateral fascia of the abdominal wall.
PROSTHETIC MESHES:
The interest of studying abdominal wall defects has existed since ancient times; the first references on the subject are from Egyptian civilization and are present in the Ebers Papyrus (1536 BC) [35]. The observation and correlation between a hernia and the weakness of the abdominal wall muscle is attributed to Galen (129–201 AD) [36]. The most recent revolution for the surgical treatment of abdominal defects emerged with the concept of tension-free surgery through the use of polypropylene mesh (Figure 5). Since then, the use of prostheses started with the aim of strengthening the abdominal wall, and the use of meshes was quickly disseminated [37].
Usher first used polypropylene meshes in 1959 in the United States, showing good results [38]. In Brazil, the first report was made by Felício Falci [39] in 1969 in a series with 100 patients who underwent inguinal hernia treatment. The tension-free correction of aponeurotic defects was widespread in the 1970s, especially in publications from Lichtenstein [37,40].
Still, in the 1950s, there was a publication describing the ideal characteristics that a prosthetic material should present: it should be chemically and biologically inert, mechanically stable, non-carcinogenic, suitable for sterilization, biocompatible, and hypoallergenic [41]. It is usually not easy to obtain a mesh that meets all of these criteria. To date, several materials have been used to manufacture prosthetic meshes, including knitted polypropylene [38], commercial polyester fabric [41], nylon, stainless steel [42], cotton [43], vicryl [44], expanded polytetrafluoroethylene (PTFE) [45], ethylene terephthalate, carbon fibers [46], oxidized cellulose, polyethylene glycol, and hylan G-F20 [47]. There are currently 3 types of meshes: synthetic, composite, and biologic.
SYNTHETIC MESHES:
Synthetic meshes can be subclassified as non-absorbable and absorbable types.
Non-absorbable meshes encompasses the following mesh types: polypropylene, polyester, and expanded PTFE [48]. Polypropylene mesh is permanent, widely used, consists of mono-, double-, or multi-filaments, and is lightweight (Vypro; Ethicon) or heavyweight (Marlex). Comparatively, the heavy mesh is thicker, with smaller pores and less elasticity, also presenting increased tensile strength. All polypropylene meshes generate a vigorous foreign body reaction and produce strong repair through rapid integration into the abdominal wall. However, these same properties also make polypropylene meshes susceptible to adhesions, obstructions, formation of fistulas, retraction, and bacterial growth.
Polyethylene terephthalate (polyester) mesh is non-absorbable and is manufactured to be more malleable, less inflammatory, and more resistant to infections. Polyester meshes include Dacron (DePoy Intl, Leeds, UK), Mersilene (Ethicon), and Symbotex (Covidien, Mansfield, MA, USA).
The expanded PTFE mesh is another permanent and synthetic mesh, manufactured with 2 surfaces, 1 ventral and 1 visceral [48]. The ventral surface faces the abdominal wall and has a rough surface with large pores to facilitate incorporation, while the visceral surface faces the peritoneal content and is designed to be smoother with smaller pores to prevent adhesions, obstructions, and fistulas. PTFE meshes include Teflon (EI DuPont de Nemours and Company, Wilmington, DE, USA) and GORE-TEX (WL Gore & Associates, Wall Township, NJ, USA).
Absorbable synthetic meshes (polyglycolic acid, polyglactin) are useful for repairing hernias for a short period because they induce little inflammatory reaction before their hydrolysis and are considered to be made of unsatisfactory material for use in permanent replacement of the abdominal wall [49].
Non-absorbable meshes are preferred for repairing uncontaminated abdominal wall defects, while absorbable meshes are preferred for repairing infected abdominal wall defects until resolution of the infection; then, they can be replaced with a non-absorbable mesh. However, the mesh is a foreign body, being an important cause of peritoneal adhesion, particularly if it is used intraperitoneally. Non-absorbable meshes also present a higher risk of infection as an additional risk factor [50,51]. The ideal mesh maintains adequate and permanent occlusion of the abdominal wall defect, with low rates of infection and adherence, and does not induce fistula formation [52–54].
COMPOSITE MESHES: The second mesh category is called composite, which can be coated in the parietal or ventral surface [48]. These meshes were produced because of the increase in laparoscopic repairs of ventral hernias as well as because of the increase in open surgery complications, culminating in the removal of the polypropylene, polyester, or PTFE mesh. The composite mesh is a dual-surface material, with the ventral surface being a non-absorbable mesh to be incorporated into the abdominal wall, and the visceral surface may be coated with a temporary resistant barrier (coated composite mesh) or a permanent barrier (double-sided mesh) against adhesions. Common coated composite meshes include Proceed (Ethicon) and Sepramesh (CR Bard), while the dual-surface composite meshes are Composix (CR Bard) and DualMesh (WL Gore & Associates).
BIOLOGIC MESHES: The last mesh type is the biologic, which emerged from the need for closure of contaminated or potentially contaminated areas with meshes [48]. Introduced in the United Kingdom in the 1990s and approved by the FDA in the 2000s [55], biologic meshes allow tissue infiltration and regeneration, attracting native fibroblasts and promoting neovascularization [56,57] and serving as a matrix for re-epithelialization. This culminates into the deposit of antibiotics and defense cells on the mesh, providing the ability to resist infection in hostile environments and not requiring removal in cases of infection [58,59]. Common meshes in this category include AlloDerm and Strattice (Lifecell Corporation, Branchburg, NJ, USA), DuraDerm (CR Bard), BioDesign (Cook Medical Inc, Bloomington, IN, USA), Tutopatch (Tutogen Medical Inc, Alachua, FL, USA), and Permacol (Medtronic, UK).
The composition of the biologic mesh is mainly of processed acellular dermal matrix derived from porcine, bovine, or human sources, making it immunologically inert. These products are supplied in non-crosslinked and crosslinked types. Crosslinking is thought to increase durability, tensile strength, and enzymatic degradation as well as to decrease bacterial contamination [59]. However, Romain et al did not observe a significant difference in hernia recurrence rates after 11 months between the groups of patients who received crosslinked and non-crosslinked porcine mesh in their series of 39 patients [59]. Although all biologic xenograft meshes provide matrices for cell growth, not all undergo similar processing techniques and, as a result, are remodeled in different ways [60].
There is no consensus on the body’s reaction to different types of meshes. For example, Mulier et al [60] found that Permacol (crosslinked) and Strattice (non-crosslinked) showed endovascular growth and collagen infiltration in the host. On the other hand, Novitsky et al [61] reported that improved inflammatory response and foreign body reaction are responsible for encapsulation and poor integration in the host of crosslinked biologic meshes. In addition, Deeken et al [62] demonstrated that crosslinked materials caused greater fibrous encapsulation in the first 6 months, although the materials of the studied mesh exhibited a substantial decrease in encapsulation between 6 and 12 months, making it similar to non-crosslinked materials for 12 months.
In contaminated wounds, the rates of infection at the surgical site and recurrence between synthetic and biologic meshes were 21.5% and 13.5% and 21% to 17.8%, respectively, favoring the use of biologic mesh in this scenario [63]. In a major single-center study of 512 patients who underwent abdominal wall reconstruction using biologic mesh, the rates of hernia recurrence were reduced to 11.5% after 3 years and 14.6% after 5 years [64]. Unfortunately, biologic meshes are exceptionally expensive. A critical aspect of any intervention analysis is the cost-benefit ratio. The cost of a non-crosslinked porcine acellular skin in 2012 was approximately US $32.40/cm2; thus, the cost of a 25×40 cm prosthesis is in the range of US $32 000 [65]. Comparatively, polypropylene mesh of a similar size costs approximately US $0.15/cm2, effectively costing 100 times less than the biologic type [66].
The use of prostheses to repair abdominal wall defects has brought new problems. Although the mesh reduces the rates of hernia occurrence, it has its own set of complications; infection is one of the most devastating complications after any mesh implantation [67]. The risk of infection in correcting abdominal wall defects appears to be greater than in other clean cases; however, there is a wide variety reported in the literature, from 1% to 10% [68], depending on the type of mesh, technique, and population. Infection of prostheses in the abdominal wall can cause serious and expensive consequences, as well as a serious impact on the patient’s life owing to prolonged hospitalizations, multiple reinterventions, and high social costs.
Postoperative wound infection usually appears on a surgical wound within 30 days to 1 year after surgery, with cases involving an implanted prosthesis [69–71]. Surgical site infections (SSI) remain the second most common type of health care-associated infection in Europe and the United States, with SSI accounting for 20% of all health care-associated infections in hospitalized patients [72]. The World Health Organization has shown that health care-associated infections are associated with an increase in morbidity, mortality (which may exceed 10% in certain infections) [72], hospitalization, and total costs [71]. In particular, the prolonged hospital stay for SSI presented an additional 9.7 days, and the extra hospitalization costs ranged between US $1087 and US $29 443 per infection [73,74]. According to the National Nosocomial Infections Surveillance System, the distribution of Tillman pathogens in surgical infections seems to have changed in the past 10 years [71,75]. The most frequently isolated bacteria are S. aureus, followed by coagulase-negative Staphylococci, E. coli, E. faecalis, Pseudomonas aeruginosa, Enterobacter spp, and Klebsiella. [73]
A study published in 2017 by Bueno-Lledó et al [76], including 3470 patients who underwent correction of an abdominal defect with a prosthesis, demonstrated that the use of corticosteroids or immunosuppressive drugs, surgical time greater than 180 min, and concomitant enterotomy are risk factors related to infection, abdominal defect recurrence, and need for mesh removal, conditions naturally found in intestinal transplantation. Moreover, the use of mesh can complicate future surgical approaches, specifically by limiting access to the graft for post-transplant abdominal exploration, retransplantation, or explantation.
USE OF MESHES IN INTESTINAL TRANSPLANTATION: In the study by Di Benedetto et al [77] conducted between December 2001 and November 2004, 27 intestinal transplantations were reported, including 20 isolated grafts, 3 multiviscerals with the liver, and 4 without the liver. Four patients who received small-intestine isolated grafts had difficulty in the abdominal wall closure, requiring prosthetic mesh; however, the 4 patients required explantation of the mesh in the first months owing to prosthesis infection.
Alexandrides et al [6] reported 8 pediatric intestinal transplantation cases; in 6 patients, the abdominal defect was treated with a PTFE mesh and in 2 patients with Silastic, both sutured at the border of the abdominal aponeurosis. The mesh was generally replaced in a series by a smaller mesh with progressive advancement of the surrounding abdominal wall, leading to closure in steps. However, most of these patients died before the final closure. The authors strongly recommend early removal when using Silastic mesh.
This technique was performed to provide safe and temporary abdominal closure, minimize fluid and electrolyte losses, and minimize contracture of the abdominal wall [78]. On the other hand, the prolonged use of mesh with the intention of secondary healing is not recommended in adults due to the potential development of enterocutaneous fistulas and infection [79,80]. This development can be an even more difficult problem for the group of transplanted patients, who are generally immunocompromised due to the administration of anti-rejection agents, such as calcineurin inhibitors and steroids.
Thus, the delay in definitive closure increases the risk of complications. Furthermore, the use of meshes in intestinal transplantation is related to higher morbidity. Additionally, the cost of the mesh and the cost of treating associated complications have been limiting factors. [7]
ABDOMINAL WALL TRANSPLANTATION:
In the last 15 years, owing to improvements in microsurgery techniques, abdominal wall transplantation has become a reality and has allowed for the restoration of the abdominal domain in complex scenarios. This type of transplantation was introduced in 1999 in the context of reconstruction of complex abdominal wall defects along with multivisceral transplantation. In their first publication, Levi et al [4] observed that approximately 20% of patients receiving intestinal transplantation or multivisceral transplantation do not have enough abdominal wall tissue for primary closure due to previous laparotomies, enterocutaneous fistulas, and ostomies [4]. Several types of abdominal wall transplantation have been described, including non-composite and non-vascularized allografts, non-composite and vascularized allografts, and composite and vascularized allografts.
NON-COMPOSITE AND NON-VASCULARIZED ALLOGRAFT: This method is based on the use of both layers of the rectus abdominis muscle fascia after muscle removal [81]. The harvesting and implantation of this type of graft is a simple procedure that does not require vascular anastomoses. This method is ideal if there is a large aponeurotic defect with mobility of the skin.
The technique for graft harvesting, described by Gondolesi et al [7,81], consists of a median thoracoabdominal incision during organ harvesting. The incision is deepened to the fascia, and the subcutaneous tissue is mobilized to the lateral border of the rectus muscle. The rectus muscle, including its anterior and posterior fascia, is incised subcostally and laterally to the rectus muscle on both sides and is then excised by dividing it suprapubically. Then, it is wrapped in gauze soaked in saline solution, placed in a sterile bag with preservation solution for cold storage, and transported in a cool box with ice.
After harvesting, bench surgery becomes a fundamental step to properly separate the anterior and posterior lamina from the rectus abdominis muscles fascia and to remove the fat layer from the anterior lamina left by the entry of the perforating arteries, which must be closed with polypropylene 6-0 sutures. At the end of bench surgery, a graft consisting of a double-layered fascia is preserved in a University of Wisconsin (UW) or HTK solution and is stored at 4°C to be used at the end of the recipient’s surgery. The recipient’s wound should be properly prepared before closure, with a prolonged dissection of the subcutaneous tissue and skin flaps to the sites, in which the gastrojejunostomy and/or ileostomy are placed to cover the entire defect with the fascia graft. The fascia is usually fixed using 2 continuous sutures with non-absorbable thread. When skin closure is not feasible, a negative-pressure wound dressing should be placed directly on the graft [7,82].
Gondolesi et al [7] published results from 16 patients who received non-vascularized rectus abdominis muscle fascia. Abdominal wall infection developed in 7 of the 16 patients, and 3 of the affected patients did not have sufficient skin coverage over the fascia; 2 of these patients required removal of the fascia graft. Since 2009, 18 other cases have been reported. The Berlin group used the rectus abdominis muscle fascia in 5 adults, with no report of infection [81]. In the updated experience of 19 patients (9 pediatric and 10 adult) who received the fascia of the rectus abdominis muscle, Gondolesi et al [81] reported that no patient had ventral hernia in an average follow-up period of about 5 years. In their study, 12 of the 19 recipients required 23 reoperations due to local complications.
More recently, in 2019, Cassar et al [83] reported 2 cases of pediatric patients who received non-vascularized fascia grafts. In both cases, the wound was completely healed. Also in 2019, a Spanish group [84] reported 2 cases of multivisceral transplantation in adults using the non-vascularized allograft for abdominal wall closure. The first patient died with multiple infections (pneumonia and intra-abdominal collections). The second patient had no complications and presented complete incorporation of the fascia graft.
The advantages of a non-vascularized rectus abdominis’ fascia graft include the fact that the grafts are potentially “non” immunogenic and that vascular anastomoses are not necessary. The fascia of the rectus abdominis muscle is avascular, poorly cellular, and composed mainly of fibrous tissue [7], which is responsible for its low immunogenicity. Moreover, the graft can come from the same or a different organ donor, can be used on the same day of the transplantation or be preserved for up to 21 days in UW or HTK at 4°C, or can even be cryopreserved. Additional advantages are that the tissue is capable of resisting multiple reoperations and wound infections and can be replaced by another compatible graft when necessary [83]. In the long term, it is integrated with the abdominal wall and does not cause adhesions when the internal peritoneal layer is preserved. However, the literature shows that abdominal infection is associated with 44% of non-vascularized allografts [7].
VASCULARIZED AND NON-COMPOSITE ALLOGRAFT: This method consists of using the posterior rectus sheath’s fascia in continuity with the falciform ligament when harvesting the liver (and other organs, if there is multivisceral harvesting) [85]. Since the fascia maintains a blood supply through the falciform ligament, there are fewer infectious complications. This type of graft is indicated when there is a combined liver-intestinal transplantation or multivisceral transplantation. The falciform ligament goes inferiorly from the liver to the umbilicus and often carries an arterial branch called the hepatic falciform artery [86]. This branch comes from the hepatic artery (usually the left) and provides blood flow to the sheath of the posterior rectus abdominis muscle. This forms the anatomical basis for the use of the posterior rectus abdominis fascia as a vascularized allograft when placed together with a liver graft.
The technique for obtaining the vascularized graft consists of an incision in the midline through the skin and subcutaneous tissue, but not through the linea alba. The sheath of the anterior rectus abdominis is incised on both sides of the linea alba, and the rectus abdominis muscles along with the anterior sheath, subcutaneous tissue, and skin are displaced to expose the sheath of the posterior rectus abdominis. Parallel incisions (through the posterior sheath and peritoneum) are made in the lateral border of the rectus abdominis sheath on both sides. A lower transverse incision is made just above the umbilicus, and an upper transverse incision is made in the xiphoid area. At this point, the sheath of the posterior rectus abdominis is connected only to the falciform ligament (which is in continuity with the donor’s liver). The liver is then harvested as usual in continuity with these structures [85].
Recipient hepatectomy and liver preparation in bench surgery are routinely performed. The flap from the posterior rectus abdominis sheath is inserted as an embedded flap for establishing adequate perfusion of the liver graft. In the recipient, as the incision is preferably vertical, the anterior and posterior sheaths is medially separated and sutured at the graft edges. The closure of skin is primary or in series, depending on the patient [85].
In 2010, Agarwal et al [87] reported their experience using this technique in a 3-year-old patient. There were no complications in the abdominal wall throughout the patient’s treatment. No tissue biopsies showed signs of acute rejection. Later, in 2013, Lee et al [85] reported their experience in using the sheath fascia of the vascularized posterior rectus abdominis in 4 pediatric patients in a 2-year period. At the time of publication, 3 of the 4 patients were still alive with a functioning liver and abdominal wall.
The vascularized transplantation of the abdominal wall has as a disadvantage the risk of ischemia secondary to thrombosis and graft necrosis [84]. In addition, it is necessary to alert the transplant team to harvest the sheath of the posterior rectus abdominis and the falciform ligament in continuity with the donor’s liver.
VASCULARIZED AND COMPOSITE ALLOGRAFT: Abdominal wall transplantation associated with intestinal transplantation was first reported in 2003 [4]. The vascularized and composite allograft of the abdominal wall comprises the peritoneum, posterior rectus abdominis sheath, both rectus abdominis muscles, anterior rectus abdominis sheath, overlapping fat and skin, and parts of the internal oblique, external oblique, and the transverse abdomen muscles (Figure 6). The combination of 2 highly immunogenic grafts, the intestine, and the abdominal wall (including the donor’s skin) at that time represented an unknown immunological risk for allograft rejection and graft-vs-host disease [88].
Skin, as an immunogenic organ with half of its cells belonging to or related to the immune system [89,90], did not appear to be transplantable at first, but a better understanding of the immune basis of rejection [90,91] made the skin an acceptable part of a graft [92].
Pioneers in this procedure, the Miami group initially used an inverted U incision, which provided a good-sized graft for transplantation, but which can also hinder or preclude the closure of abdominal defects in donors, an important aesthetic issue [93]. In many countries, this would have a low acceptability rate with coordinators and family members of donors. The Oxford group used an elliptical, longitudinal incision over the 2 rectus abdominis muscles, leaving the pubic insertion inferiorly and the lower epigastric vessels bilaterally entering the deep surface of the graft [93]. The harvesting of abdominal organs is performed as usual and the flap of the abdominal wall is perfused with the other organs. After finishing the harvesting of organs, the detachment of the flap from the abdominal wall is completed. The vessels are divided with minimal dissection at the origin of the external iliac arteries. The abdominal wall is flushed with UW preservation solution, stored in UW solution, and placed in a cool box with ice [93].
In 2003, Levi et al [4] described transplanting an abdominal wall graft using the donor’s lower epigastric vessels (maintained in continuity with femoral and iliac vessel grafts) and implanting them to the recipient’s common iliac artery and vein. Subsequently, the technique was modified by Cipriani et al [94], using a microsurgical technique: the donor’s epigastric pedicles were anastomosed directly with the recipient’s epigastric vessels, without the need for access to the recipient’s femoral and iliac vessels.
In 2014, Giele et al [95] from Oxford faced a different issue related to abdominal wall transplantation: storage and subsequent ischemia-reperfusion injury of the abdominal wall graft during the procedure was longer than 5 h. The presence of teams working at the same time on the recipient was an alternative to minimize the ischemia time, with 1 team performing the intestinal transplantation and the other remotely revascularizing the abdominal wall in the blood vessels of the recipient’s forearm. The duration of the procedure was an average of 50 min (range, 30–60 min). At the end of the transplantation, the wall graft was revascularized in the abdomen.
Other groups reported series of abdominal wall transplantation [96]. In a review of 35 full-thickness vascularized abdominal wall transplantations after intestinal/multivisceral transplantations [93], the successful rate of abdominal closure after abdominal wall transplantation was very high, with 88% graft survival and no related mortality [97]; follow-up generally ranged from 6 to 7 months.
Moreover, an advantage is that the cutaneous component of the abdominal wall can serve as an immune modulator: a recent study [93] analyzed a small cohort of 29 intestinal/multivisceral transplantations, 14 of which were combined with abdominal wall transplantation. The advantage of carrying a wall graft was demonstrated by the lower rate of intestine rejection (7% vs 27%) and lower rate (14% vs 33%) of diagnostic errors (viral infection vs rejection), followed by better survival of the intestinal graft (79% vs 60%).
Without noninvasive markers that are reliable, rejection has always been the most feared complication after intestinal transplantation. Clinical symptoms such as diarrhea, abdominal distension, and fever have often been associated and preventively considered as substitute markers for rejection [98]. The histological analysis of mucosa remains the criterion standard for detecting rejection [99], but it also creates the risk of ulceration or perforation of the graft as well as a diagnostic pitfall. Conversely, the cutaneous component of the transplanted allograft is easily accessible and can be monitored in a more consistent and less harmful way than that of the visceral organs during the rejection process [100] (Figure 7). Although the monitoring of small intestine rejection through the skin allograft is a fascinating hypothesis, the use of skin allograft to monitor the function of the small intestine should be considered with caution [94].
Abdominal wall transplantation appears to have some disadvantages: at the time of harvesting, the flap is disconnected from its nerve connections located on the sides of the abdomen, resulting in the denervation of the abdominal graft. The absence of nerve stimuli leads to progressive flap hypotrophy, resulting in an abdominal wall with no muscle tone [94]. In addition, the procedure is still limited to a few transplantation centers, where the expertise of the transplant team is well integrated with the plastic surgery team. Owing to the small number of cases presented to date, it is not possible to make a definitive statement related to the best technique.
Conclusions
Expanding the abdominal domain or replacing the missing or damaged abdominal wall is one of the challenges of intestinal transplantation. Several techniques are available to overcome this issue, and whichever approach is performed, it is important to notice that they may not be mutually exclusive and that both approaches can be combined in the same recipient to ensure success of the procedure. The results obtained with these techniques have been encouraging, but a high incidence of wound complications in some reports has raised concerns. Moreover, there is no consensus among transplantation centers regarding which technique would be ideal with higher success rates and lower rates of complications.
Figures
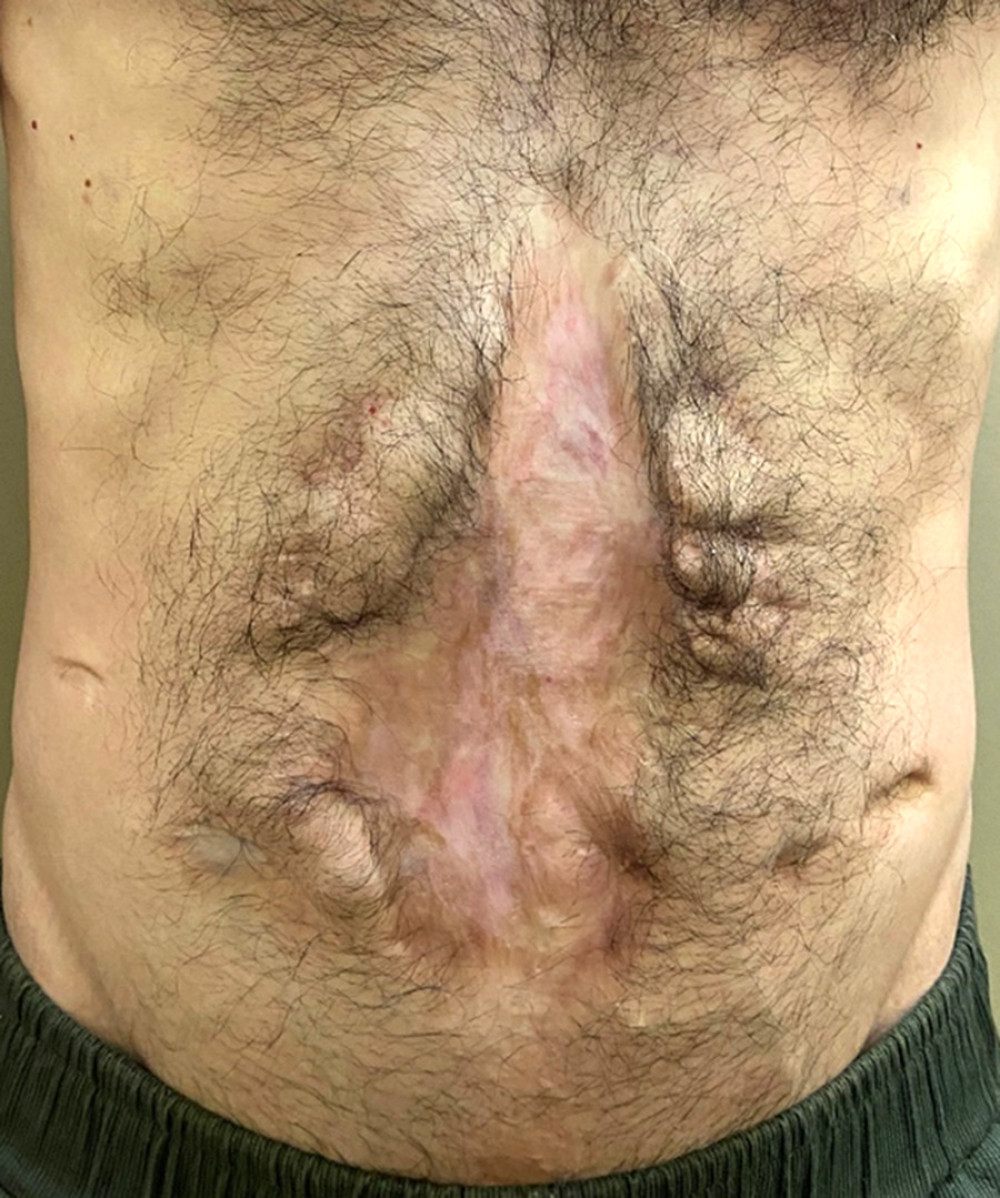 Figure 1. Extensive abdominal fibrosis resulting from second intention healing of peritoneostomy and ostomy scars. (Arsenal of the department.)
Figure 1. Extensive abdominal fibrosis resulting from second intention healing of peritoneostomy and ostomy scars. (Arsenal of the department.) 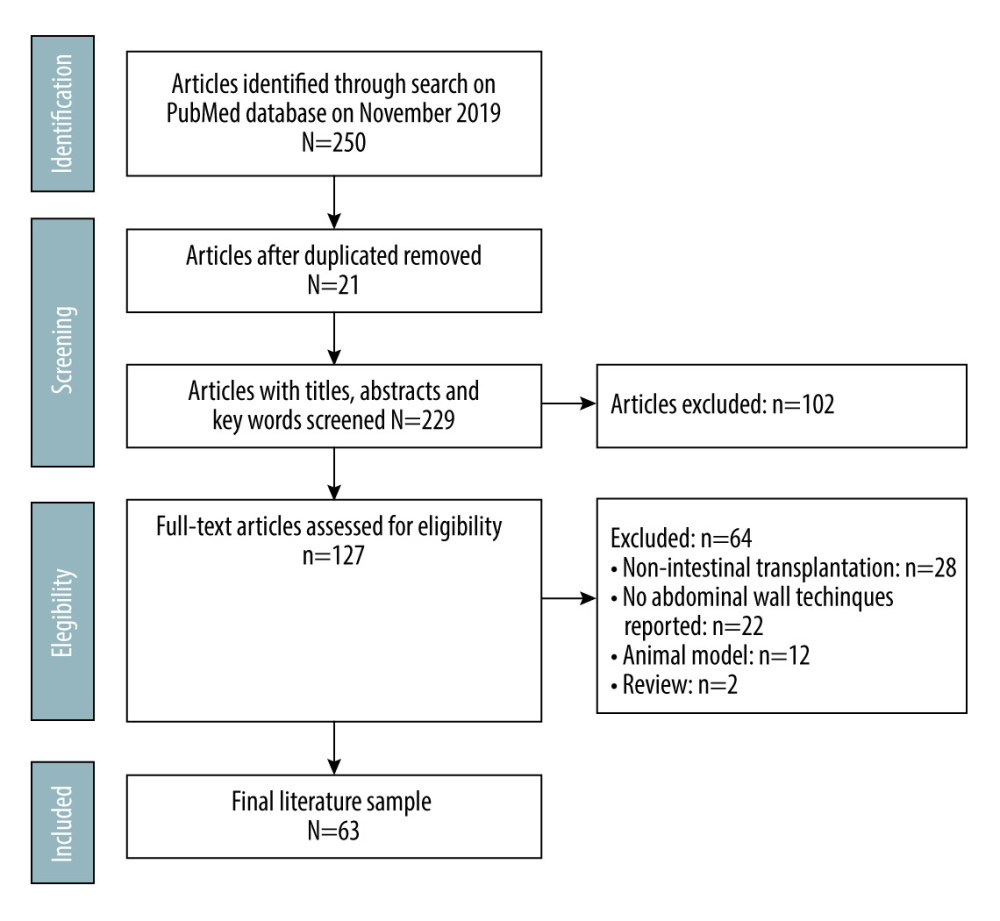 Figure 2. Study design.
Figure 2. Study design. 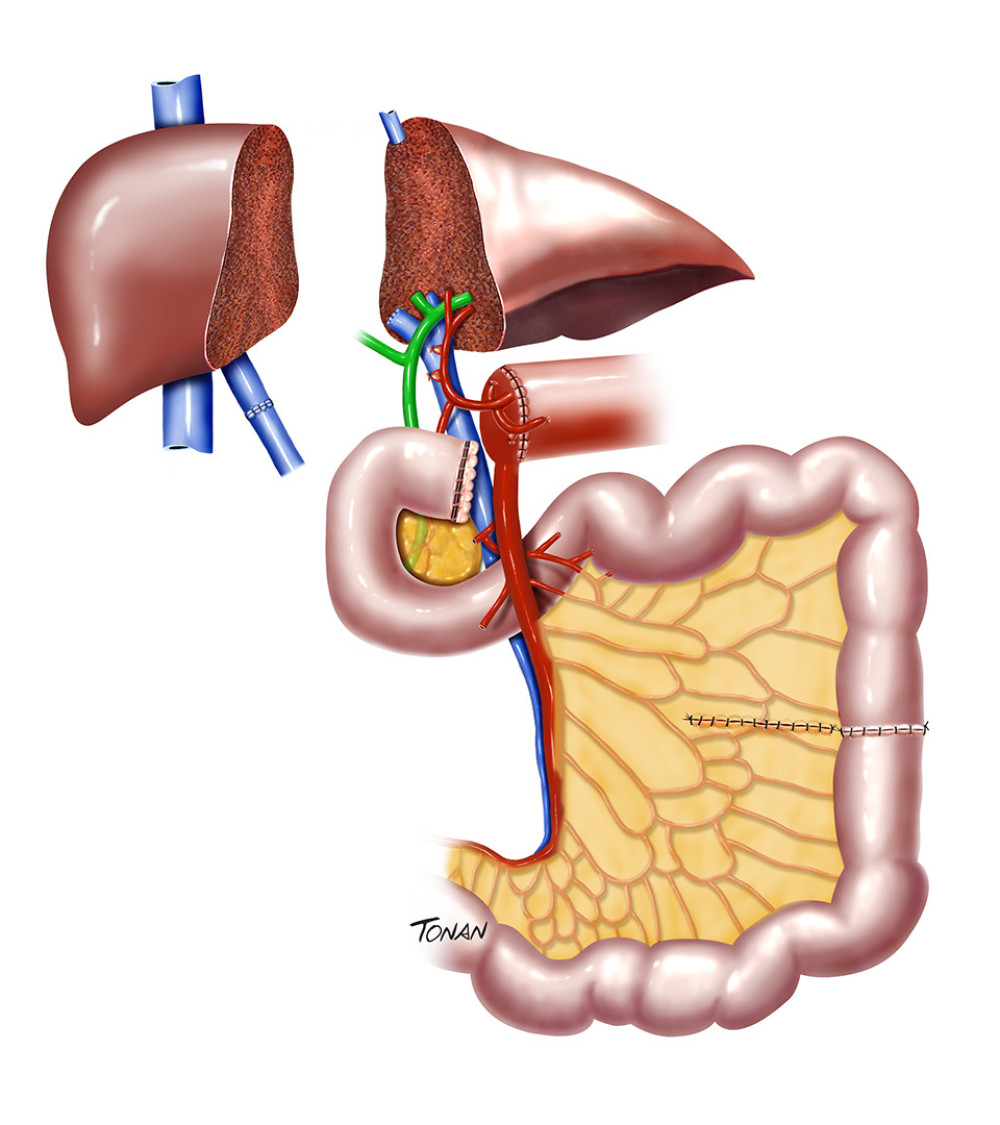 Figure 3. Reduced-size graft. Created with Adobe® Photoshop.
Figure 3. Reduced-size graft. Created with Adobe® Photoshop.  Figure 4. Expander tissue. Created with Adobe® Photoshop.
Figure 4. Expander tissue. Created with Adobe® Photoshop. 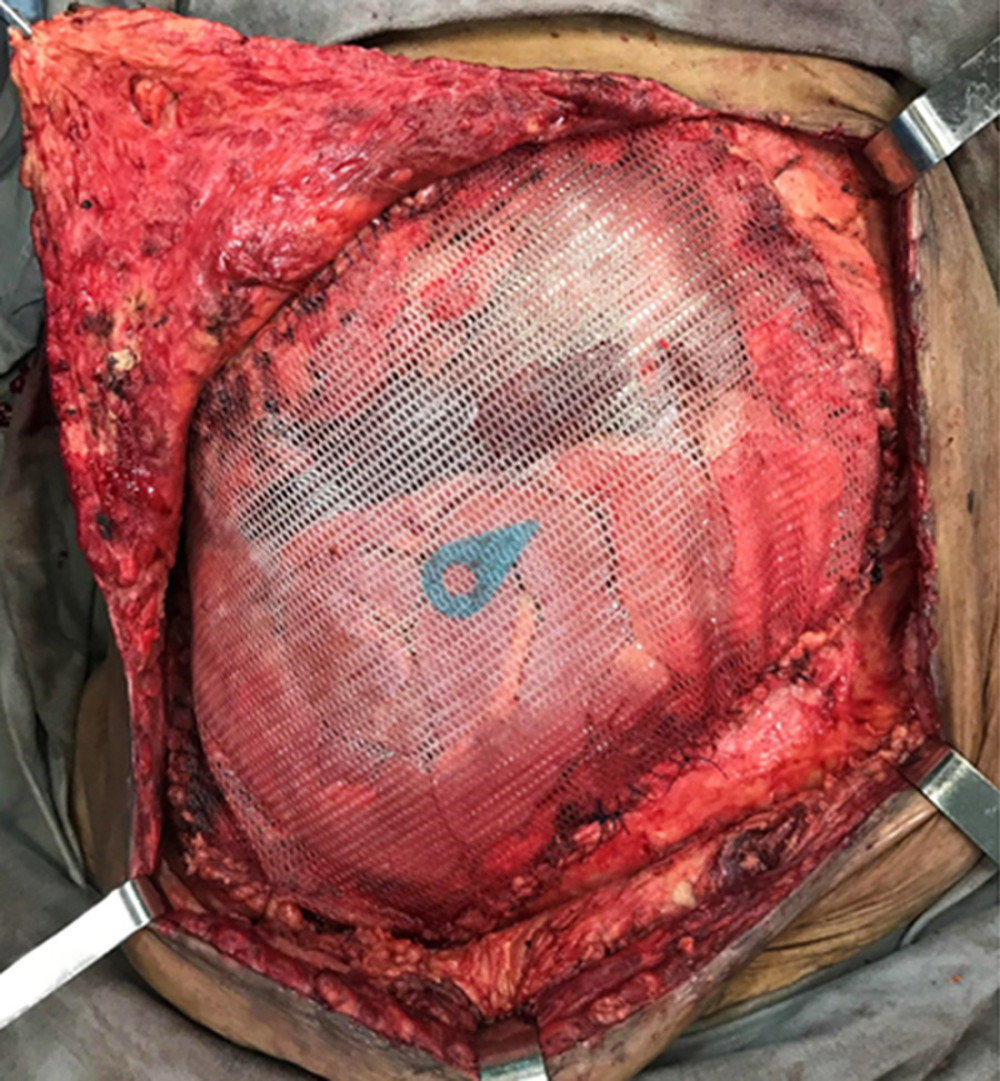 Figure 5. Prosthetic meshes. (Arsenal of the department.)
Figure 5. Prosthetic meshes. (Arsenal of the department.) 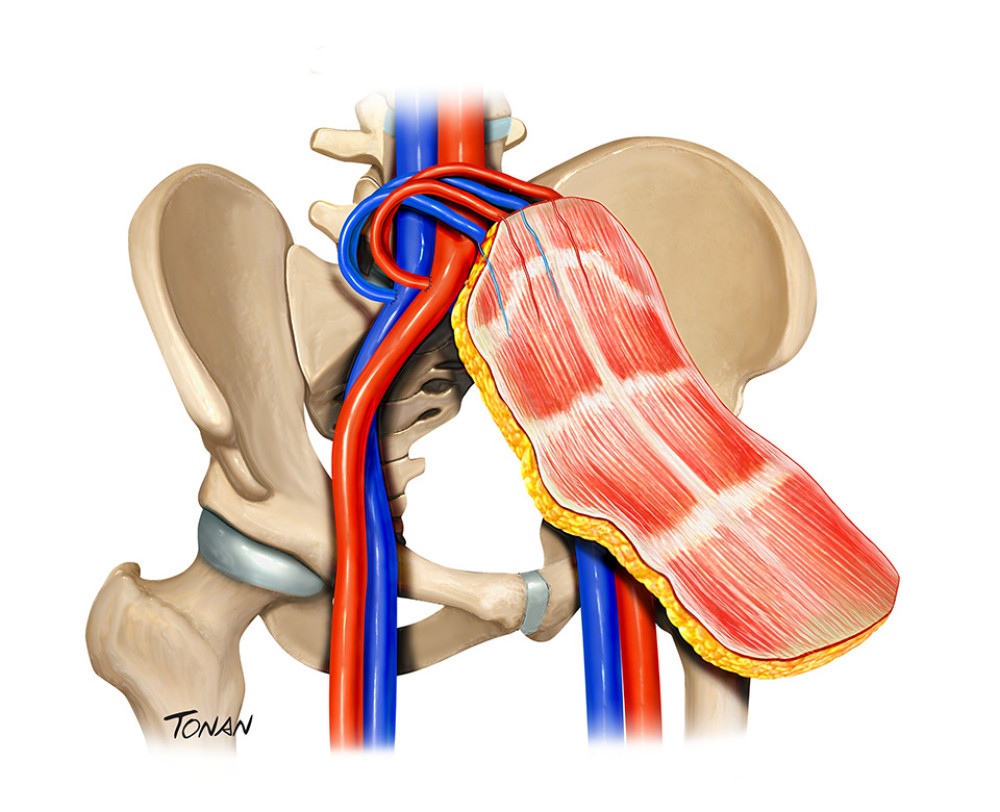 Figure 6. Abdominal wall transplantation. Created with Adobe® Photoshop.
Figure 6. Abdominal wall transplantation. Created with Adobe® Photoshop. 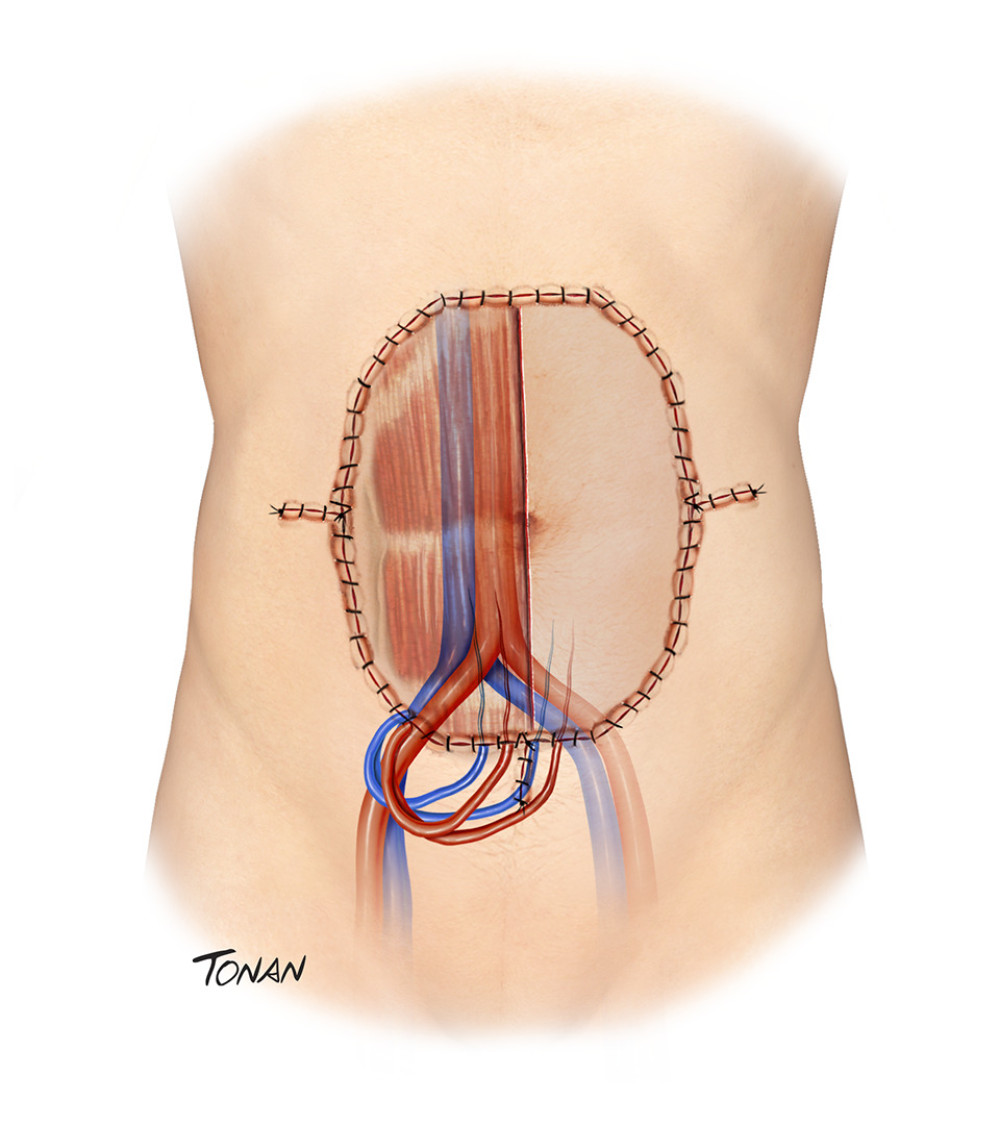 Figure 7. Abdominal wall transplantation. Created with Adobe® Photoshop.
Figure 7. Abdominal wall transplantation. Created with Adobe® Photoshop. References
1. Bond G, Reyes J, Mazariegos G, The impact of positive T-cell lymphocytotoxic crossmatch on intestinal allograft rejection and survival: Transplant Proc, 2000; 32(6); 1197-98
2. Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE, Long-term total parenteral nutrition with growth, development, and positive nitrogen balance: Surgery, 1968; 64(1); 134-42
3. Goulet O, Ruemmele F, Causes and management of intestinal failure in children: Gastroenterology, 2006; 130(2 Suppl 1); S16-28
4. Levi DM, Tzakis AG, Kato T, Transplantation of the abdominal wall: Lancet, 2003; 361(9376); 2173-76
5. Ysebaert D, Duysburgh I, Prognosis of patients with non-malignant chronic intestinal failure receiving long-term home parenteral nutrition. Gastroenterology. 1995;108:1005–10: Clin Nutr, 1995; 14(5); 319
6. Alexandrides IJ, Liu P, Marshall DM, Abdominal wall closure after intestinal transplantation: Plast Reconstr Surg, 2000; 106(4); 805-12
7. Gondolesi G, Selvaggi G, Tzakis A, Use of the abdominal rectus fascia as a nonvascularized allograft for abdominal wall closure after liver, intestinal, and multivisceral transplantation: Transplantation, 2009; 87(12); 1884-88
8. Gupte GL, Haghighi KS, Sharif K, Surgical complications after intestinal transplantation in infants and children – UK experience: J Pediatr Surg, 2010; 45(7); 1473-78
9. Sheth J, Sharif K, Lloyd C, Staged abdominal closure after small bowel or multivisceral transplantation: Pediatr Transplant, 2012; 16(1); 36-40
10. Gerlach UA, Pascher A, Technical advances for abdominal wall closure after intestinal and multivisceral transplantation: Curr Opin Organ Transplant, 2012; 17(3); 258-67
11. Moher D, Liberati A, Tetzlaff J, Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement: PLoS Med, 2009; 6(7); e1000097
12. Mazariegos GV, Intestinal transplantation: Current outcomes and opportunities: Curr Opin Organ Transplant, 2009; 14(5); 515-21
13. Fishbein TM, Bodian CA, Miller CM, National sharing of cadaveric isolated intestinal allografts for human transplantation: A feasibility study: Transplantation, 2000; 69(5); 859-63
14. Smith JM, Weaver T, Skeans MA, OPTN/SRTR 2018 annual data report: Intestine: Am J Transplant, 2020; 20(s1); 300-39
15. Delrivière L, Muiesan P, Marshall M, Size reduction of small bowels from adult cadaveric donors to alleviate the scarcity of pediatric size-matched organs: An Anatomical and Feasibility Study: Transplantation, 2000; 69(7); 1392-96
16. Reyes J, Fishbein T, Bueno J, Reduced-size orthotopic composite liver-intestinal allograft: Transplantation, 1998; 66(4); 489-92
17. de Goyet JdV, Mitchell A, Mayer AD, En bloc combined reduced-liver and small bowel transplants: From large donors to small children: Transplantation, 2000; 69(4); 555-59
18. Xenos ES, Khan F, Nery J, Cadaveric small bowel/split liver transplantation in a child: Transpl Int, 1999; 12(1); 63-67
19. Byrd HS, Hobar PC, Abdominal wall expansion in congenital defects: Plast Reconstr Surg, 1989; 84(2); 347-52
20. Ceulemans LJ, Deferm NP, Miserez M, The role of osmotic self-inflatable tissue expanders in intestinal transplant candidates: Transplant Rev (Orlando), 2016; 30(4); 212-17
21. Clifton MS, Heiss KF, Keating JJ, Use of tissue expanders in the repair of complex abdominal wall defects: J Pediatr Surg, 2011; 46(2); 372-77
22. Albanese ARGigantic median xipho-umbilical eventration; Method for treatment: Rev Asoc Med Argent, 1951; 65(709–710); 376-78 [in Spanish]
23. Young D, Repair of epigastric incisional hernia: Br J Surg, 1961; 48(211); 514-16
24. Ramirez OM, Ruas E, Dellon AL, “Components separation” method for closure of abdominal-wall defects: An anatomic and clinical study: Plast Reconstr Surg”, 1990; 86(3); 519-26
25. Nguyen V, Shestak KC, Separation of anatomic components method of abdominal wall reconstruction – clinical outcome analysis and an update of surgical modifications using the technique: Clin Plast Surg, 2006; 33(2); 247-57
26. Shestak KC, Edington HJ, Johnson RR, The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: Anatomy, surgical technique, applications, and limitations revisited: Plast Reconstr Surg, 2000; 105(2); 731-38 ; quiz 739
27. Gonzalez R, Rehnke RD, Ramaswamy A, Components separation technique and laparoscopic approach: A review of two evolving strategies for ventral hernia repair: Am Surg, 2005; 71(7); 598-605
28. den Hartog D, Eker HH, Tuinebreijer WE, Isokinetic strength of the trunk flexor muscles after surgical repair for incisional hernia: Hernia, 2010; 14(3); 243-47
29. Oliveira LT, Essu FF, de Mesquita GHA, Component separation of abdominal wall with intraoperative botulinum A presents satisfactory outcomes in large incisional hernias: a case report: Int J Surg Case Rep, 2017; 41; 99-104
30. Black CK, Zolper EG, Walters ET, Utility of a modified components separation for abdominal wall reconstruction in the liver and kidney transplant population: Arch Plast Surg, 2019; 46(5); 462-69
31. Scheuerlein H, Thiessen A, Schug-Pass C, Köckerling F, What do we know about component separation techniques for abdominal wall hernia repair?: Front Surg, 2018; 5; 24
32. Brewer MB, Rada EM, Milburn ML, Human acellular dermal matrix for ventral hernia repair reduces morbidity in transplant patients: Hernia, 2011; 15(2); 141-45
33. Zolper EG, Black CK, Devulapalli C, Long term outcomes of abdominal wall reconstruction using open component separation and biologic mesh in the liver, kidney, and small bowel transplant population: Hernia, 2020; 24(3); 469-79
34. Khansa I, Janis JE, Complex open abdominal wall reconstruction: Management of the skin and subcutaneous tissue: Plast Reconstr Surg, 2018; 142(3 Suppl); 125s-32s
35. Lyerly MD, David C, Kim H, Sabitson MD, Hernias: Textbook of Surgery – Biological Basis of Modern Surgical Practice, 1986; 1231-32, Igaku-Shoin/Saunders
36. Restrepo JFP, Hérnias abdominais: Aparelho Digestivo – Clínica e Cirurgia: Medsi, 1996; 1569-70 [in Portuguese]
37. Lichtenstein IL, Shulman AG, Ambulatory outpatient hernia surgery. Including a new concept, introducing tension-free repair: Int Surg, 1986; 71(1); 1-4
38. Usher FC, Ochsner J, Tuttle LL, Use of marlex mesh in the repair of incisional hernias: Am Surg, 1958; 24(12); 969-74
39. Falci FUse of marlex-mesh in the therapy of inguinal hernia in adults (analysis of 100 operated cases): Hospital (Rio J), 1969; 75(1); 147-59 [in Portuguese]
40. Lichtenstein IL, Shulman AG, Amid PK, Montllor MM, The tension-free hernioplasty: Am J Surg, 1989; 157(2); 188-93
41. Shokry MM, Khalil IA, El-Kasapy A, Multifunctional prosthetic polyester-based hybrid mesh for repairing of abdominal wall hernias and defects: Carbohydr Polym, 2019; 223; 115027
42. Sheen AJ, Prosthetics in hernia repair: Surg Today, 2005; 35(3); 196-98
43. Stephenson BM, Kingsnorth AN, Safety and sterilization of mosquito net mesh for humanitarian inguinal hernioplasty: World J Surg, 2011; 35(9); 1957
44. Liu L, Petro C, Majumder A, The use of Vicryl mesh in a porcine model to assess its safety as an adjunct to posterior fascial closure during retromuscular mesh placement: Hernia, 2016; 20(2); 289-95
45. Kennedy GM, Matyas JA, Use of expanded polytetrafluoroethylene in the repair of the difficult hernia: Am J Surg, 1994; 168(4); 304-6
46. Mohsina A, Tamilmahan P, Mathew DD, Biomaterials for hernia repair in animals; A review: Adv Anim Vet, 2014; 2(4S); 48-54
47. Altinli E, Sümer A, Köksal N, Prevention of adhesion to prosthetic mesh: Comparison of oxidized generated cellulose, polyethylene glycol and hylan GF 20: Ulus Travma Acil Cerrahi Derg, 2011; 17(5); 377-82
48. Cevasco M, Itani KMF, Ventral hernia repair with synthetic, composite, and biologic mesh: Characteristics, indications, and infection profile: Surg Infect, 2012; 13(4); 209-15
49. Lamb JP, Vitale T, Kaminski DL, Comparative evaluation of synthetic meshes used for abdominal wall replacement: Surgery, 1983; 93(5); 643-48
50. Tyrell J, Silberman H, Chandrasoma P, Absorbable versus permanent mesh in abdominal operations: Surg Gynecol Obstet, 1989; 168(3); 227-32
51. Baykal A, Onat D, Rasa K, Effects of polyglycolic acid and polypropylene meshes on postoperative adhesion formation in mice: World J Surg, 1997; 21(6); 579-82 ; discussion 582–83
52. LeBlanc KA, Bellanger D, Rhynes KV, Tissue attachment strength of prosthetic meshes used in ventral and incisional hernia repair. A study in the New Zealand White rabbit adhesion model: Surg Endosc, 2002; 16(11); 1542-46
53. Greene MA, Mullins RJ, Malangoni MA, Laparotomy wound closure with absorbable polyglycolic acid mesh: Surg Gynecol Obstet, 1993; 176(3); 213-18
54. Jenkins SD, Klamer TW, Parteka JJ, Condon RE, A comparison of prosthetic materials used to repair abdominal wall defects: Surgery, 1983; 94(2); 392-98
55. Schembari E, Santangelo A, Pesce A, Biological mesh combined with topical negative pressure therapy in complex abdominal wounds: A short series and a review of the literature: Wounds, 2019; 32(4); 93-100
56. Rosen MJ, Biologic mesh for abdominal wall reconstruction: A critical appraisal: Am Surg, 2010; 76(1); 1
57. Smart NJ, Marshall M, Daniels IR, Biological meshes: A review of their use in abdominal wall hernia repairs: Surgeon, 2012; 10(3); 159-71
58. Bachman S, Ramshaw B, Prosthetic material in ventral hernia repair: How do I choose?: Surg Clin North Am, 2008; 88(1); 101-12
59. Romain B, Story F, Meyer N, Comparative study between biologic porcine dermal meshes: Risk factors of postoperative morbidity and recurrence: J Wound Care, 2016; 25(6); 320-25
60. Mulier KE, Nguyen AH, Delaney JP, Marquez S, Comparison of Permacol™ and Strattice™ for the repair of abdominal wall defects: Hernia, 2011; 15(3); 315-19
61. Novitsky YW, Orenstein SB, Kreutzer DL, Comparative analysis of histopathologic responses to implanted porcine biologic meshes: Hernia, 2014; 18(5); 713-21
62. Deeken CR, Melman L, Jenkins ED, Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair: J Am Coll Surg, 2011; 212(5); 880-88
63. Bondre IL, Holihan JL, Askenasy EP, Suture, synthetic, or biologic in contaminated ventral hernia repair: J Surg Res, 2016; 200(2); 488-94
64. Garvey PB, Giordano SA, Baumann DP, Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix: J Am Coll Surg, 2017; 224(3); 341-50
65. Chatterjee A, Krishnan NM, Rosen JM, Complex ventral hernia repair using components separation with or without synthetic mesh: a cost-utility analysis: Plast Reconstr Surg, 2014; 133(1); 137-46
66. Hiles M, Briggs CM, The overall cost of complex ventral hernia repair with biological grafts: Gen Surg News, 2010; 37(12); 24-25
67. Krpata DM, Blatnik JA, Novitsky YW, Rosen MJ, Evaluation of high-risk, comorbid patients undergoing open ventral hernia repair with synthetic mesh: Surgery, 2013; 153(1); 120-25
68. Brown RH, Subramanian A, Hwang CS, Comparison of infectious complications with synthetic mesh in ventral hernia repair: Am J Surg, 2013; 205(2); 182-87
69. Quyn AJ, Johnston C, Hall D, The open abdomen and temporary abdominal closure systems – historical evolution and systematic review: Colorectal Dis, 2012; 14(8); e429-38
70. Mees J, Mardin WA, Senninger N, Treatment options for postoperatively infected abdominal wall wounds healing by secondary intention: Langenbecks Arch Surg, 2012; 397(8); 1359-66
71. Bagnall NM, Vig S, Trivedi P, Surgical-site infection: Surgery (Oxford), 2009; 27(10); 426-30
72. Klevens RM, Edwards JR, Richards CL, Estimating health care-associated infections and deaths in U.S. hospitals, 2002: Public Health Rep, 2007; 122(2); 160-66
73. World Health Organization: Global guidelines for the prevention of surgical site infection, 2016, World Health Organization
74. Scott RD: The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention, 2009, Center for Disease Control and Prevention
75. Mangram AJ, Horan TC, Pearson ML, Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee: Am J Infect Control, 1999; 27(2); 97-132 quiz 133–34: discussion 96
76. Bueno-Lledó J, Torregrosa-Gallud A, Sala-Hernandez A, Predictors of mesh infection and explantation after abdominal wall hernia repair: Am J Surg, 2017; 213(1); 50-57
77. Di Benedetto F, Lauro A, Masetti M, Use of prosthetic mesh in difficult abdominal wall closure after small bowel transplantation in adults: Transplant Proc, 2005; 37(5); 2272-74
78. Mansberger AR, Kang JS, Beebe HG, Le Flore I, Repair of massive acute abdominal wall defects: J Trauma, 1973; 13(9); 766-74
79. Voyles CR, Richardson JD, Bland KI, Emergency abdominal wall reconstruction with polypropylene mesh: Short-term benefits versus long-term complications: Ann Surg, 1981; 194(2); 219-23
80. Fansler RF, Taheri P, Cullinane C, Polypropylene mesh closure of the complicated abdominal wound: Am J Surg, 1995; 170(1); 15-18
81. Gondolesi GE, Aguirre NF, Techniques for abdominal wall reconstruction in intestinal transplantation: Curr Opin Organ Transplant, 2017; 22(2); 135-41
82. Farinelli PA, Rubio JS, Padín JM, Use of nonvascularized abdominal rectus fascia after liver, small bowel, and multiorgan transplantation: Long-term follow-up of a single-center series: Transplant Proc, 2017; 49(8); 1810-14
83. Cassar N, Cortes-Cerisuelo M, Bambridge C, The difficult abdominal closure after paediatric intestinal transplantation: Use of abdominal rectus muscle fascia and literature review: Pediatr Transplant, 2019; 23(5); e13473
84. Justo I, Manrique A, Calvo J, Abdominal wall transplantation in organ transplantation: Our experience: Cir Esp, 2019; 97(5); 247-53
85. Lee JC, Olaitan OK, Lopez-Soler R, Expanding the envelope: The posterior rectus sheath – liver vascular composite allotransplant: Plast Reconstr Surg, 2013; 131(2); 209e-18e
86. Michels NA, Newer anatomy of the liver and its variant blood supply and collateral circulation: Am J surg, 1966; 112(3); 337-47
87. Agarwal S, Dorafshar AH, Harland RC, Liver and vascularized posterior rectus sheath fascia composite tissue allotransplantation: Am J Transplant, 2010; 10(12); 2712-16
88. Selvaggi G, Levi DM, Cipriani R, Abdominal wall transplantation: surgical and immunologic aspects: Transplant Proc, 2009; 41(2); 521-22
89. Seneschal J, Clark RA, Gehad A, Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells: Immunity, 2012; 36(5); 873-84
90. Starzl R, Brandacher G, Lee WPA, Review of the early diagnoses and assessment of rejection in vascularized composite allotransplantation: Clin Dev Immunol, 2013; 2013; 402980
91. Schneeberger S, Morelon E, Landin L, Committee EC, Vascularized composite allotransplantation: A member of the transplant family?: Transplantation, 2012; 93(11); 1088-91
92. Sarhane KA, Khalifian S, Ibrahim Z, Diagnosing skin rejection in vascularized composite allotransplantation: Advances and challenges: Clin Transplant, 2014; 28(3); 277-85
93. Giele H, Vaidya A, Reddy S, Vrakas G, Friend P, Current state of abdominal wall transplantation: Curr Opin Organ Transplant, 2016; 21(2); 159-64
94. Cipriani R, Contedini F, Santoli M, Abdominal wall transplantation with microsurgical technique: Am J Transplant, 2007; 7(5); 1304-7
95. Giele H, Bendon C, Reddy S, Remote revascularization of abdominal wall transplants using the forearm: Am J Transplant, 2014; 14(6); 1410-16
96. Haveman JW, Tempelman TM, Hofker HSFirst combined intestinal and abdominal wall transplantation in the Netherlands: Ned Tijdschr Geneeskd, 2016; 160; A9788 [in Dutch]
97. Berli JU, Broyles JM, Lough D, Current concepts and systematic review of vascularized composite allotransplantation of the abdominal wall: Clin Transplant, 2013; 27(6); 781-89
98. Sudan D, Long-term outcomes and quality of life after intestine transplantation: Curr Opin Organ Transplant, 2010; 15(3); 357-60
99. Ruiz P, Updates on acute and chronic rejection in small bowel and multivisceral allografts: Curr Opin Organ Transplant, 2014; 19(3); 293-302
100. Gerlach UA, Vrakas G, Sawitzki B, Abdominal wall transplantation: skin as a sentinel marker for rejection: Am J Transplant, 2016; 16(6); 1892-900
Figures
 Figure 1. Extensive abdominal fibrosis resulting from second intention healing of peritoneostomy and ostomy scars. (Arsenal of the department.)
Figure 1. Extensive abdominal fibrosis resulting from second intention healing of peritoneostomy and ostomy scars. (Arsenal of the department.) Figure 2. Study design.
Figure 2. Study design. Figure 3. Reduced-size graft. Created with Adobe® Photoshop.
Figure 3. Reduced-size graft. Created with Adobe® Photoshop. Figure 4. Expander tissue. Created with Adobe® Photoshop.
Figure 4. Expander tissue. Created with Adobe® Photoshop. Figure 5. Prosthetic meshes. (Arsenal of the department.)
Figure 5. Prosthetic meshes. (Arsenal of the department.) Figure 6. Abdominal wall transplantation. Created with Adobe® Photoshop.
Figure 6. Abdominal wall transplantation. Created with Adobe® Photoshop. Figure 7. Abdominal wall transplantation. Created with Adobe® Photoshop.
Figure 7. Abdominal wall transplantation. Created with Adobe® Photoshop. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860