12 September 2023: Original Paper
Living Donor Liver Transplantation in Patients with Preformed Donor-Specific Anti-Human Leukocyte Antigen Antibodies Using Preoperative Desensitization Therapy According to Intensity of Donor-Specific Antibodies: A Single-Center Study
Kohei OgawaDOI: 10.12659/AOT.941346
Ann Transplant 2023; 28:e941346
Abstract
BACKGROUND: In liver transplantation (LT), preoperative desensitization therapy is considered necessary in patients positive for donor-specific anti-human leukocyte antigen antibodies (DSAs). However, the relationship between DSA intensity and the necessary desensitization therapy is unclear.
MATERIAL AND METHODS: A total of 37 adult living donor (LD) LTs performed between January 2016 and March 2022 were examined. Mycophenolate mofetil (MMF) was administered preoperatively in DSA-positive cases with positive lymphocyte cross-matching who underwent LDLT. In those with strongly positive DSA (mean fluorescence intensity 10 000), rituximab was administered 2 weeks before LDLT in addition to MMF. Cross-reactive epitope group antigen (CREG)-alone-positive cases were also treated with preoperative MMF when lymphocyte cross-matching was positive.
RESULTS: Of the 37 patients, 9 were DSA-positive, 7 were CREG-alone-positive, and the others were double-negative. Of 9 DSA-positive cases, desensitization therapy was performed in 7, among which rituximab administration was performed in 3 strongly DSA-positive cases. Of 7 CREG-alone-positive cases, 2 were lymphocyte cross-match-positive and underwent desensitization therapy. The 1-year survival rate was 100% in both DSA- and CREG-alone-positive cases. The frequency of T-cell mediated rejection in DSA-positive, CREG-alone-positive, and double-negative cases was 22%, 43%, and 29%, respectively, with no significant difference. Antibody-mediated rejection occurred in only 1 patient, who was strongly DSA-positive and blood-group incompatible. There was also no significant difference among the 3 groups in terms of the frequency of biliary complications or 90-day mortality.
CONCLUSIONS: Satisfactory LDLT results were achieved in DSA- and CREG-alone-positive cases following desensitization therapy.
Keywords: Desensitization, Immunologic, Liver Transplantation, Living Donors, Adult, Humans, rituximab, HLA Antigens, Antibodies
Background
High frequencies of antibody-mediated rejection (AMR) and graft loss have been reported in lymphocyte cross-match-positive cases in kidney transplantation, and strategies to overcome this problem have shown progress [1–4]. In the field of liver transplantation (LT), however, opinion is equally divided on whether lymphocyte cross-matching is associated with graft loss [5–11]. Recent technological improvements in detecting anti-human leukocyte antigen (HLA) antibodies have made it possible to determine whether a recipient has a high titer of donor-specific anti-HLA antibodies (DSAs) or cross-reactive epitope group (CREG) antibodies before LT, and there are increasing reports of poor results and a complicated postoperative course following LT in DSA-positive recipients [12–18]. Therefore, a consensus has recently been reached that preoperative desensitization therapy is necessary for DSA-positive cases.
We have previously reported an association between preformed DSAs and 90-day mortality in living donor (LD) LT [19]. Based on this result, we started desensitization therapy for DSA-positive patients prior to LDLT. Rituximab has been used as desensitization therapy in patients with blood-group-incompatible LDLT, and good results have been reported [20,21]. However, it is unclear which strengths of DSA-positivity require rituximab, because not all DSA-positive patients develop AMR. Patients with strongly positive DSAs may require rituximab. However, milder desensitization may be sufficient in moderately positive cases, and desensitization may not be necessary in patients with a low mean fluorescence intensity (MFI).
We used different preoperative desensitization therapies for DSA- and CREG-positive cases depending on the results of MFI values. In this report, we describe the results of our tailor-made preoperative desensitization therapy and subsequent LDLT in DSA- and CREG-positive cases at a single institution.
Material and Methods
PATIENTS:
We have previously reported the results of LDLT in 8 DSA-positive patients up to the end of 2015 [17]. During that period, no desensitization therapy was performed for DSA-positive cases other than blood-group-incompatible cases. In the present study, 37 adult patients who underwent LDLT between January 2016, when desensitization therapy for DSA-positive cases was introduced, and March 2022 were targeted. Eight DSA-positive patients who underwent LDLT before December 2015 were included as historical controls.
GRAFT SELECTION AND RECIPIENT SURGICAL PROCEDURE:
The selection criteria for living donors and the surgical procedure in recipients were as follows: the graft-to-recipient weight ratio (GRWR) was ≥0.7%; and donor residual liver volume was ≥30%. Splenectomy was performed only when the portal vein pressure was >20 mmHg after revascularization, and not for the purpose of desensitization in blood group incompatibility or DSA-positive cases.
PERIOPERATIVE IMMUNOSUPPRESSION:
Our usual postoperative immunosuppression is two-drug administration of tacrolimus and steroids. Patients with blood type incompatibility and who were also DSA-positive or CREG-positive underwent postoperative immunosuppressive therapy with 3 agents: tacrolimus, MMF, and steroids. The target whole-blood trough level for tacrolimus was 10–15 ng/mL for the first 2 weeks, approximately 8–10 ng/mL for the next 3 to 8 weeks, and 5–8 ng/mL after the second month. MMF was administered at 1500 mg/body/day. Steroid therapy was initiated at a dose of 10 mg/kg before graft reperfusion and tapered from 1 mg/kg/day on days 1–3 to 0.5 mg/kg/day on days 4–6, and then to 0.3 mg/kg/day on day 7. The same dose was then given orally during weeks 2 to 4 and tapered to 0.1 mg/kg/day after 4 weeks. Oral steroid treatment was stopped after 3 months.
MEASUREMENT OF ANTI-HLA ANTIBODIES:
All living donors and recipients were routinely typed for HLA-A, HLA-B, HLA-C, HLA-DR, HLA-DQ, and HLA-DP before LDLT. Anti-HLA antibody screening for both HLA class I and class II was performed preoperatively using the FlowPRA screening test (One Lambda, Inc., West Hills, CA, USA), and a single-antigen bead assay on a Luminex analyzer was added for positive cases. These tests were outsourced to ReproCELL Co., Ltd. (Kanagawa, Japan). Antibodies with an MFI ≥1000 were considered positive, of which positive antibodies against the donor HLA antigen were termed DSA-positive. CREG was identified by referring to the CREG map described in the result report by ReproCELL, and those with an MFI ≥1000 were termed CREG-positive. Measurement of anti-HLA antibodies was performed postoperatively whenever AMR due to DSA or CREG was suspected. In addition, such cases were followed up at intervals of 6 months to 1 year.
LYMPHOCYTE CROSS-MATCHING:
In addition to anti-HLA antibody screening and identification of DSA and CREG, evaluation of lymphocyte cross-matching was also performed. Until the end of 2017, the complement-dependent cytotoxicity (CDC) method was used, and a positive diagnosis was made when ≥20% of the lymphocytes were killed. Flow cytometry cross-match, which is more sensitive than CDC, was started in 2018. A positive diagnosis was made when the ratio of the MFI of the negative control to the MFI of the patient’s serum was ≥2 for B cells and ≥1.5 for T cells.
FOR DSA-POSITIVE CASES: For DSA-positive cases, preoperative treatment was selected according to the MFI value (Figure 1). For strongly DSA-positive cases with an MFI of DSA of around 10 000 or more, rituximab 375 mg/m2 was administered at least 2 weeks before LDLT, and tacrolimus and MMF were started 1 week before LDLT. The target trough level of tacrolimus was 5–10 ng/mL, and the dose of MMF was 1000 mg/body/day. In addition, intravenous immune globulin (IVIG) was administered intraoperatively and postoperatively (0.6 g/kg ×5 days). For patients who had an MFI of DSA ≥1000 and <10,000 (moderately DSA-positive) and positive lymphocyte cross-matching, only preoperative MMF and perioperative IVIG were generally administered. A negative lymphocyte cross-match with an MFI of DSA ≥1000 was considered not to be significant DSA, and desensitization was not performed.
FOR CREG-ALONE-POSITIVE CASES:
For cases that were DSA-negative but CREG-positive, if the lymphocyte cross-match was positive, preoperative MMF and perioperative IVIG was performed, as for moderately DSA-positive cases.
FOR BLOOD-GROUP-INCOMPATIBLE CASES:
For blood-group-incompatible cases, irrespective of DSA and CREG status, preoperative treatment was performed with rituximab, MMF, and tacrolimus in the same manner as in strongly DSA-positive cases. Moreover, plasmapheresis with type AB plasma was performed just before LDLT, with the aim of achieving an anti-donor blood-group antibody titer of 8 times or less. IVIG was also administered perioperatively.
DIAGNOSIS OF REJECTION:
T-cell-mediated rejection (TCMR) was diagnosed based on pathological evidence. If liver enzymes and bilirubin were elevated postoperatively, and rejection was suspected, an ultrasound-guided percutaneous liver biopsy was performed. TCMR was diagnosed by the Banff classification [22]. AMR was diagnosed based on: (i) the presence of detectable DSAs in serum; (ii) evidence of periportal edema and necrosis in hematoxylin-eosin-stained tissue [23], endothelial cell hypertrophy/enlargement, capillary dilatation, leukocyte sludging and/or leukocyte margination, and edema according to the Banff meeting report [24]; (iii) diffuse portal microvasculature C4d staining; and (iv) exclusion of similar types of injury [25].
MONITORING OF INFECTION:
For cytomegalovirus (CMV) monitoring, pp65 antigenemia was examined weekly for the first month after LT. In the present study, positive pp65 antigenemia was defined as CMV infection. Blood cultures were obtained when fever with temperature ≥38°C appeared. A positive blood culture was defined as bacteremia.
MANAGEMENT OF INFECTION:
During the perioperative period, ampicillin and sulbactam sodium were used as prophylactic antibacterial agents, and micafungin sodium hydrate was used as a prophylactic antifungal agent for 4 days postoperatively. Tube feeding was started the day after the operation, and the central venous catheter inserted during the operation was removed within 1 week after LDLT. Prophylactic antiviral drugs against cytomegalovirus were not administered. When pp65 antigenemia became positive, valganciclovir hydrochloride was administered.
DEFINITION OF BILIARY COMPLICATIONS:
Biliary complications were defined as perioperative bile leakage, biliary stricture requiring treatment (either anastomotic or non-anastomotic), or findings of sclerosing cholangitis on cholangiography.
STUDY DESIGN:
The primary endpoint of this study was overall survival after LDLT for DSA-positive and CREG-alone-positive cases after starting desensitization therapy. Secondary endpoints were the frequency of complications such as rejection and infectious complications in DSA-positive and CREG-alone-positive cases. These data were compared with those of double-negative cases. The data of the present DSA-positive cases were also compared with those of the historical controls prior to December 2015 [17].
STATISTICAL ANALYSIS:
Cumulative probability curves of overall survival were calculated using Kaplan–Meier methods, and differences between curves were evaluated using the log rank test. Continuous variables are presented as the median and range values and compared using the Mann-Whitney U test or Kruskal-Wallis test. Categorical variables are presented as the number and ratio values and compared by the chi-squared test or Fisher’s exact test. A
ETHICAL CONSIDERATIONS:
This study was approved by the institutional review board of Ehime University (approval No. 2209021) and conducted in accordance with the Declaration of Helsinki of 1975, as revised in 1996.
Results
PATIENT CHARACTERISTICS:
Table 1 summarizes the patients’ characteristics. The 37 patients consisted of 17 males and 20 females, with a median age of 54 (range, 24–70) years. The primary disease was liver cirrhosis in 21 patients, acute liver failure in 3, primary biliary cholangitis in 3, biliary atresia in 3, autoimmune hepatitis in 3, re-transplantation in 1, and other in 3. The median Model for End-stage Liver Disease (MELD) score was 13 (range, 6–40). The blood group combinations comprised 24 identical cases, 5 compatible cases, and 8 incompatible cases. Of the 37 patients, 25 had anti-HLA antibodies. Seven had only class I antibodies, 5 had only class II antibodies, and 13 had both class I and class II antibodies. Detected anti-HLA antibodies were against HLA-A in 9 cases, HLA-B in 10 cases, and HLA-C in 7 cases for class I, and HLA-DR in 14 cases, HLA-DQ in 10 cases, and DP in 8 cases for class II. Significantly more females than males had anti-HLA antibodies. Patients with a history of pregnancy also frequently had anti-HLA antibodies, but there was no significant difference when limited to women. There was no significant difference in history of blood transfusion between anti-HLA antibody-positive and -negative patients.
CLINICAL FEATURES AND OUTCOMES OF DSA-POSITIVE CASES:
Of the 25 anti-HLA antibody-positive cases, 9 were DSA-positive, of which 5 were also CREG-positive. Table 2 lists the details of the 9 DSA-positive cases. Lymphocyte cross-matching was positive in 7 of the 9 DSA-positive cases. There were 3 strongly DSA-positive cases, including 1 blood group-incompatible case (cases 1–3). For these 3 cases, desensitization therapy including rituximab was performed. The other 6 DSA-positive cases were moderately DSA-positive (cases 4–9), of which 3 (cases 4–6) were cross-match-positive and treated preoperatively with MMF. Case 7 was also cross-match-positive but did not receive preoperative MMF due to spontaneous bacterial peritonitis before LDLT. In case 8, despite a low MFI of DSA and a negative cross-match result, desensitization therapy was performed due to blood group incompatibility. The remaining patient (case 9) was cross-match-negative and did not undergo desensitization therapy. In all DSA-positive patients who were treated with rituximab, CD20-positive cells were less than 2% just before LDLT. Of the 3 strongly DSA-positive cases, 2 patients developed TCMR (cases 2 and 3). AMR was also observed in 1 patient (case 3) who was blood group-incompatible. Case 2 was cured with steroid pulse therapy, but case 3 was refractory to steroid pulse therapy and required administration of antithymocyte globulin (ATG). TCMR or AMR was not observed in any patient with moderately positive DSA, either with (cases 4–6 and 8) or without (cases 7 and 9) preoperative desensitization therapy.
CLINICAL FEATURES AND OUTCOMES OF CREG-ALONE-POSITIVE CASES:
Table 3 lists the details of the 7 DSA-negative but CREG-positive patients. MMF was started preoperatively only in the 2 patients (cases 10 and 11) in whom lymphocyte cross-matching was positive. No rejection occurred in these 2 patients, whereas TCMR occurred in 3 of the remaining 5 patients who were not desensitized.
GRAFT SURVIVAL:
Survival curves for DSA-positive, CREG-alone-positive, and double-negative cases are shown in Figure 2. The median follow-up duration after LDLT was 1253 (range, 66–2520) days. There was no 90-day mortality among the DSA- or CREG-alone-positive cases. The 1-year survival rate was 100% in both DSA- and CREG-alone-positive cases, and there was no significant difference compared with double-negative cases.
COMPARISONS OF CLINICAL FACTORS AND OUTCOMES AMONG DSA-POSITIVE, CREG-ALONE-POSITIVE, AND DOUBLE-NEGATIVE PATIENTS:
Findings of the comparisons of perioperative clinical factors, postoperative complications, and graft outcomes among the DSA-positive, CREG-alone-positive, and double-negative patients are presented in Table 4. There were no significant differences among the groups in terms of preoperative or surgical factors except for the frequency of desensitization therapy. Regarding postoperative rejections, TCMR was observed in 2/7 (22.2%) DSA-positive cases, in 3/7 (42.9%) CREG-alone-positive cases, and in 6/21 (28.6%) double-negative cases, with no significant difference. Regarding infectious complications, there was no significant difference in the frequency of cytomegalovirus (CMV) infection or in bacteremia after LDLT among the DSA-positive, CREG-alone-positive, and double-negative cases. There was also no significant difference among the 3 groups in terms of the frequency of biliary complications or 90-day mortality.
COMPARISON OF DSA-POSITIVE CASES BEFORE AND AFTER INTRODUCTION OF PREOPERATIVE DESENSITIZATION THERAPY:
There was no significant difference in preoperative factors except for the frequency of desensitization therapy between DSA-positive cases up to the end of 2015 (historical controls) and those after 2016. Regarding surgical factors, however, operative time was significantly shorter, and blood loss also tended to be less in the present cases. There were significantly fewer severe postoperative complications (≥ grade IIIb of the Clavien–Dindo classification) in the present cases, and 90-day mortality and overall survival were significantly improved in the present cases compared with the historical controls (1-, 3-, and 5-year overall survival: 100%, 100%, 100% vs 50%, 50%, 50%, respectively, p=0.02). There were no significant differences between the present cases and the historical controls in terms of the rejection rate (TCMR: 50% vs 20%, p=0.23, AMR: 13% vs 10%, respectively, p=0.93) (Table 5).
:
A 57-year-old woman with alcoholic cirrhosis was scheduled for LDLT from her daughter. The blood type combination was identical, but the preoperative single-antigen Luminex test was positive for broad-spectrum anti-HLA antibodies in both Class I and Class II, with an MFI ≥10 000, including DSA and CREG. The flow cytometry lymphocyte cross-match was also positive for both T and B cells. Desensitization therapy including rituximab for strongly DSA-positive cases was performed. She underwent LDLT using a right lobe graft from her 24-year-old daughter without splenectomy. Because her serum bilirubin level was elevated from postoperative day (POD) 7, steroid pulse therapy was started for suspected rejection. Subsequently, the bilirubin level decreased gradually, but the transaminase levels increased after POD 15 and a liver biopsy was performed, followed by second steroid pulse therapy. Liver biopsy showed rejection with a rejection activity index (RAI) score of 2 (P=1, B=0, V=1), which was considered to be the result of the initial steroid pulse therapy. After the second steroid pulse therapy, her transaminase levels improved, and she was discharged in good condition on POD 36. Although the single-antigen Luminex test performed immediately before transplantation showed no significant change in the MFI for almost all anti-HLA antibodies compared to before rituximab administration, the MFI of anti-HLA antibodies decreased for many anti-HLA antibodies, including DSA on POD 7 and 6 months after LDLT. However, for some anti-HLA antibodies in Class I, including CREG, the MFI was increasing to higher than the preoperative value.
:
A 52-year-old man with HCV-related liver cirrhosis was scheduled for LDLT from his nephew. This patient was blood group-incompatible, and the preoperative single-antigen Luminex test was also strongly positive for Class II DSA (DQ6 11620). The flow cytometry lymphocyte cross-match was positive for B cells. According to the protocol for blood group incompatibility, preoperative plasmapheresis was performed in addition to desensitization with rituximab, tacrolimus, and MMF. The MFI of anti-HLA antibodies was markedly decreased after plasmapheresis. LDLT using a right lobe graft from the 24-year-old nephew was performed without splenectomy. The patient developed steroid-resistant rejection and required ATG administration. His liver function then improved gradually, and he was discharged on POD 59. Liver biopsy performed on POD 14 showed findings suggestive of TCMR with an RAI score of 5 (P=2, V=2, B=1). As C4d staining was also lightly positive, AMR was suspected in addition to TCMR. Regarding changes in the MFI of anti-HLA antibodies, the MFI of DSA and some anti-HLA antibodies that were preoperatively negative increased at POD 7, but decreased again at POD 37 after rejection treatment.
Discussion
We have previously reported that 90-day mortality of LDLT was significantly higher in DSA-positive recipients than in DSA-negative recipients [19]. Based on this result, we started desensitization therapy in DSA-positive patients prior to LDLT in 2016. In general, DSA with an MFI ≥1000 is considered DSA-positive. However, the recent anti-HLA antibody identification test and lymphocyte cross-matching by flow cytometry are highly sensitive, and not all DSA-positive patients are considered to be at high risk for AMR. We considered that the risk of sensitization varied depending on the intensity of DSA, and not all DSA-positive patients were at high risk of AMR. McCaughan et al reported that, in DSA-positive cases, an MFI ≥10 000 was associated with mortality at 1 year after LT [16]. In LDLT, Yoshizawa et al reported a high mortality rate in class I DSA-positive cases with an MFI ≥10 000 [14]. Therefore, in the present series, rituximab was used for those with an MFI ≥10 000. DSA-positive cases with an MFI ≥1000 and <10 000 were desensitized with MMF alone. Furthermore, if the lymphocyte cross-match was negative, it was determined that the patient had no significant DSA, and desensitization was not performed.
There have been several reports of preoperative desensitization therapy in DSA-positive cases [20,21,26]. Although preoperative desensitization therapy with rituximab may reduce the risk of AMR, it can increase the risk of post-transplant infectious complications. Akamatsu et al [21] reviewed the use of rituximab for desensitization in DSA-positive cases in Japan and they reported a 1-year survival rate of 85% in 47 rituximab-treated cases. Six of these 47 patients (13%) developed AMR, of whom 3 died. They reported that rituximab doses below 300 mg/m2 were associated with significantly higher rates of AMR and recommended rituximab doses above 300 mg/m2. However, their report included many DSA-positive cases with an MFI <10 000, and it is questionable whether such cases required rituximab. In contrast, Del Bello et al [20] reported that the use of polyclonal antibodies or rituximab as an induction therapy for preformed DSAs did not reduce the risk of acute rejection, but increased the risk of infectious complications. Therefore, rituximab should be used only in patients at high risk of AMR. For 3 strongly DSA-positive cases in the present series, rituximab was administered at a dose of 375 mg/m2, which resulted in no graft loss due to AMR. Furthermore, using MMF alone without rituximab in moderately DSA-positive cases minimized the risk of infectious complications in DSA cases overall.
DSA-positive and cross-match-positive status has also been reported to be associated with increased incidence of TCMR [26,27]. Kubal et al reported that overall low rejection rates could be achieved in cross-match-positive LT with the use of rabbit ATG with or without rituximab induction. In the present series, the frequency of TCMR in DSA-positive and CREG-alone-positive cases did not differ from that in double-negative cases. Moreover, no rejection occurred in patients with an MFI <10 000 who received preoperative MMF administration. These results might suggest that desensitization with MMF alone may be sufficient for DSA-positive cases with a moderate MFI. However, of 3 strongly DSA-positive cases, 2 cases developed TCMR despite intensive desensitization therapy. Of them, 1 incompatible case (case 3) also developed AMR and required ATG administration. In this case, the postoperative titer of anti-blood group antibody was 2 to 4 times, which is not particularly high. Therefore, DSA might have been the cause of AMR. Regarding the MFI levels of anti-HLA antibody, the MFI of DSA, which had decreased after plasmapheresis, increased again on POD 7; and non-DSA anti-HLA antibody, which is thought to be caused by blood transfusion, also appeared. These results indicate that, in strongly DSA-positive cases, desensitization therapy is essential to suppress postoperative rejection, although it seems difficult to achieve 100% prevention.
The usefulness of IVIG in blood-type-incompatible LT has been reported by Ikegami et al [28], and the results of blood-type-incompatible LT in our department have also improved significantly since IVIG has been used in combination with rituximab. The usefulness of IVIG for cross-match-positive cases has been reported in renal transplantation [29]. The suggested mechanisms of IVIG against alloimmune responses include reduction of alloantibodies through anti-idiotypic circuits, inhibition of inflammatory cytokine generation, inhibition of complement-mediated injury, and inhibition of antibody production. Therefore, IVIG may be an effective tool in strongly DSA-positive cases, especially in deceased-donor LT patients in whom preoperative desensitization therapy including rituximab is not sufficiently performed. Future investigations are needed to determine how to desensitize patients with a high MFI of broad-spectrum anti-HLA antibodies who are awaiting deceased-donor LT.
The literature on the relevance of CREG in the field of LT is scarce. CREG positivity has been reported by Nainani et al as a risk factor for AMR in renal transplantation [30]. In their report, severe AMR developed in 4 out of 9 renal transplant recipients with preformed CREG antibodies and 2 patients achieved clinical remission with combined use of rituximab and IVIG. Therefore, they recommended that patients with CREG antibodies might be candidates for prophylactic desensitization regimens such as rituximab and IVIG. In our present series, preoperative desensitization therapy was performed only for cross-match-positive cases among CREG-positive cases. Although only 2 patients underwent desensitization therapy, they survived without rejection. Interestingly, in case 2, who was strongly positive for CREG, as well as for DSA, the MFI of DSA decreased after LT, but there was some increase in CREG. This might suggest that DSA was adsorbed to the graft, and the MFI decreased, whereas CREG differed in the degree of antibody production and the degree of adsorption to the graft. Further investigations are needed to determine whether desensitization therapy against CREG-positive cases is required in LT.
This research has some limitations. First, it was a retrospective study with a small number of cases at a single institution. The population of DSA-positive cases was heterogeneous in their MFI values, and desensitization therapy also varied from case to case. Therefore, the statistical results might be unreliable. As DSA-positive was defined as having an MFI ≥1000, 2 cases with a low MFI of about 1000–2000 and that were cross-match-negative were included. When discussing the outcome of DSA-positive cases, it might have been better to exclude such low MFI cases. Second, the desensitization therapy was different for each case, because the study was conducted during a transitional period in treatment policies. Moreover, the operation time of DSA-positive cases in the present study was significantly shorter than for the historical controls, and the good results in the present study may be attributed to the influence of the operative procedure learning curve, as well as the factors of desensitization therapy. It is expected that further investigations will clarify which cases require rituximab and which are suitable for MMF alone among DSA- and CREG-positive cases.
Conclusions
Desensitization therapy was performed according to the MFI of DSA in LDLT, and the results of LDLT were satisfactory. In LDLT, where donors are limited, even a strongly DSA-positive donor-recipient combination is not considered a contraindication if appropriate preoperative desensitization therapy is performed.
Figures
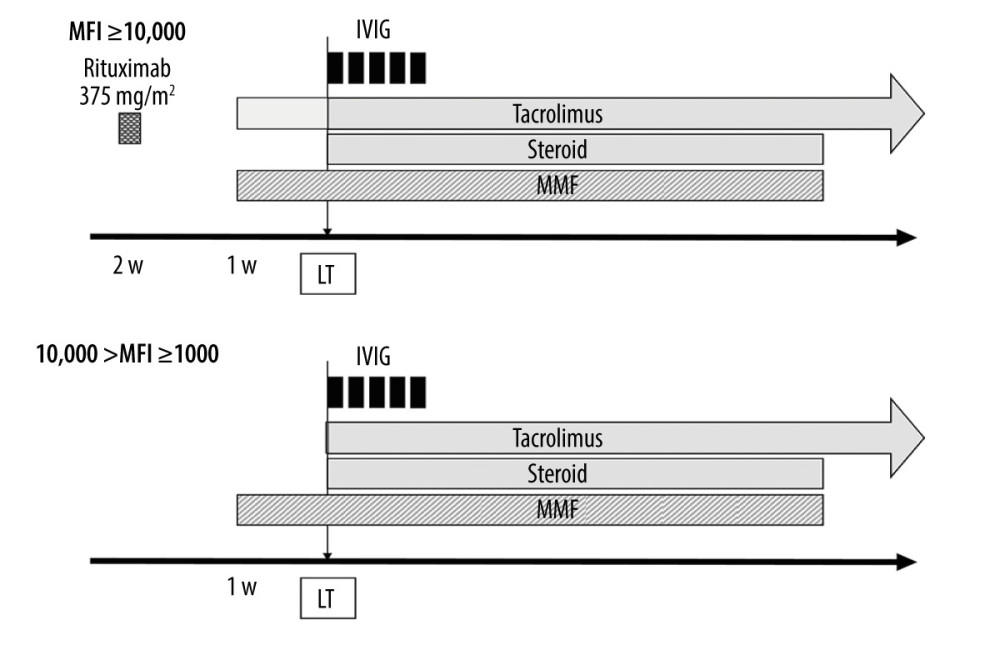 Figure 1. Desensitization protocol for donor-specific anti-human leukocyte antigen antibody (DSA)-positive cases. MFI – mean fluorescence intensity; IVIG – intravenous immune globulin; MMF – mycophenolate mofetil; LT – liver transplantation (Microsoft, PowerPoint for Mac, ver. 16.48).
Figure 1. Desensitization protocol for donor-specific anti-human leukocyte antigen antibody (DSA)-positive cases. MFI – mean fluorescence intensity; IVIG – intravenous immune globulin; MMF – mycophenolate mofetil; LT – liver transplantation (Microsoft, PowerPoint for Mac, ver. 16.48). 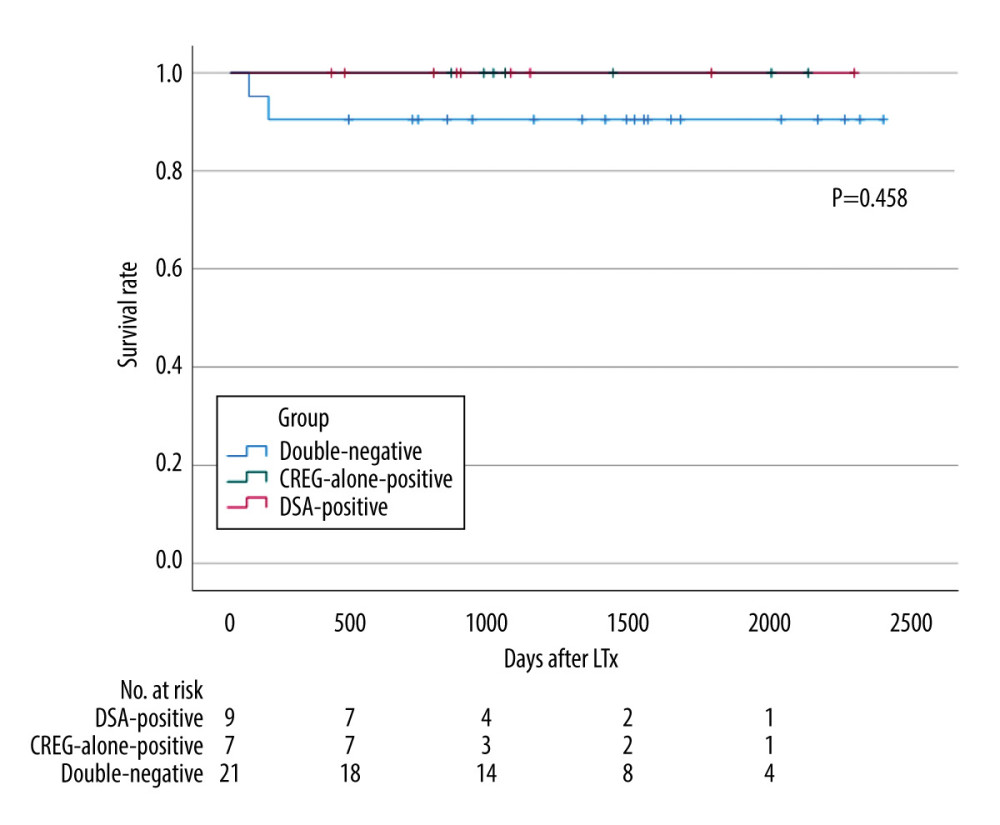 Figure 2. Graft survival in donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients (IBM, SPSS statistics, ver. 28.0.1.0). The 1-year graft survival rate is 100% in both DSA-positive and CREG-alone-positive cases, and 87.5% in double-negative cases (p=0.289). DSA, donor-specific anti-human leukocyte antigen antibody; CREG, cross-reactive epitope group; LTx, liver transplantation.
Figure 2. Graft survival in donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients (IBM, SPSS statistics, ver. 28.0.1.0). The 1-year graft survival rate is 100% in both DSA-positive and CREG-alone-positive cases, and 87.5% in double-negative cases (p=0.289). DSA, donor-specific anti-human leukocyte antigen antibody; CREG, cross-reactive epitope group; LTx, liver transplantation. 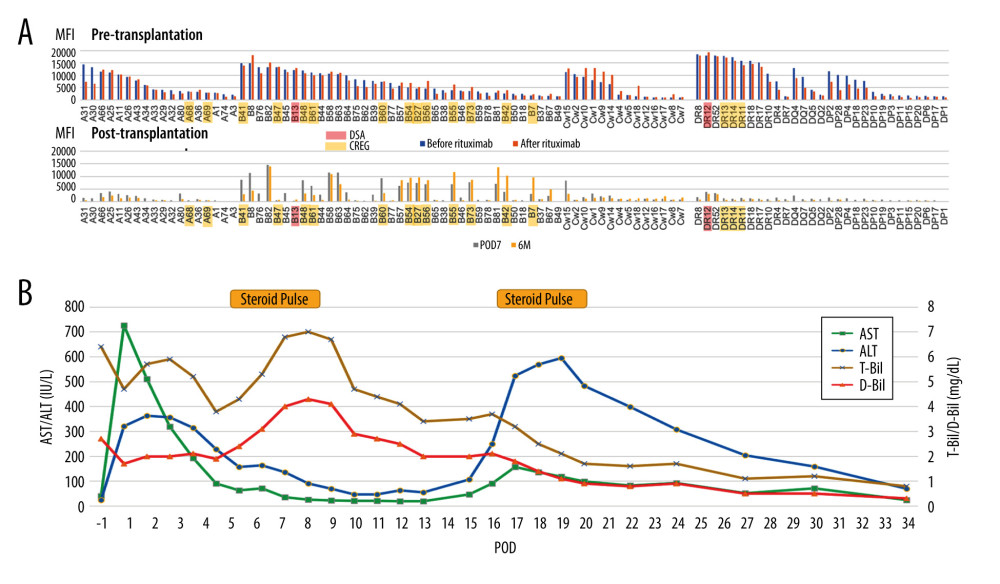 Figure 3. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 2 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Pre- and post-transplant anti-HLA antibodies. Pre-Transplantation; on admission (before rituximab administration) and just before liver transplantation (LT) (after rituximab administration). After transplantation; on postoperative day (POD) 7 and 6 months after LT. (B) Clinical course of case 2. Serum bilirubin levels increased from POD 5, but improved with steroid pulse therapy. Serum transaminases increase from POD 15, but improved with repeated steroid pulse therapy. DSA – donor-specific anti-human leukocyte antigen antibody; CREG – cross-reactive epitope group; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin.
Figure 3. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 2 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Pre- and post-transplant anti-HLA antibodies. Pre-Transplantation; on admission (before rituximab administration) and just before liver transplantation (LT) (after rituximab administration). After transplantation; on postoperative day (POD) 7 and 6 months after LT. (B) Clinical course of case 2. Serum bilirubin levels increased from POD 5, but improved with steroid pulse therapy. Serum transaminases increase from POD 15, but improved with repeated steroid pulse therapy. DSA – donor-specific anti-human leukocyte antigen antibody; CREG – cross-reactive epitope group; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin. 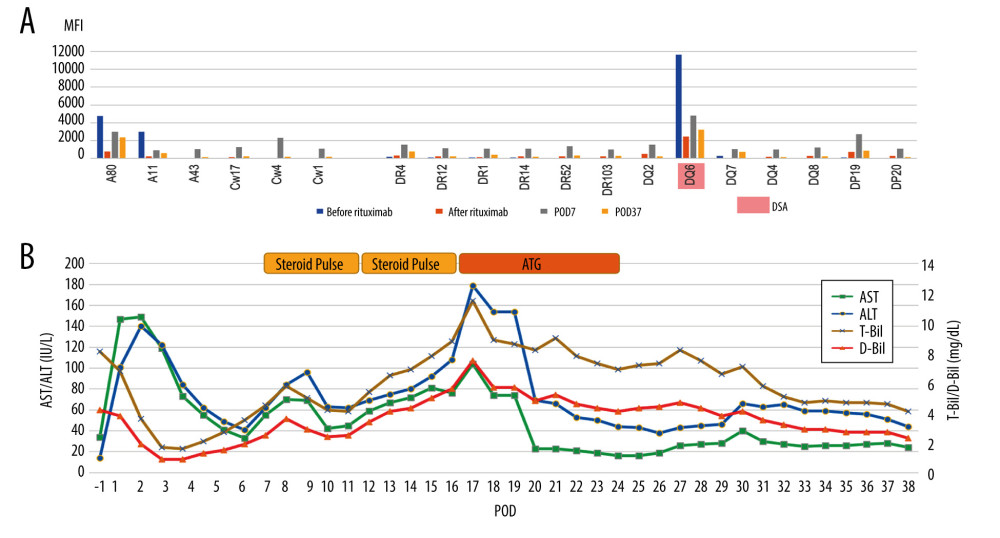 Figure 4. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 3 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Anti-HLA antibodies on admission (before rituximab administration), just before liver transplantation (LT) (after plasmapheresis), on postoperative day (POD) 7, and on POD 37. (B) Clinical course of case 3. Serum bilirubin and transaminase levels increase from POD 7. Although they improved after steroid pulse therapy, they were re-elevated during steroid tapering. They were not improved after repeated steroid pulse therapy, and antithymocyte globulin (ATG) was administered. They improved gradually after ATG administration; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin.
Figure 4. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 3 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Anti-HLA antibodies on admission (before rituximab administration), just before liver transplantation (LT) (after plasmapheresis), on postoperative day (POD) 7, and on POD 37. (B) Clinical course of case 3. Serum bilirubin and transaminase levels increase from POD 7. Although they improved after steroid pulse therapy, they were re-elevated during steroid tapering. They were not improved after repeated steroid pulse therapy, and antithymocyte globulin (ATG) was administered. They improved gradually after ATG administration; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin. Tables
Table 1. Characteristics of the 37 living donor liver transplant recipients since 2016.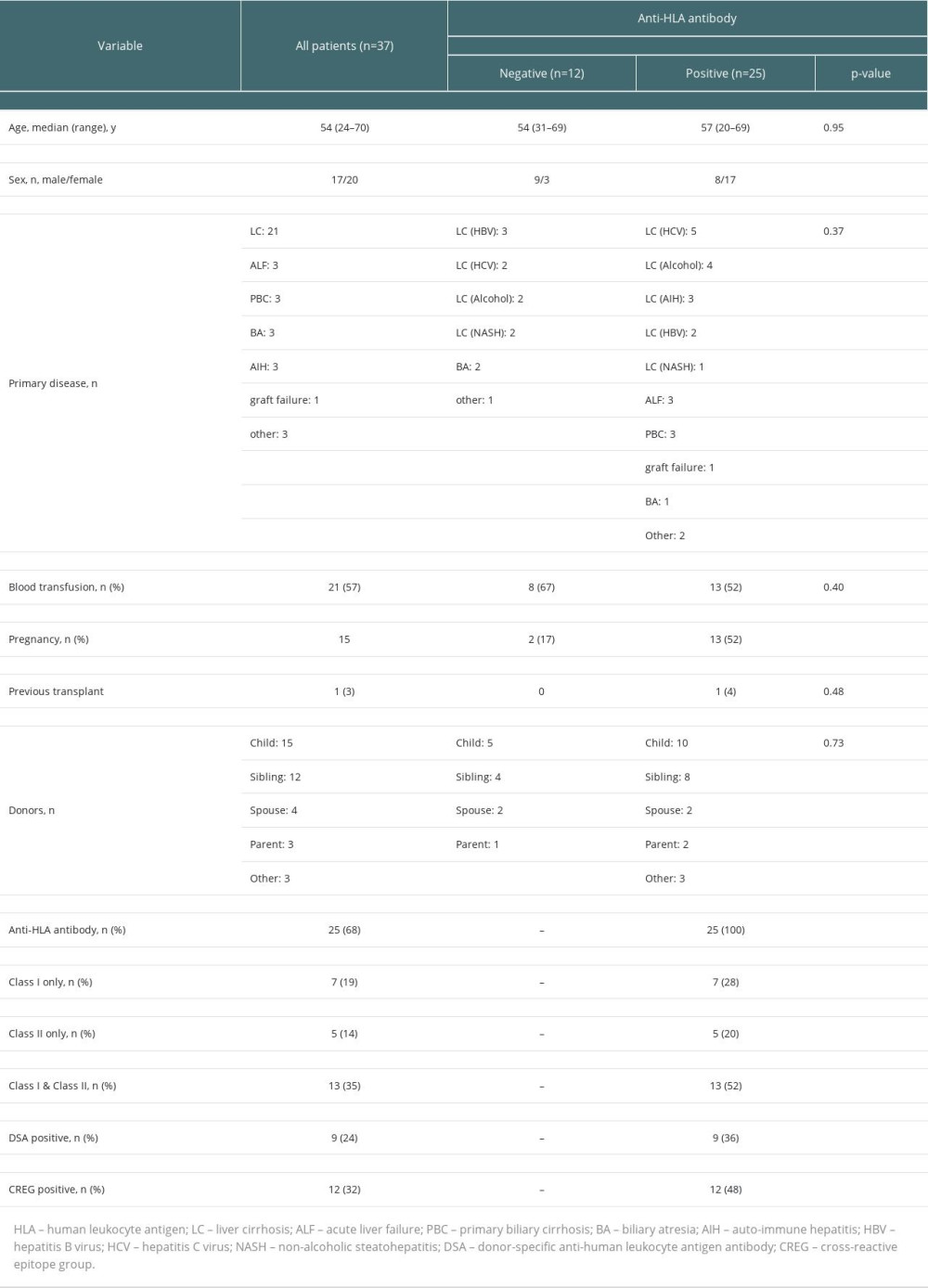 Table 2. Clinical features and outcomes of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients.
Table 2. Clinical features and outcomes of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients.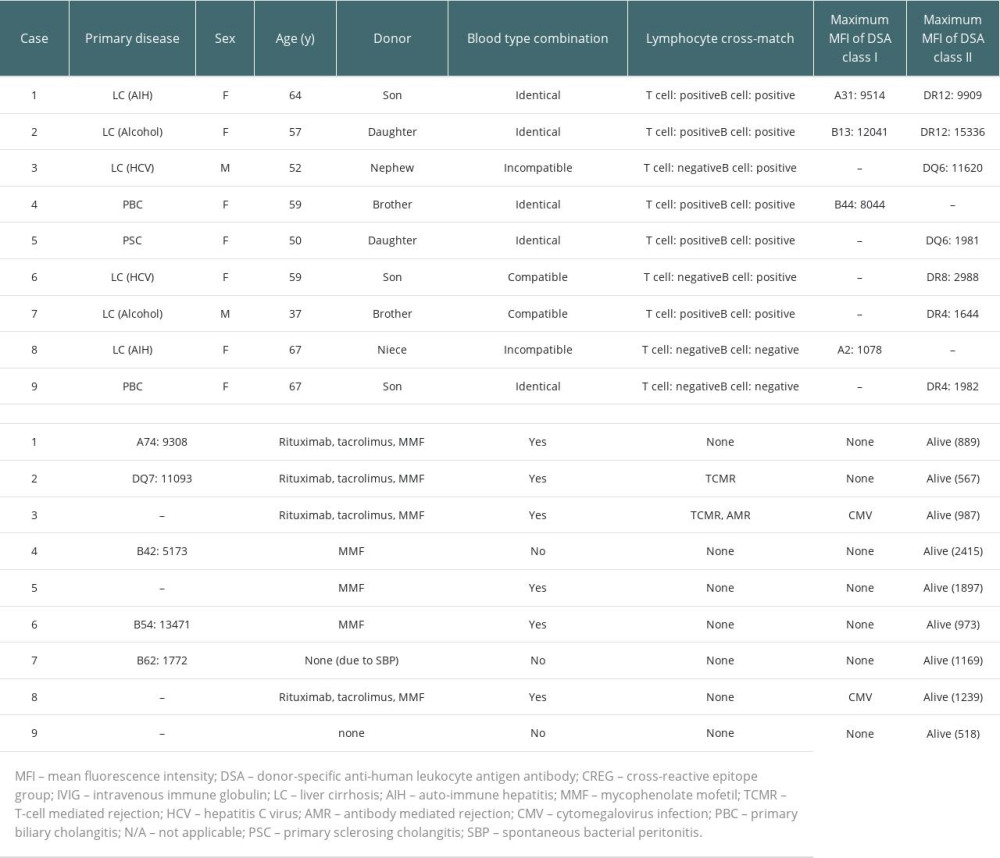 Table 3. Clinical features and outcomes of cross-reactive epitope group antibody (CREG)-alone-positive liver transplant recipients.
Table 3. Clinical features and outcomes of cross-reactive epitope group antibody (CREG)-alone-positive liver transplant recipients.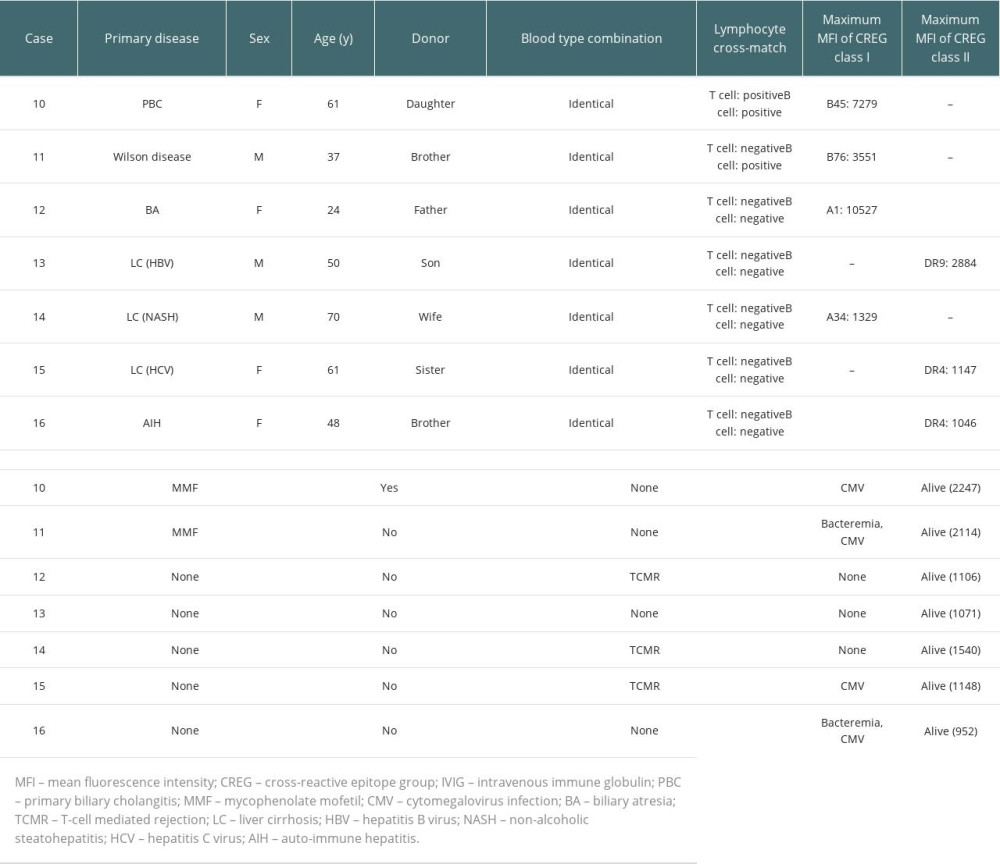 Table 4. Comparisons of clinical factors and outcomes among donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients.
Table 4. Comparisons of clinical factors and outcomes among donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients.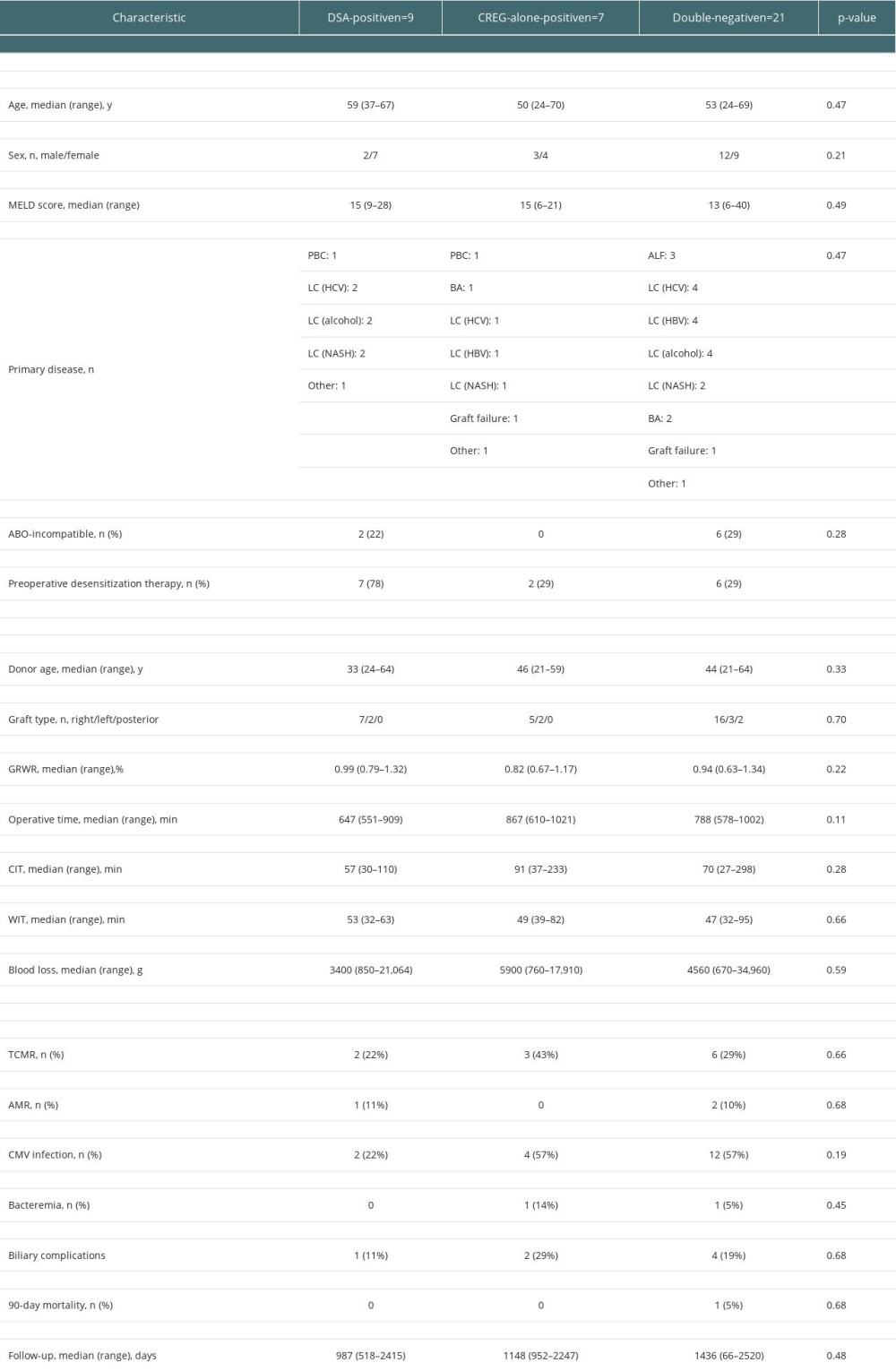 Table 5. Comparisons of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients by time period.
Table 5. Comparisons of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients by time period.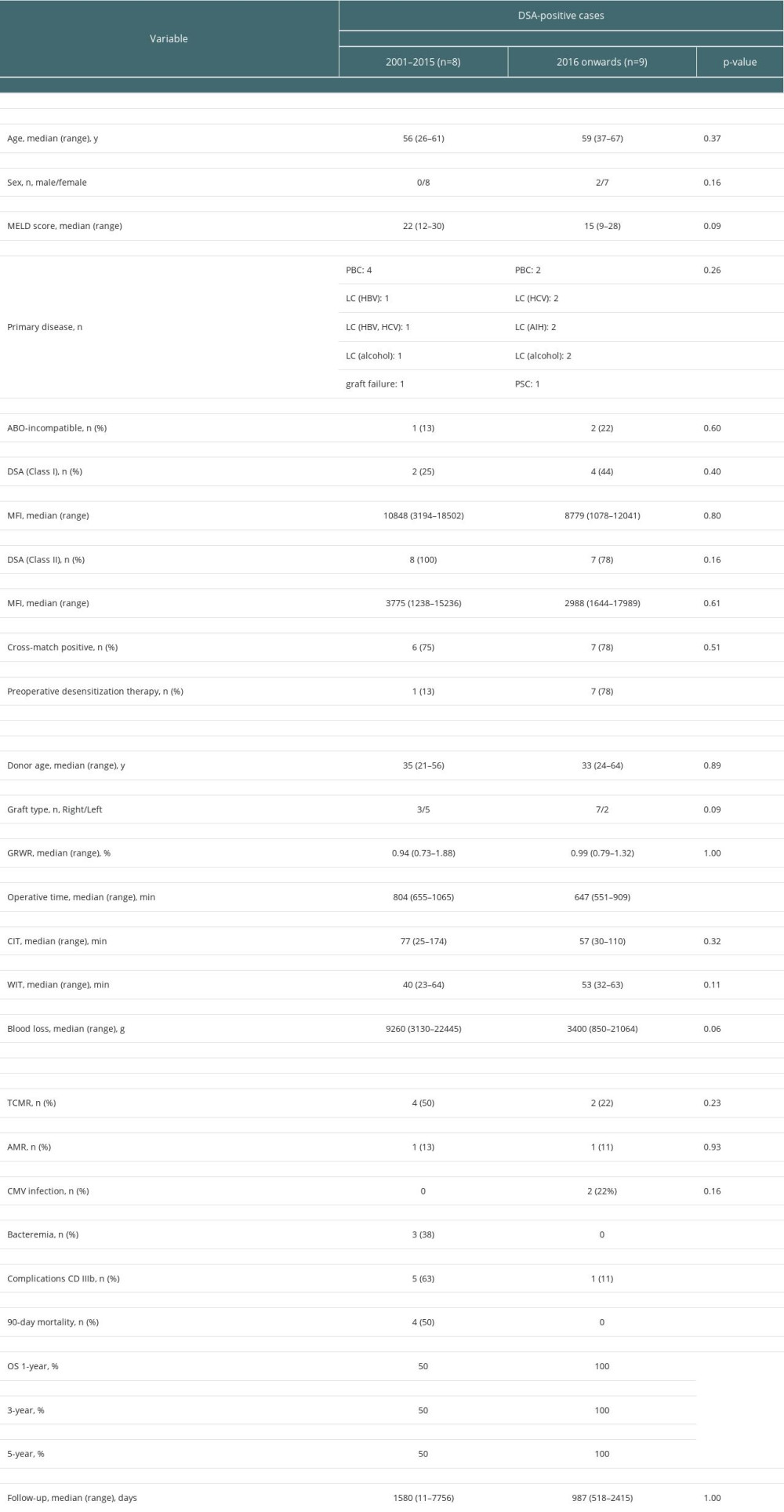
References
1. Colvin RB, Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis: J Am Soc Nephrol, 2007; 18(4); 1046-56
2. Gloor J, Cosio F, Lager DJ, Stegall MD, The spectrum of antibody-mediated renal allograft injury: Implications for treatment: Am J Transplant, 2008; 8(7); 1367-73
3. Jordan SC, Reinsmoen N, Peng A, Advances in diagnosing and managing antibody-mediated rejection: Pediatr Nephrol, 2010; 25(10); 2035-45 quiz 2045–48
4. Vo AA, Choi J, Cisneros K, Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients: Transplantation, 2014; 98(3); 312-19
5. Katz SM, Kimball PM, Ozaki C, Positive pretransplant crossmatches predict early graft loss in liver allograft recipients: Transplantation, 1994; 57(4); 616-20
6. Fujita S, Rosen C, Reed A, Langham MR, Significance of preformed anti-donor antibodies in liver transplantation: Transplantation, 1997; 63(1); 84-88
7. Kasahara M, Kiuchi T, Takakura K, Postoperative flow cytometry crossmatch in living donor liver transplantation: Clinical significance of humoral immunity in acute rejection: Transplantation, 1999; 67(4); 568-75
8. Doran TJ, Geczy AF, Painter D, A large, single center investigation of the immunogenetic factors affecting liver transplantation: Transplantation, 2000; 69(7); 1491-98
9. Evrard V, Otte JB, Sokal E, Impact of surgical and immunological parameters in pediatric liver transplantation: A multivariate analysis in 500 consecutive recipients of primary grafts: Ann Surg, 2004; 239(2); 272-80
10. Muro M, Marin L, Miras M, Liver recipients harbouring anti-donor preformed lymphocytotoxic antibodies exhibit a poor allograft survival at the first year after transplantation: Experience of one centre: Transpl Immunol, 2005; 14(2); 91-97
11. Ashihara E, Tsuji H, Sakashita H, Antidonor antibody in patients receiving ABO-identical and HLA-mismatched living donor liver transplants: Effect on survival: Transplantation, 2007; 83(4); 506-9
12. Kozlowski T, Rubinas T, Nickeleit V, Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies: Liver Transpl, 2011; 17(4); 357-68
13. Leonard GR, Shike H, Uemura T, Liver transplantation with a strongly positive crossmatch: case study and literature review: Liver Transpl, 2013; 19(9); 1001-10
14. Yoshizawa A, Egawa H, Yurugi K, Significance of semiquantitative assessment of preformed donor-specific antibody using luminex single bead assay in living related liver transplantation: Clin Dev Immunol, 2013; 2013; 972705
15. O’Leary JG, Kaneku H, Demetris AJ, Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss: Liver Transpl, 2014; 20(2); 218-27
16. McCaughan JA, Robertson V, Falconer SJ, Preformed donor-specific HLA antibodies are associated with increased risk of early mortality after liver transplantation: Clin Transplant, 2016; 30(12); 1538-44
17. Muro M, Moya-Quiles MR, Mrowiec A, Humoral response in liver allograft transplantation: A review of the role of anti-human leukocyte antigen (HLA) antibodies: Curr Protein Pept Sci, 2016; 17(8); 776-84
18. Legaz I, Boix F, Lopez M, Influence of preformed antibodies in liver transplantation: J Clin Med, 2020; 9(3); 708
19. Tamura K, Tohyama T, Watanabe J, Preformed donor-specific antibodies are associated with 90-day mortality in living-donor liver transplantation: Hepatol Res, 2019; 49(8); 929-41
20. Del Bello A, Neau-Cransac M, Lavayssiere L, Outcome of liver transplant patients with preformed donor-specific anti-human leukocyte antigen antibodies: Liver Transpl, 2020; 26(2); 256-67
21. Akamatsu N, Hasegawa K, Sakamoto S, Rituximab desensitization in liver transplant recipients with preformed donor-specific HLA antibodies: A Japanese Nationwide Survey: Transplant Direct, 2021; 7(8); e729
22. , Banff schema for grading liver allograft rejection: An international consensus document: Hepatology, 1997; 25(3); 658-63
23. Haga H, Egawa H, Shirase T, Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation: Liver Transpl, 2004; 10(1); 16-27
24. Demetris AJ, Bellamy C, Hubscher SG, 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of antibody-mediated rejection: Am J Transplant, 2016; 16(10); 2816-35
25. Del Bello A, Congy-Jolivet N, Danjoux M, Donor-specific antibodies and liver transplantation: Hum Immunol, 2016; 77(11); 1063-70
26. Kubal CA, Mangus RS, Saxena R, Crossmatch-positive liver transplantation in patients receiving thymoglobulin-rituximab induction: Transplantation, 2014; 97(1); 56-63
27. Schluckebier D, Cousin VL, Petit LM, Preformed and de novo DSA are associated with T-cell-mediated rejection in pediatric liver transplant recipients requiring clinically indicated liver biopsy: Pediatr Transplant, 2020; 24(1); e13611
28. Ikegami T, Taketomi A, Soejima Y, Rituximab, IVIG, and plasma exchange without graft local infusion treatment: A new protocol in ABO incompatible living donor liver transplantation: Transplantation, 2009; 88(3); 303-7
29. Kakuta Y, Satoh S, Watarai Y, Successful desensitization of T cell flow cytometry crossmatch positive renal transplant recipients using plasmapheresis and super high-dose intravenous immunoglobulin: Transplant Direct, 2018; 4(1); e336
30. Nainani N, Singh N, Shanahan T, Cross Reactive Epitope Group antibodies in sensitized kidneys transplant recipients was associated with early acute Antibody Mediated Rejection: Transpl Immunol, 2009; 20(3); 113-17
Figures
 Figure 1. Desensitization protocol for donor-specific anti-human leukocyte antigen antibody (DSA)-positive cases. MFI – mean fluorescence intensity; IVIG – intravenous immune globulin; MMF – mycophenolate mofetil; LT – liver transplantation (Microsoft, PowerPoint for Mac, ver. 16.48).
Figure 1. Desensitization protocol for donor-specific anti-human leukocyte antigen antibody (DSA)-positive cases. MFI – mean fluorescence intensity; IVIG – intravenous immune globulin; MMF – mycophenolate mofetil; LT – liver transplantation (Microsoft, PowerPoint for Mac, ver. 16.48). Figure 2. Graft survival in donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients (IBM, SPSS statistics, ver. 28.0.1.0). The 1-year graft survival rate is 100% in both DSA-positive and CREG-alone-positive cases, and 87.5% in double-negative cases (p=0.289). DSA, donor-specific anti-human leukocyte antigen antibody; CREG, cross-reactive epitope group; LTx, liver transplantation.
Figure 2. Graft survival in donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients (IBM, SPSS statistics, ver. 28.0.1.0). The 1-year graft survival rate is 100% in both DSA-positive and CREG-alone-positive cases, and 87.5% in double-negative cases (p=0.289). DSA, donor-specific anti-human leukocyte antigen antibody; CREG, cross-reactive epitope group; LTx, liver transplantation. Figure 3. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 2 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Pre- and post-transplant anti-HLA antibodies. Pre-Transplantation; on admission (before rituximab administration) and just before liver transplantation (LT) (after rituximab administration). After transplantation; on postoperative day (POD) 7 and 6 months after LT. (B) Clinical course of case 2. Serum bilirubin levels increased from POD 5, but improved with steroid pulse therapy. Serum transaminases increase from POD 15, but improved with repeated steroid pulse therapy. DSA – donor-specific anti-human leukocyte antigen antibody; CREG – cross-reactive epitope group; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin.
Figure 3. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 2 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Pre- and post-transplant anti-HLA antibodies. Pre-Transplantation; on admission (before rituximab administration) and just before liver transplantation (LT) (after rituximab administration). After transplantation; on postoperative day (POD) 7 and 6 months after LT. (B) Clinical course of case 2. Serum bilirubin levels increased from POD 5, but improved with steroid pulse therapy. Serum transaminases increase from POD 15, but improved with repeated steroid pulse therapy. DSA – donor-specific anti-human leukocyte antigen antibody; CREG – cross-reactive epitope group; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin. Figure 4. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 3 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Anti-HLA antibodies on admission (before rituximab administration), just before liver transplantation (LT) (after plasmapheresis), on postoperative day (POD) 7, and on POD 37. (B) Clinical course of case 3. Serum bilirubin and transaminase levels increase from POD 7. Although they improved after steroid pulse therapy, they were re-elevated during steroid tapering. They were not improved after repeated steroid pulse therapy, and antithymocyte globulin (ATG) was administered. They improved gradually after ATG administration; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin.
Figure 4. Changes in mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA) antibody and the clinical course of case 3 (Microsoft, PowerPoint for Mac, ver. 16.48). (A) Anti-HLA antibodies on admission (before rituximab administration), just before liver transplantation (LT) (after plasmapheresis), on postoperative day (POD) 7, and on POD 37. (B) Clinical course of case 3. Serum bilirubin and transaminase levels increase from POD 7. Although they improved after steroid pulse therapy, they were re-elevated during steroid tapering. They were not improved after repeated steroid pulse therapy, and antithymocyte globulin (ATG) was administered. They improved gradually after ATG administration; AST – aspartate transaminase; ALT – alanine transaminase; T-Bil – total bilirubin, D-Bil – direct bilirubin. Tables
 Table 1. Characteristics of the 37 living donor liver transplant recipients since 2016.
Table 1. Characteristics of the 37 living donor liver transplant recipients since 2016. Table 2. Clinical features and outcomes of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients.
Table 2. Clinical features and outcomes of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients. Table 3. Clinical features and outcomes of cross-reactive epitope group antibody (CREG)-alone-positive liver transplant recipients.
Table 3. Clinical features and outcomes of cross-reactive epitope group antibody (CREG)-alone-positive liver transplant recipients. Table 4. Comparisons of clinical factors and outcomes among donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients.
Table 4. Comparisons of clinical factors and outcomes among donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients. Table 5. Comparisons of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients by time period.
Table 5. Comparisons of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients by time period. Table 1. Characteristics of the 37 living donor liver transplant recipients since 2016.
Table 1. Characteristics of the 37 living donor liver transplant recipients since 2016. Table 2. Clinical features and outcomes of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients.
Table 2. Clinical features and outcomes of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients. Table 3. Clinical features and outcomes of cross-reactive epitope group antibody (CREG)-alone-positive liver transplant recipients.
Table 3. Clinical features and outcomes of cross-reactive epitope group antibody (CREG)-alone-positive liver transplant recipients. Table 4. Comparisons of clinical factors and outcomes among donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients.
Table 4. Comparisons of clinical factors and outcomes among donor-specific anti-human leukocyte antigen antibody (DSA)-positive, cross-reactive epitope group antibody (CREG)-alone-positive, and double-negative liver transplant recipients. Table 5. Comparisons of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients by time period.
Table 5. Comparisons of donor-specific anti-human leukocyte antigen antibody (DSA)-positive liver transplant recipients by time period. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








