03 March 2023: Original Paper
Comparison of Biosimilar Filgrastim with Innovator Fligrastim for Peripheral Blood Stem Cells Mobilization, Collection of CD34+ Stem Cells, and Engraftment in Patients Undergoing Autologous and Allogeneic Stem Cell Transplantation: A Single-Center Experience
Maha M. IslamiDOI: 10.12659/AOT.938585
Ann Transplant 2023; 28:e938585
Abstract
BACKGROUND: In the Middle East, there is lack of data on peripheral blood CD34+stem cells mobilization by using biosimilar filgrastim. We have been using both Neupogen and a biosimilar G-CSF) Zarzio® (as a mobilizing agent since February 2014 for both allogenic and autologous stem cell transplantations.
MATERIAL AND METHODS: This was a single-center retrospective study. All patients and healthy donors who received either the biosimilar G-CSF (Zarzio®) or original G-CSF (Neupogen®) for mobilization of CD34+ stem cells were included in the study. The primary goal was to determine and compare the rate of successful harvest and amount of CD34+ stem cells collected in either adult cancer patients or healthy donors between Zarzio® and Neupogen® groups.
RESULTS: A total of 114 patients, including 97 cancer patients and 17 healthy donors, underwent successful CD34+ stem cell mobilization using G-CSF with chemotherapy (35 with Zarzio® +chemotherapy, 39 with Neupogen® +chemotherapy) or G-CSF as monotherapy (14 with Zarzio®, 9 with Neupogen®) in autologous transplantation. In an allogeneic stem cell transplantation, successful harvest was achieved by using G-CSF monotherapy (8 with Zarzio®, 9 with Neupogen®). There was no difference between Zarzio® and Neupogen® in the amount of CD34+ stem cells collected at leukapheresis. There was no difference with regards to secondary outcomes between the 2 groups.
CONCLUSIONS: Our study showed that biosimilar G-CSF (Zarzio®) has comparable efficacy to the original G-CSF (Neupogen®) when used for mobilization in both autologous and allogenic stem cell transplantation and was associated with significant cost saving.
Keywords: Biosimilar Filgrastim, Zarzio, Neupogen, CD34+ Mobilization, Stem Cell Transplantation, Adult, Humans, Filgrastim, Biosimilar Pharmaceuticals, peripheral blood stem cells, Granulocyte Colony-Stimulating Factor, Hematopoietic Stem Cell Transplantation, Stem Cell Transplantation, Antigens, CD34, Cell Adhesion Molecules
Background
Hematopoietic stem cell transplantation (HSCT), either autologous or allogenic, provides a curative therapy for various hematological malignancies. The most widely used source for HSCT is peripheral blood stem cells (PBSCs). Autologous HSCT uses hematopoietic peripheral blood stem cells collected from patients and is widely used to treat many kinds of hematologic neoplasms. Allogenic HSCT uses hematopoietic PBSCs that are collected from healthy donors, not from patients. Mobilization of PBSCs (CD34+ stem cells) is successfully attained using a granulocyte-colony stimulating factor (G-CSF) alone or in combination with chemotherapy. The original G-CSF (Neupogen®) is widely used as a mobilizer of CD34+ stem cells among healthy donors in allogenic stem cell transplants or among cancer patients in autologous stem cell transplants as a standard of care [1]. Alternatively, biosimilar filgrastim (G-CSF) can be used instead of originator filgrastim. A biosimilar is highly similar to but not identical in quality, safety, and efficacy to the originator, which is already a licensed reference biotherapeutic product [2]. Biosimilars’ approval is attained based on comparable quality, safety, and efficacy data to the originator rather than on the basis of a positive risk-benefit assessment, which has already been done with the originator [2]. Various biosimilars of G-CSF have been widely used in Europe since 2009 after receiving approval from the European Medicines Agency (EMA) in 2008 and were recently approved by the US Food and Drug Administration (FDA) for the same indication as that of Neupogen®. They are intended to decrease the incidence of infection‚ as manifested by febrile neutropenia‚ in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a significant incidence of severe neutropenia with fever. They also reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy treatment for patients with acute myeloid leukemia (AML) and reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg‚ febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by blood marrow transplantation (BMT); to mobilize autologous hematopoietic peripheral blood stem cells into the peripheral blood for collection by leukapheresis; and to reduce the incidence and duration of sequelae of severe neutropenia (eg‚ fever‚ infection‚ oropharyngeal ulcers) in symptomatic patients with congenital neutropenia‚ cyclic neutropenia‚ or idiopathic neutropenia. Zarzio® was the first biosimilar, approved by the USFDA in 2015 [1,3]. Biosimilar Filgrastim (Zarzio®) has been used in the Princess Norah Oncology Centre since 2015 for peripheral blood progenitor cells mobilization for healthy donors and cancer patients, as well as mobilization for both types allogenic and autologous stem cell transplantation for many types of cancer.
Material and Methods
OUTCOMES:
The primary outcome was to achieve successful harvest, defined as collection a minimum of 2×106/kg CD34+ cells. Secondary outcomes were time in days to achieve neutrophil and platelet engraftment. Neutrophil engraftment was considered complete when the absolute neutrophil count (ANC) was equal to or greater than 0.5×109/L for 3 consecutive days after infusion of PBSCs, whereas platelet engraftment was considered complete when the platelet count was 20×109/L or more for 7 consecutive days without platelet transfusion. We compared the safety of filgrastim (Zarzio®) versus Neupogen® in terms of allergic reaction and pain, number of leukapheresis sessions, and we compared the rescue use of plerixafor in both groups and the impact of biosimilar filgrastim (Zarzio®) in terms of cost savings.
STATISTICAL ANALYSIS:
Descriptive analysis was done using SPSS software. The data are represented as median and percentage. All statistical analysis was completed using SPSS software version 23 and Excel 2019.
Results
BASELINE CHARACTERISTICS:
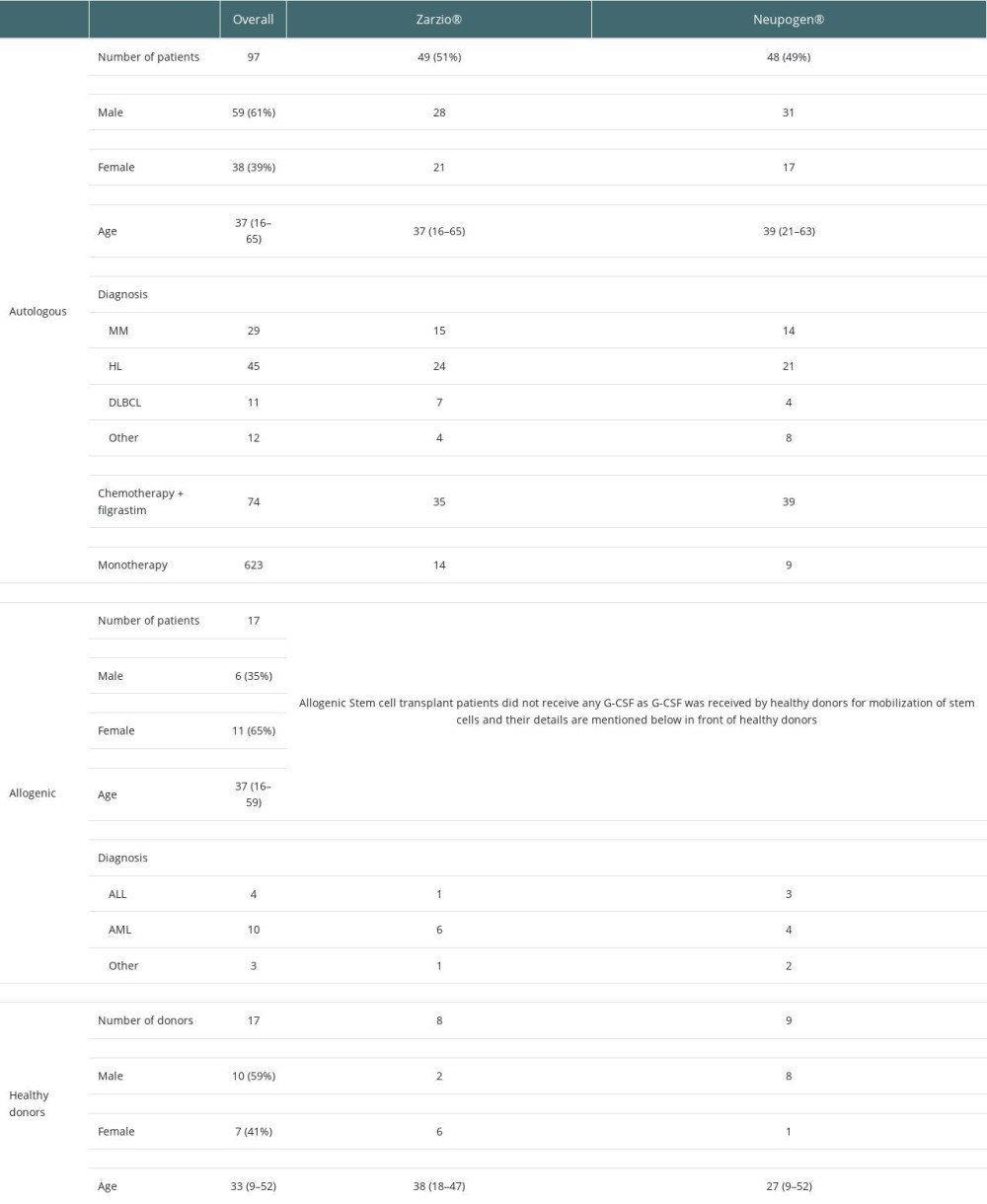
A total of 114 patients, including 97 cancer patients, most of whom had Hodgkin lymphoma (n= 45), multiple myeloma (n=29), or other hematological leukemia (n=14), and 17 healthy donors, underwent mobilization from February 1, 2014 to December 31, 2018. In autologous transplantation, 49 patients received Zarzio® while 48 patients received Neupogen®. In the allogenic setting, 8 healthy donors received Zarzio® while 9 healthy donors received Neupogen®. Baseline characteristics were comparable in both groups (Table 1).
SUCCESSFUL MOBILIZATION:
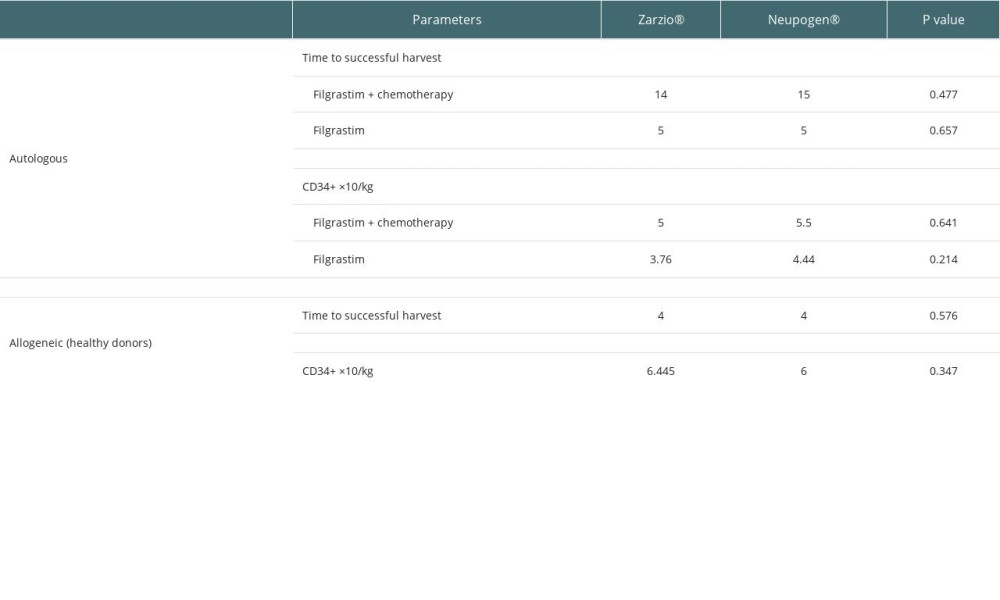
Ninety-seven cancer patients underwent successful CD34+ stem cell mobilization using G-CSF with chemotherapy (35 with Zarzio® +chemotherapy, 39 with Neupogen® +chemotherapy) or G-CSF as monotherapy (14 with Zarzio®, 9 with Neupogen®) in autologous transplantation. For allogeneic stem cell transplantation, successful harvest was achieved by using G-CSF monotherapy (8 with Zarzio®, 9 with Neupogen®). The mobilization success was similar across both groups, and the use of rescue plerixafor was similar across both groups in the autologous transplant setting. Plerixafor was used for 2 patients in the Zarzio® group and 2 patients in the Neupogen® group (Table 2).
HARVEST AND LEUKAPHERESIS RESULTS:
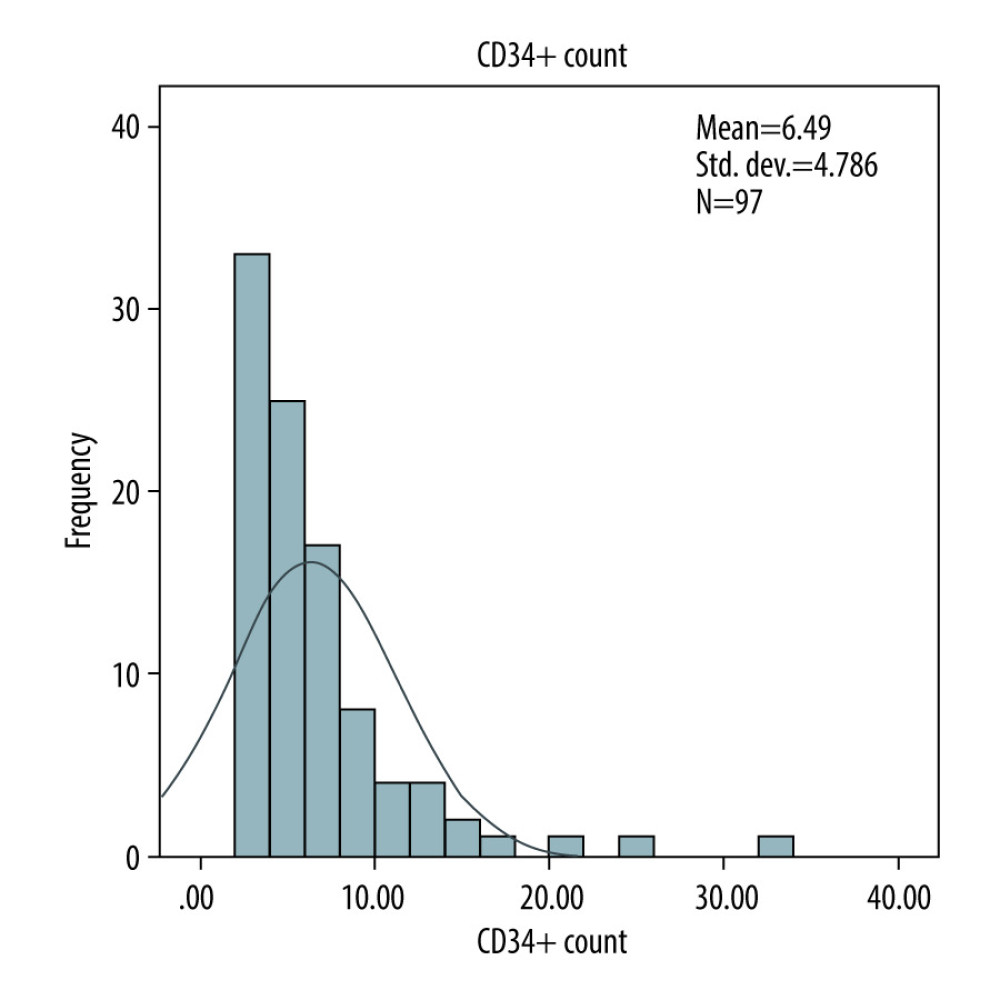
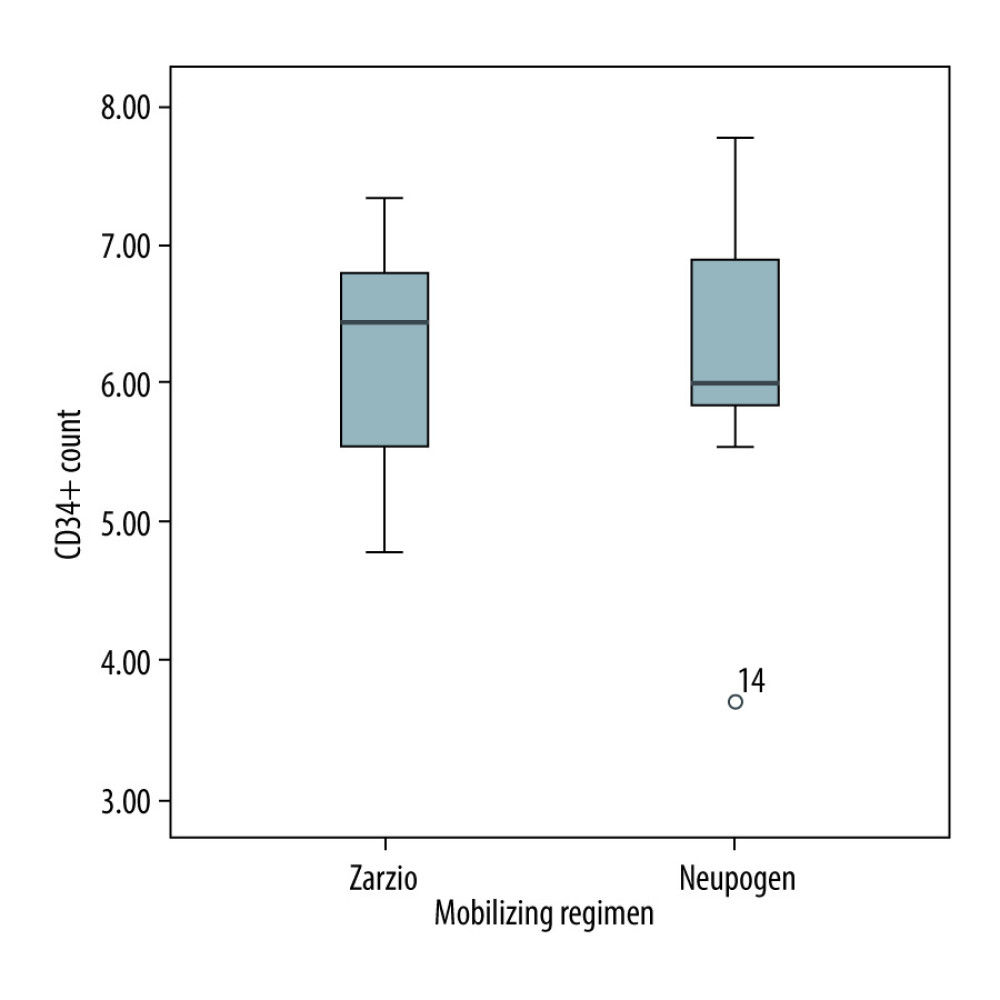
There was no difference between Zarzio® and Neupogen® in the amount of CD34+ stem cells collected at leukapheresis. The total CD34+ stem cells collected with chemotherapy in autologous were 5×106/kg Zarzio® vs 5.5×106/kg Neupogen® (p=0.64); the total CD34+ stem cells collected using G-CSF as monotherapy in autologous stem cell transplants were 3.76×106/kg Zarzio® vs 4.44×106/kg Neupogen® (P=0.21) (Figure 1). In allogeneic stem cell transplant, the total CD34+ stem cells collected were 6.4×106/kg Zarzio® vs 6×106/kg Neupogen® (p=0.34) (Figure 2). Results of the successful harvest are shown in Table 2. In both groups, the median number of apheresis sessions was similar.
ENGRAFTMENT RESULTS:
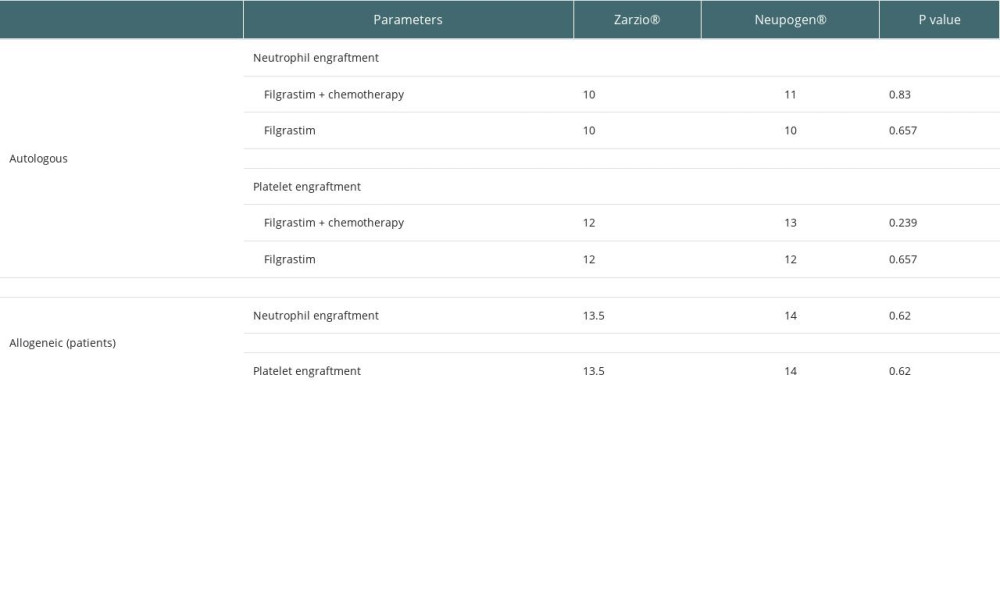
There was no difference in median time to achieve neutrophil and platelet engraftment between the groups, as shown in Table 3.
SAFETY AND COST PROFILE:
There were no allergic reactions observed in either group. Two patients in the Neupogen® group and 1 patient in the Zarzio® group received analgesics for bone pain. Hence, safety issues regarding allergic reactions and bone pain were comparable in both groups. The total direct cost of Zarzio® was 3 times less than that of Neupogen®. The price of Neupogen® was 450 SAR/vial before the addition of Zarzio®, and then reduced to 225 SAR/vial after Zarzio® was added to the formulary in 2014. The price of Zarzio® was 96 SAR/vial when introduced to our formulary. We would have spent SAR 180 000 had we used Zarzio® in all 114 patients as mobilizing agent vs 600 000 SAR if only Neupogen® had been used in all 114 patients as the mobilizing agent. The use of biosimilar filgrastim reduces healthcare expenditure without affecting patient outcomes.
Discussion
LIMITATIONS:
This was a single-center retrospective study.
Conclusions
Biosimilar G-CSF (Zarzio®) has comparable efficacy to the original G-CSF (Neupogen®) when used for mobilization in both autologous and allogenic stem cell transplantation and was associated with significant cost saving. Further prospective comparisons are needed.
References
1. Gascón P, Tesch H, Verpoort K: Support Care Cancer, 2013; 21(10); 2925-32
2. World Health Organization. Expert Committee on Biological Standardization: Guidelines on evaluation of similar biotherapeutic products (SBPs), 2009
3. Cilia M, Ruiz S, Richardson P, Quality issues identified during the evaluation of biosimilars by the European Medicines Agency’s Committee for Medicinal Products for Human Use: AAPS PharmSciTech, 2018; 19(2); 489-511
4. Khan MA, Aseeri MA, Alshamrani MA, Emerging role of biosimilars in oncology-hematology in Saudi Arabia: A practical perspective: Global Journal on Quality and Safety in Healthcare, 2020; 3(1); 22-29
5. Ismail S, Abu Esba L, An institutional guide for formulary decisions of biosimilars: Hosp Pharm, 2022; 58(1); 38-48
6. Shaw BE, Confer DL, Hwang WY, Concerns about the use of biosimilar granulocyte colony-stimulating factors for the mobilization of stem cells in normal donors: Position of the World Marrow Donor Association: Haematologica, 2011; 96(7); 942
7. Nasillo V, Paolini A, Riva G, Effectiveness of originator (Neupogen) and biosimilar (Zarzio) filgrastim in autologous peripheral blood stem cell mobilization in adults with acute myeloid leukemia: A single-center retrospective study: Leuk Lymphoma, 2018; 59(1); 225-28
8. Pham T, Patil S, Fleming S, Comparison of biosimilar filgrastim with originator filgrastim for peripheral blood stem cell mobilization and engraftment in patients with multiple myeloma undergoing autologous stem cell transplantation: Transfusion, 2015; 55(11); 2709-13
9. Antelo ML, Zabalza A, Sánchez Antón MP: J Clin Apher, 2016; 31(1); 48-52
10. Becker P, Schwebig A, Brauninger S, Healthy donor hematopoietic stem cell mobilization with biosimilar granulocyte-colony-stimulating factor: Safety, efficacy, and graft performance: Transfusion, 2016; 56(12); 3055-64
11. Schmitt M, Hoffmann JM, Lorenz K, Publicover A, Mobilization of autologous and allogeneic peripheral blood stem cells for transplantation in haematological malignancies using biosimilar G-CSF: Vox Sang, 2016; 111(2); 178-86
12. Severson C, Clinical Practice: The role of biosimilar granulocyte colony stimulating factor (G-CSF) Zarzio for progenitor cell mobilization and the treatment of therapy-induced neutropenia in adult hematopoietic stem cell transplantation: Can Oncol Nurs J, 2015; 25(4); 443-48
13. Henry D, Taylor C, Pharmacoeconomics of cancer therapies: Considerations with the introduction of biosimilars: Semin Oncol, 2014; 41(Suppl 3); S13-S20
14. Khan MA, Aseeri MA, Alshamrani MA, Emerging role of biosimilars in oncology-hematology in Saudi Arabia: A practical perspective: Global Journal on Quality and Safety in Healthcare, 2020; 3(1); 22-29
Figures
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860