18 July 2023: Original Paper
Recurrent Membranoproliferative Glomerulonephritis After Kidney Transplantation: Risk Factors and Impact on Graft Survival
Lais Ceccatto de Paula1ABCDEF, Marilda MazzaliDOI: 10.12659/AOT.940502
Ann Transplant 2023; 28:e940502
Abstract
BACKGROUND: Membranoproliferative glomerulonephritis (MPGN) is an uncommon cause of end-stage renal disease (ESRD). Recurrence rates after transplantation range from 11.8% to 18.9% after 5 and 15 years, respectively. This study aimed to assess the risk factors of MPGN recurrence after kidney transplantation and its impact on graft survival.
MATERIAL AND METHODS: This was a single-center retrospective cohort, including renal transplant recipients older than 18 years, with a diagnosis of MPGN in native kidneys. Data were obtained from medical records during the first 5-year post-transplant follow-up. Primary endpoints were graft function and survival. Secondary endpoints were MPGN recurrence risk factors and these cases’ clinical, laboratory, and histological features.
RESULTS: Twenty-eight patients were included; the majority male (60.7%), with a mean age of 24.0±9.4 years. At MPGN native diagnosis, all patients presented proteinuria, with C3 consumption in 42.9%. Histological analysis showed 13 (42.9%) MPGN type I and 5 (17.9%) type II, with no cases of type III. MPGN recurrence occurred in 7 (25.0%) patients; 85.7% were male, 57.1% were recipients from a living donor, all presenting nephrotic syndrome and hematuria, with C3 consumption in 71.4%. The graft function was similar between the groups. Two (28.6%) patients progressed to graft failure in the recurrence group, and 1 died with a functioning graft.
CONCLUSIONS: The MPGN recurrence rate was 25%, most of them recipients of kidneys from living donors. Nephrotic syndrome and C3 consumption were frequent at recurrence. The graft function was similar between the groups, and the 5-year graft survival rate in the recurrence group was higher than in other studies.
Keywords: Glomerulonephritis, Graft Survival, Kidney Transplantation, nephrotic syndrome, Humans, Male, Adolescent, young adult, Adult, Female, Glomerulonephritis, Membranoproliferative, Retrospective Studies, Risk Factors, Recurrence
Background
Membranoproliferative glomerulonephritis (MPGN) is a pattern of glomerular injury characterized by mesangial expansion due to hypercellularity and increased matrix, with lobular accentuation of the glomerular tufts [1,2]. Deposits of immunoglobulins or complement factors in glomerular mesangium and subendothelial space results in endocapillary proliferation and capillary-wall remodeling with double contours [2]. According to electron microscopy morphology, MPGN is traditionally classified into primary MPGN types I, II, or III and secondary MPGN. The MPGN I is the most common form, characterized by subendothelial deposits. The electron-dense deposition occurs in the glomerular basement membrane in MPGN II. In contrast, in MPGN III, electron-dense deposition occurs in both subepithelial and subendothelial areas, secondary to autoimmune processes or infections [2,3].
The new classification of MPGN considers the immunofluorescence microscopy patterns: immune-complex mediated MPGN, with complement factor 3 and immunoglobulins depositions, and MPGN associated with dysregulation of the alternative complement pathway, with isolated deposits of complement factor [2–4]. MPGN caused by an alternative complement pathway dysregulation may be divided into C3 dense deposit disease (DDD) and C3 glomerulonephritis. While DDD is characterized by osmiophilic, sausage-shaped, wavy dense deposits on the glomerular basement membrane and mesangium, in C3 glomerulonephritis (C3GN) deposits can be mesangial, subendothelial, and sometimes subepithelial and intramembranous [2].
The MPGN can be idiopathic or secondary to systemic diseases, such as autoimmune diseases, chronic infections, lymphoproliferative disorders, and plasma cell dyscrasias [1]. The clinical course varies according to the histological damage, including nephritic and nephrotic phenotypes or rapidly progressive glomerulonephritis [2]. The post-transplantation MPGN recurrence rates range from 11.8% to 18.9% after 5 and 15 years, respectively, with a negative impact on graft survival [4,5], especially in cases of MPGN I recurrence associated with crescents [4]. The late recurrence rate of DDD (or MPGN II) approaches 100%, whereas C3GN recurrence tends to occur early after transplant [6]. For immune-complex mediated GN, the glomerular monoclonal immunoglobulin deposition with glomerular C4d deposition is associated with early disease recurrence and poor outcomes [6]. This study aimed to assess the risk factors of MPGN recurrence after kidney transplantation and its impact on graft survival.
Material and Methods
This was a single-center retrospective cohort including kidney transplant recipients older than 18 years and end-stage kidney disease (ESKD) secondary to biopsy-proven MPGN. Cases of secondary MPGN, associated systemic lupus erythematous or infections by Hepatitis B virus (HBV), Hepatitis C virus (VHC), Human Immunodeficiency virus (HIV), and
Clinical, laboratory, and histological data were retrospectively collected from medical records and Renal Transplant Program databases, at the time of transplantation and during the first 5 years after transplant. Data were transcribed and organized into a Microsoft Excel worksheet. The local Ethics Committee approved the study.
Primary outcomes were allograft function, estimated using the study equation of the Chronic Kidney Disease Epidemiology Collaboration [7], and graft survival. Secondary endpoints included MPGN recurrence risk factors and clinical, laboratory, and histological characteristics of these cases. For analysis, included patients were divided into 2 groups according to recurrence of MPGN post-transplant: the MPGN recurrence group and the non-recurrence group.
Immunosuppressive therapy has changed over time. From 1985 to 1998, induction therapy was donor-specific transfusion or CD3 monoclonal antibody; between 1998 and 2008 it consisted of monoclonal anti-IL-2 receptor antibodies. From 2009, induction therapy was adjusted according to the recipient’s anti-human leukocyte antigen (HLA) antibodies profile and the donor’s characteristics. Patients with a panel reactive antibody (PRA) lower than 50%, without preformed anti-HLA donor-specific antibodies (DSA), and kidney recipients from a standard deceased donor received monoclonal anti-IL-2 receptor antibodies. In cases of expanded criteria donors, non-identical HLA living donors, PRA ≥50%, or preformed DSA, induction therapy was i.v. anti-thymocyte globulin 3–7 mg/kg, dose-adjusted by lymphocytes count. All recipients received methylprednisolone 500 mg i.v. at the time of transplantation, and steroid therapy was maintained during follow-up. The use of steroids in maintenance immunosuppressive therapy is routinely applied in the center, regardless of the CKD etiology. Maintenance immunosuppression consisted of a calcineurin inhibitor (tacrolimus or cyclosporine) and an antiproliferative drug (sodium mycophenolate or azathioprine).
The indications of post-transplant allograft biopsies were an increase in serum creatinine >20% from the previous level or new-onset proteinuria, detected by measurement in a 24-h urine collection or by protein-to-creatine ratio on a spot sample. Two renal pathologists analyzed allograft biopsies based on Banff 2013 classification, revised in 2015 [8]. Cell-mediated acute rejection was treated with i.v. Methylprednisolone or anti-thymocyte globulin. Antibody-mediated acute rejection treatment consisted of 5–7 sessions of plasmapheresis associated with 2 g/kg intravenous immunoglobulin (IVIG).
Diagnosis of MPGN in the allograft was made through glomerular hypercellularity and increased mesangial matrix on light microscopy. MPGN was classified according to immunofluorescence and electron microscopy patterns. Therapy of MPGN recurrence consisted of steroids in pulse therapy i.v. or with a transient increase in daily oral doses.
In statistical analysis, continuous variables were expressed as mean±standard deviation or median and range or percentages. Continuous variables among the groups were compared by unpaired
Results
Among 2870 renal transplants performed between 1985 and 2019, 49 (1.7%) patients had ESKD secondary to biopsy-proven MPGN. Nineteen cases of secondary MPGN were excluded, 8 (42.1%) with HBV infection, 2 (10.5%) with HCV infection, 7 (36.9%) with
Clinical presentation of MPGN in native kidneys was of edema and proteinuria (n=28, 100%), nephrotic syndrome (n=22, 78.6%), hematuria (n=25, 89.3%), and systemic hypertension (n=25, 89.3%). The complement profile showed consumption of C3 (n=12, 42.9%) and C4 (n=2, 7.2%). Histological analysis showed 15 (53.6%) cases of native MPGN type I, and 6 (21.4%) cases of MPGN type II, without cases of MPGN type III described. In 7 cases, only light microscopy data was available. Twenty (71.4%) patients presented mesangial hypercellularity at native MPGN diagnosis, with chronic histologic lesions detected in 53.6% of the biopsies (Table 1). Sixteen patients (57.1%) received a kidney from a deceased donor, with a mean cold ischemia time of 21.1±8.0 h. Most included patients (n=19, 67.9%) received only steroid pulse therapy without additional induction immunosuppression at the kidney transplantation. The maintenance immunosuppression consisted of an association of steroids (n=28, 100%), antiproliferative drugs (n=27, 96.4%), and/or calcineurin inhibitors (n=21,75.0). Nine (32.1%) patients had delayed graft function.
The MPGN recurrence occurred in 7 (25.0%) patients, with a median of 15 months (2 to 72 months) after transplantation. Most of these patients were male (n=6, 85.7%), recipients of a kidney from a living related donor (n=4, 57.1%), white race (n=7, 100%), with a report of nephrotic syndrome and hematuria (n=7, 100%) at diagnosis of MPGN in native kidneys. The pre-transplant complement profile showed that consumption of C3 fraction was more frequent in the recurrent group (71.4% vs 33.3%, p<0.01). Also, reduced levels of C4 were observed only in the recurrence group (n=2, 28.6%) (Table 1). Analysis of native kidney biopsies showed no significant difference in MPGN classification or frequency of crescents, mesangial hypercellularity, and interstitial fibrosis between groups. Considering the induction of immunosuppression in the recurrence group, 5 (71.4%) received a donor-specific transfusion or CD3 monoclonal antibody, 1 (14.3%) received monoclonal anti-IL-2 receptor antibodies, and 1 (14.3%) received i.v. anti-thymocyte globulin 3 mg/kg. In this group, all patients received azathioprine on the initial immunosuppressive therapy, and most (n=5, 71.4%) received a calcineurin inhibitor.
Post-transplant clinical and laboratory data showed a higher incidence of nephrotic syndrome (57.1% vs 9.5%, p=0.02), hematuria (100% vs 38.1%), and C3 complement consumption in the recurrent group (n=4, 57.1% vs n=3, 14.3%, p=0.04). C4 consumption was rare; the only case was observed in the recurrent group. Allograft biopsies from the recurrence group exhibited mesangial hypercellularity (n=7, 100%) and crescents (n=2, 28.6%). Interstitial fibrosis was more common in the recurrence group (85.7
During the follow-up, 9 patients (32.1%) presented graft failure, 2 (22.2%) due to MPGN recurrence. There was 1 death with a functioning graft in the MPGN recurrence group. There was no significant difference in graft survival between the groups. In a 5-year follow-up, 19 (67.8%) patients in the non-recurrence group and 4 (57.1%) in the recurrence group remained with a functioning graft, with a serum creatinine of 1.9±0.9 mg/dl (Figure 2). At the end of 5 years of follow-up, the estimated glomerular filtration rate was 67.5±35.1 mL/min/1.73 m2 in the non-recurrence group and 52.9±8.4 mL/min/1.73 m2 in the recurrence group (p=0.45). All patients with a functioning graft in a 5-year follow-up in the recurrence group presented graft failure due to chronic dysfunction in the long-term follow-up, with a median of 75 (64–147) months after MPGN recurrence diagnosis.
Discussion
Post-transplant MPGN recurrence is common, reaching 50% during the first 2 years [9]. In this series, however, the recurrence of MPGN occurred in 25% of transplant recipients with a confirmed diagnosis of primary MPGN in the native kidney. There were no significant differences in the general characteristics between the MPGN recurrence and non-recurrence groups. All the patients with post-transplant MPGN recurrence presented nephrotic syndrome at native MPGN diagnosis, suggesting more severe disease in this group. After kidney transplantation, the prevalence of nephrotic syndrome was also significantly higher in the MPGN recurrence group. Thus, in this series, nephrotic syndrome at the diagnosis of MPGN in the native kidney was associated with a higher risk for recurrence after transplantation.
Complement levels were regularly monitored in this series, usually every half year, with empiric immunosuppressive therapy adjustment according to its results. We observed that the higher percentage of patients with reduced complement C3 levels pre-transplant was in the recurrence group. Also, complement C4 pre-transplant consumption was observed only in the recurrence group, suggesting a more severe immune response. A previous study by our group analyzing the risk for lupus nephropathy post-transplant showed an increased risk in patients with reduced complement levels at transplant [10].
The introduction of a new classification of MPGN, with the identification of patterns of immunoglobulins and complement product deposition, enabled a more accurate assessment of the disease after kidney transplantation [9]. It was not possible to reclassify our MPGN cases according to the new classification due to the lack of a detailed description of the immunofluorescence patterns. Despite the differences in clinical and laboratory presentation, native kidney biopsy morphology, including the prevalence of mesangial hypercellularity, crescents, and interstitial fibrosis, was similar between the groups.
The incidence of cell-mediated acute rejection was similar between the groups, and there were no cases of antibody-mediated acute rejection in the MPGN recurrence group. Some histologic features of active antibody-mediated rejection (AMR), such as glomerular basement membrane duplication, with or without cellular proliferation, may be indistinguishable from MPGN by light microscopy [11]. In this series, the absence of C4d deposits in renal tissue and complete analysis of biopsies by immunofluorescence and electron microscopy confirmed the diagnosis of MPGN recurrence. Also, the absence of donor-specific anti-HLA antibodies helped to exclude the chance of superimposed AMR.
The MPGN recurrence after transplantation is associated with an impaired graft function, with a reported 5-year allograft survival after diagnosis of 30% [9]. In this series, however, the graft survival in the MPGN recurrence group was higher than expected. This difference may result from including less severe forms of MPGN in this study or from the maintenance of steroids as immunosuppressive therapy. The limiting points of this study are its retrospective design, the small number of patients included, and the absence of reclassification of the MPGN cases according to the new criteria. However, this study provides important information about MPGN recurrence after kidney transplantation and its impact on graft survival. Accurate diagnosis of native glomerulopathy is essential for correctly assessing the risk of disease recurrence after transplantation. Applying digital pathology and artificial intelligence tools seems to contribute to this process [12,13]. In addition, monitoring complement levels before and after transplant could help identify patients at risk for recurrence.
Conclusions
MPGN recurrence is common after kidney transplantation, impacting graft survival. In this series, the MPGN recurrence was lower than previously reported. Risk factors for recurrence in this series were nephrotic syndrome and complement consumption at presentation in the native kidney, suggesting a more severe disease. Maintenance of steroids after transplant could explain the lower incidence of recurrence. Regularly monitoring of proteinuria and complement C3 and C4 levels after transplant could be associated with the better graft survival observed in this series due to maintenance immunosuppression adjustment in cases of complement consumption.
Figures
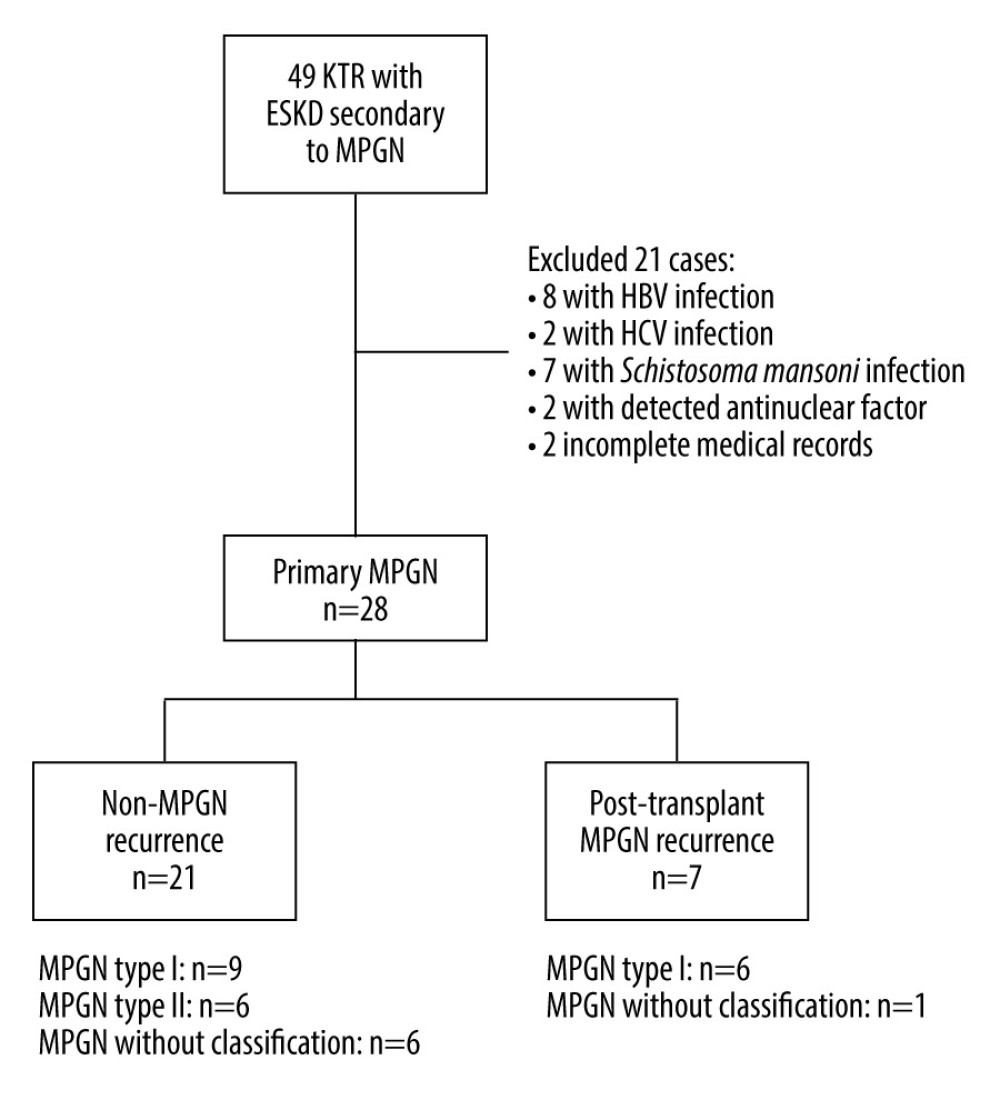 Figure 1. Study population and analyzed groups. MPGN – membranoproliferative glomerulonephritis; KTR – kidney transplant recipients; ESKD – end-stage kidney disease, HBV – hepatitis B virus; HCV – hepatitis C virus. (Created with Microsoft™ Word).
Figure 1. Study population and analyzed groups. MPGN – membranoproliferative glomerulonephritis; KTR – kidney transplant recipients; ESKD – end-stage kidney disease, HBV – hepatitis B virus; HCV – hepatitis C virus. (Created with Microsoft™ Word). 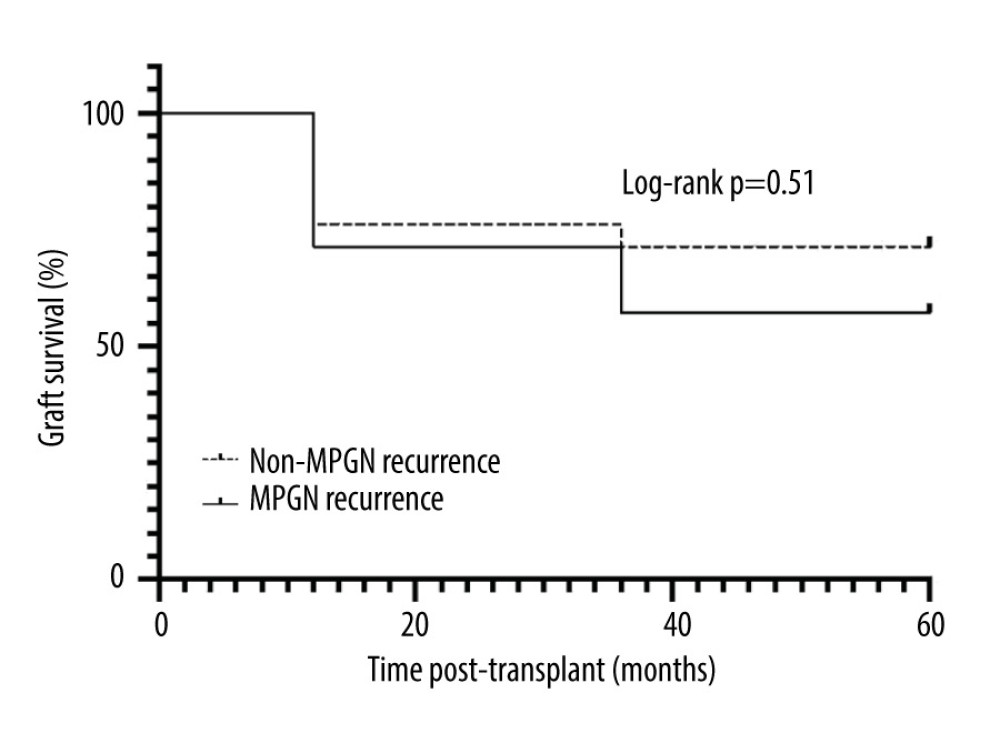 Figure 2. Graft survival of patients with pre-transplant MPGN diagnosis, according to MPGN recurrence and time post-transplant follow-up. (Created with GraphPad Prism 7.0c™ for Mac).
Figure 2. Graft survival of patients with pre-transplant MPGN diagnosis, according to MPGN recurrence and time post-transplant follow-up. (Created with GraphPad Prism 7.0c™ for Mac). Tables
Table 1. Pre-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.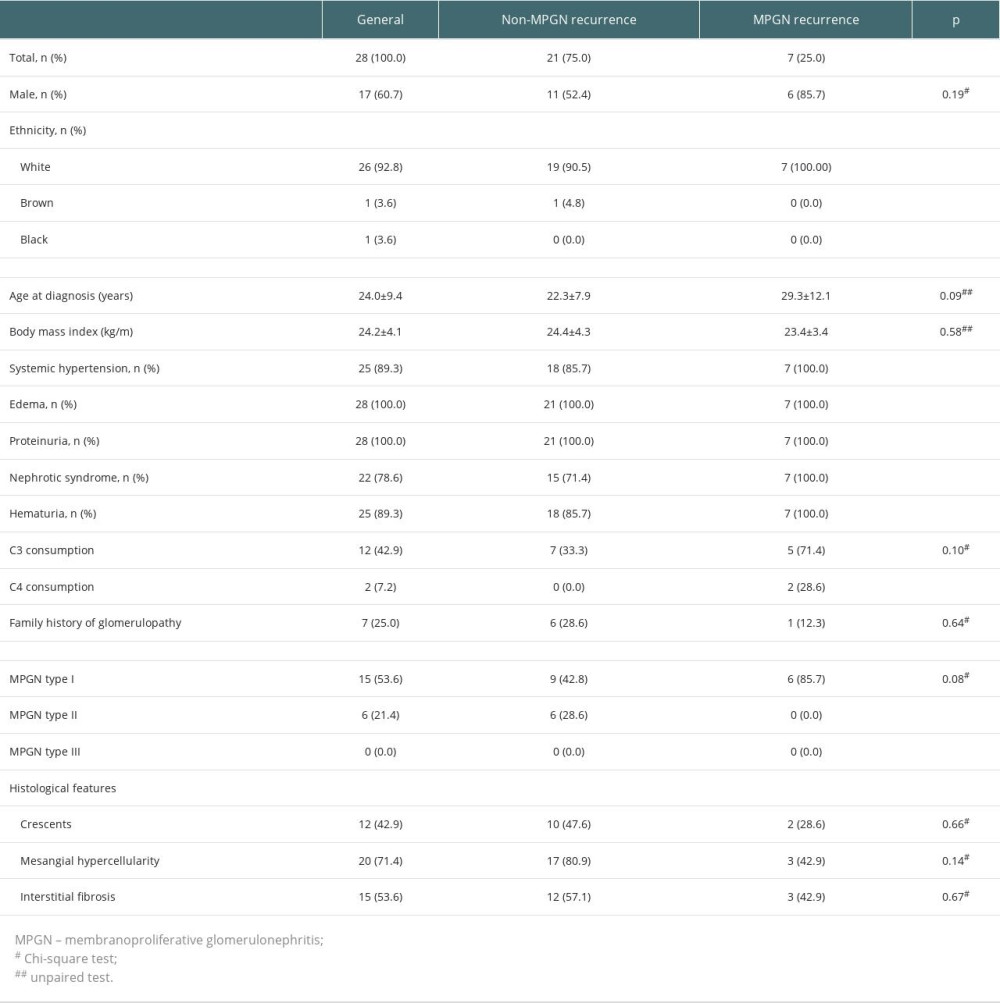 Table 2. Post-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.
Table 2. Post-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.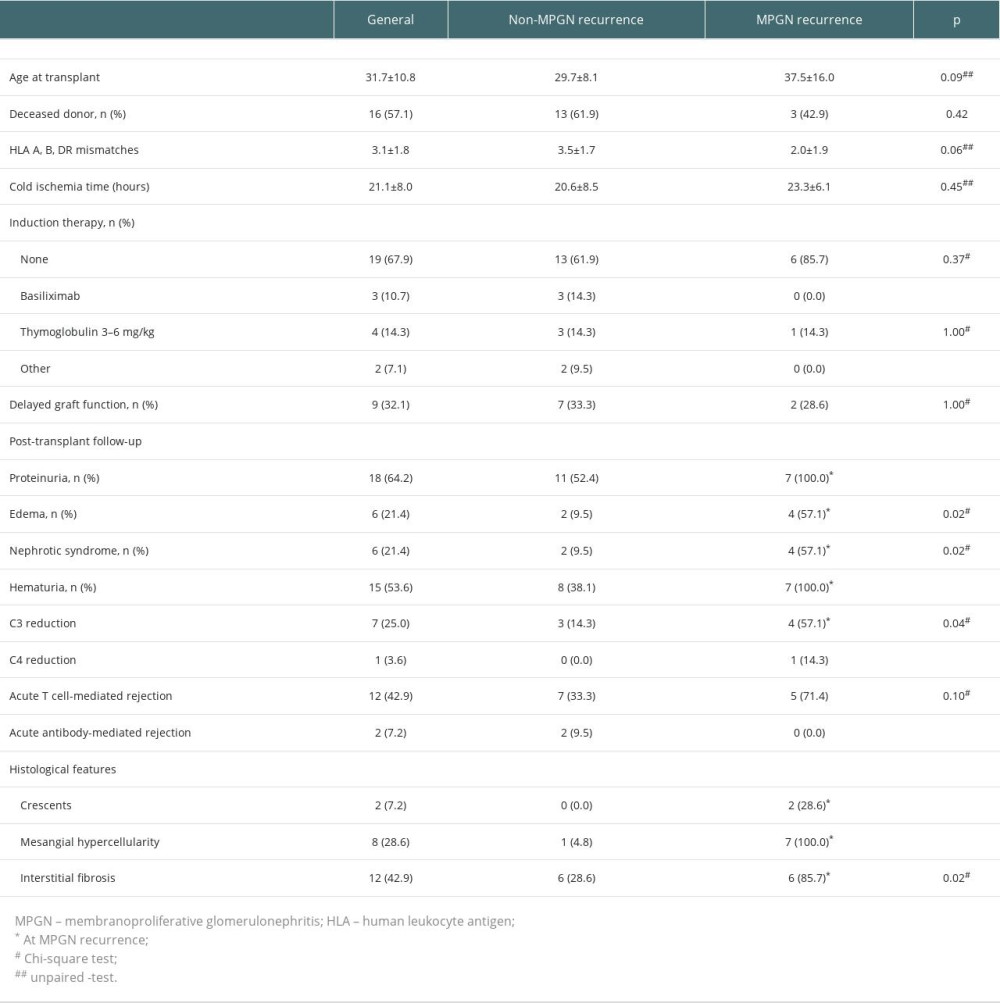
References
1. Lorenz EC, Sethi S, Leung N, Recurrent membranoproliferative glomerulonephritis after kidney transplantation: Kidney Int, 2010; 77(8); 721-28
2. Sethi S, Fervenza FC, Membranoproliferative glomerulonephritis – a new look at an old entity: N Engl J Med, 2012; 366(12); 1119-31
3. Aziz F, Singh T, Garg N, Post-transplant idiopathic immune complex membrano-proliferative glomerulonephritis: Characteristics and outcomes: Clin Nephrol, 2022; 99(2); 69-77
4. Sprangers B, Kuypers DR, Recurrence of glomerulonephritis after renal transplantation: Transplant Rev, 2013; 27(4); 126-34
5. Allen PJ, Chadban SJ, Craig JC, Recurrent glomerulonephritis after kidney transplantation: Risk factors and allograft outcomes: Kidney Int, 2017; 92(2); 461-69
6. De Souza L, Prunster J, Chan D, Recurrent glomerulonephritis after kidney transplantation: Curr Opin Organ Transplant, 2021; 26(4); 360-80
7. Levey AS, Stevens LA, Schmid CH, A new equation to estimate glomerular filtration rate: Ann Intern Med, 2009; 150(9); 604-12
8. Haas M, Sis B, Racusen LC, Banff 2013 Meeting Report: Inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions: Am J Transplant, 2014; 14(2); 272-83
9. Lim WH, Shingde M, Wong G, Recurrent and de novo glomerulonephritis after kidney transplantation: Front Immunol, 2019; 10; 1944
10. Signori Baracat AL, Ribeiro-Alves MAVF, Alves-Filho G, Mazzali M, Systemic lupus erythematosus after renal transplantation: Is complement a good marker for graft survival?: Transplant Proc, 2008; 40(3); 746-48
11. Infante B, Rossini M, Leo S, Recurrent glomerulonephritis after renal transplantation: The clinical problem: Int J Mol Sci, 2020; 21(17); 5954
12. Eccher A, Neil D, Ciangherotti A, Digital reporting of whole-slide images is safe and suitable for assessing organ quality in preimplantation renal biopsies: Hum Pathol, 2016; 47(1); 115-20
13. Girolami I, Pantanowitz L, Marletta S, Artificial intelligence applications for pre-implantation kidney biopsy pathology practice: A systematic review: J Nephrol, 2022; 35(7); 1801-8
Figures
 Figure 1. Study population and analyzed groups. MPGN – membranoproliferative glomerulonephritis; KTR – kidney transplant recipients; ESKD – end-stage kidney disease, HBV – hepatitis B virus; HCV – hepatitis C virus. (Created with Microsoft™ Word).
Figure 1. Study population and analyzed groups. MPGN – membranoproliferative glomerulonephritis; KTR – kidney transplant recipients; ESKD – end-stage kidney disease, HBV – hepatitis B virus; HCV – hepatitis C virus. (Created with Microsoft™ Word). Figure 2. Graft survival of patients with pre-transplant MPGN diagnosis, according to MPGN recurrence and time post-transplant follow-up. (Created with GraphPad Prism 7.0c™ for Mac).
Figure 2. Graft survival of patients with pre-transplant MPGN diagnosis, according to MPGN recurrence and time post-transplant follow-up. (Created with GraphPad Prism 7.0c™ for Mac). Tables
 Table 1. Pre-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.
Table 1. Pre-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant. Table 2. Post-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.
Table 2. Post-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant. Table 1. Pre-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.
Table 1. Pre-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant. Table 2. Post-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant.
Table 2. Post-transplant characteristics of the kidney transplant recipients with native biopsy-proven membranoproliferative glomerulonephritis according to its recurrence after transplant. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








