07 November 2023: Case Report
Intrahepatic Venous Collateral Circulation and Reverse Blood Flow After Main Hepatic Vein Obstruction: A Case Report with Literature Review
Jianghong Ning1DE, Yibulayin Aini1BF, Tiemin Jiang1AG*, Yingmei Shao12A, Tuerganaili Aji12AD, Hao Wen12CDDOI: 10.12659/AOT.941521
Ann Transplant 2023; 28:e941521
Abstract
BACKGROUND: Alveolar echinococcosis, a lethal parasitic disease, can invade important vessels in the liver. A liver vascular anomaly causes compensatory changes in other blood vessels connected to it because of the close relationship between them. Obstruction of the retrohepatic inferior vena cava and the second hilum can form the intrahepatic venous network and the vertebral venous plexus pathway, which can be demonstrated by hepatic venography and anatomical and autopsy studies.
CASE REPORT: A Tibetan woman, age 31, with hepatic alveolar echinococcosis and unique intrahepatic hemodynamic features, was referred to our center and underwent successful ex vivo liver resection and autotransplantation. We report our experience and review the literature. In this clinical case, we performed an ex vivo liver resection and autotransplantation without hepatic inferior vena cava reconstruction. After surgery, the circulatory system hemodynamic remained stable, and blood flow in the liver and trunk was unhindered. The patient underwent an uneventful hospitalization and recovery.
CONCLUSIONS: This clinical case demonstrates the unique venous access, hemodynamic alterations, and surgical decision-making that follow the invasion of significant hepatic vessels by alveolar echinococcosis lesions. HAE exhibits unique collateral vessels, which are uncommon in other diseases. Additionally, this kind of therapy offers fresh perspectives for the surgical treatment of end-stage HAE.
Keywords: Collateral Circulation, Hemodynamics, alveolar echinococcosis
Background
In endemic regions, alveolar echinococcosis (AE), a fatal zoonotic disease that causes severe organ damage when infected with the larval form of
Case Report
A 31-year-old Tibetan woman with liver disease identified during a health examination 1 year ago was admitted to our department from an AE-endemic area. Upon physical examination, the middle and upper abdomen were painful without any noticeable rebound soreness. Routine examinations of blood, urine, and stool, liver and kidney function, coagulation profiles, and tumor markers including alpha fetoprotein (AFP), and cancer antigen 19-9 were all normal. Additionally, the patient showed no signs of ascites, edema in the lower limbs, or portal venous hypertension. Computed tomography (CT) revealed a massive AE lesion in the left lobe and caudate lobe of the liver, which invaded the entire middle hepatic vein (MHV), left hepatic vein (LHV), and RHIVC, upward and surrounded the right hepatic vein (RHV), and there was compensatory proliferation of the right hepatic lobe (Figure 1A–1C). IVC angiography revealed RHIVC occlusion, unrestricted blood flow beneath the level of the renal vein, and compensatory vertebral venous plexus opening; intrahepatic hemodynamics were changed, RHV and short hepatic veins (SHV) were considerably thickened, and the IVC pressure was 18.5 cmH2O (Figure 2A, 2B). A 15-min indocyanine green (ICG) clearance test showed a 7% retention rate. Imaging showed the lesion had already invaded the RHIVC, and the dilated SHV and RHV formed various transportation networks. Because the RHV orifice was also invaded, intrahepatic blood had circulated within the liver through these networks before returning to the infrahepatic vena cava through the SHV and flowing up to the right atrium via the vertebral venous plexus.
After preoperative evaluation including multi-disciplinary treatment (MDT), the left hemihepatectomy was performed first. However, in vivo resection is extremely challenging and risky, so ex vivo liver resection and autotransplantation (ELRA) may be performed according to intraoperative conditions. In both, surgeons must resect the RHIVC, and predetermine not to reconstruct it. Allogeneic liver transplantation is also feasible, but it had considerable limitations for this case, such as donor shortage and the fact that postoperative immunological agents increase the recurrence rate of AE. Ultimately, the patient received ELRA without IVC reconstruction.
First, intraoperative ultrasound reconfirmed the flow rate of the reserved hepatic vein with 11.1cm/s and the portal vein with 13.4 cm/s, which suggested intrahepatic venous hypertension and poor hepatic venous outflow. Bleeding of the liver wound was more serious during the liver dissection, and venous invasion and circumstances in vivo resection are more challenging than preoperative evaluation. Thus, ELRA was performed according to the alternative scheme. After dissecting and removing the liver from the body, the temporary porta-caval shunt was performed with an artificial vessel between the end of the infrahepatic vena cava and the portal vein (PV) (Figure 2C), and there were no subsequent hemodynamic changes in the circulatory system. The suprahepatic vena cava was left aside; therefore, the IVC was not rebuilt.
The RHV orifice was repaired after the RHIVC was dissected along the lesion. The hepatic parenchyma was separated along the right side of the MHV, and the vessels were ligated off along the way. Then, dilated collateral vessels were seen on the wound of the right lobe, and 1125 g donor liver repair was completed using the hyperplastic right hepatic lobe.
The donor liver was placed into the body and the IVC was not reconstructed. The RHV was anastomosed to the infradiaphragmatic IVC end-to-end (Figure 2D), and the anhepatic phase was terminated (lasting 7 h 30 min) by opening the PV after anastomosing the main PV with the main autologous PV. The hepatic artery and the bile duct were anastomosed. Intraoperative ultrasound showed hepatic artery flow rate of 19 cm/s, PV flow rate of 21 cm/s, and hepatic vein flow rate of 15 cm/s, which were normal. The patient was observed in Intensive Care Unit (ICU) for 4 days after surgery, and then was taken to the general ward. Mild biliary leakage and right pleural effusion were found, and both were improved after conservative treatment. The drainage tubes in the operative area were all removed, and the patient was discharged on the 14th day after surgery, with no abnormality found in CT examination before discharge (Figure 3A, 3B). The patient had been taking Albendazole orally to prevent AE recurrence after discharge, and the most recent 6-month follow-up data indicated that the patient was still alive without significant postoperative problems or recurrence (Figure 3C, 3D).
Discussion
Hepatic AE is a rare parasitic disease caused by the larval stage of
In our case, the gradual obstruction of the RHIVC and the second hilum stimulated development of intrahepatic collaterals between the RHV and SHV. Preoperative venography revealed that the contrast medium in the IVC flowed into the right liver lobe through the SHV, then flowed out through the other SHV to the infrahepatic vena cava after circling in the liver via intrahepatic collaterals, appearing as a reverse outflow different from the typical upward flow (Figure 4A, 4B). ELRA without IVC reconstruction was performed in accordance with the vascular compensation and blood flow features mentioned above. After surgery, the circulatory system hemodynamic remained stable, and blood flow in the liver and trunk was unhindered.
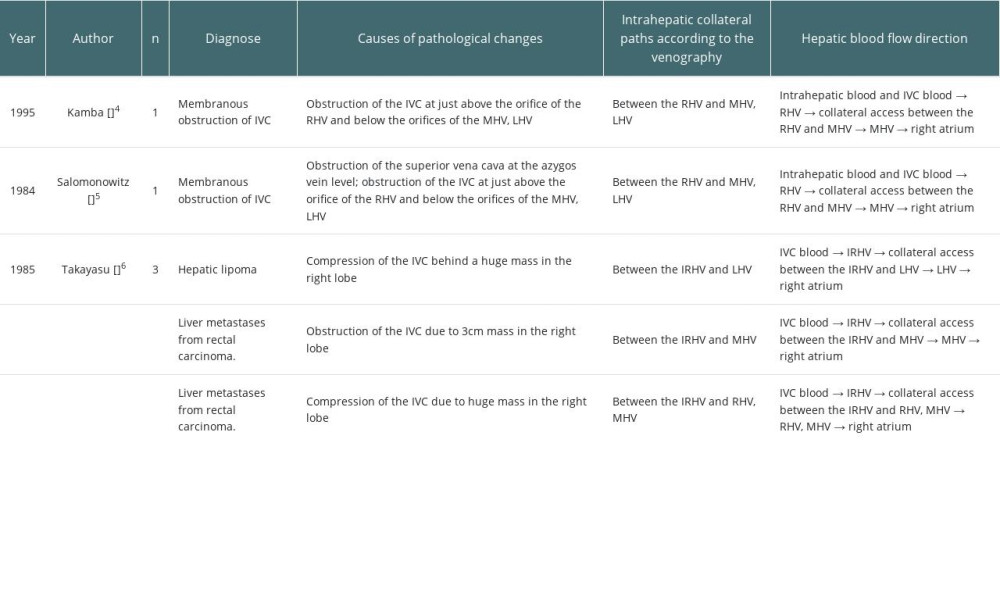
According to previous reports, the above-mentioned intrahepatic collaterals are common in liver tumor invasion, thrombosis, and membranous occlusion of IVC. A literature review (nonsystematic) was performed using PubMed and Google Scholar for papers published in English up to 2022. A total of 3 articles were identified, in which 5 cases were reported [4–6] (Table 1). There were no reports of hepatic AE, and all cases were membranous obstruction of the IVC and neoplastic disease. Two of them were membranous occlusion of IVC, the obstructions were located between the orifice of RHV and MHV-LHV, and there were horizontal collateral pathways formed from the RHV to the MHV-LHV. The outflow direction of intrahepatic blood in these 5 patients was consistent with the typical upward blood flow, and no there were no reverse hemodynamic characteristics, similar to our case. Ascites, lower-limb edema, and other symptoms were absent in all of them, highlighting the crucial function of the collateral vessels. Based on the characteristics of hepatic venous obstruction in prior cases and our case, the conditions for the formation of the intrahepatic venous collaterals appear to be: (a) at least 1 main hepatic vein outlet is unobstructed, and (b) the obstruction of the IVC is located above the confluence of the IVC and the SHV.
It has been reported that ELRA can treat end-stage hepatic AE without IVC reconstruction. Xianwei Yang et al [7,8] reported on their experience with the procedure: 1 patient died of circulatory failure due to multi-organ combined resection and excessive blood loss; 1 had anastomotic stenosis between the hepatic vein and suprahepatic IVC, and an intravenous stent was implanted after surgery; the others had no vessel-related complications. They suggested that if the stenosis is lighter and shorter, this complicated operation is not necessary and local resection with reconstruction of IVC is sufficient. Even if there is adequate collateral circulation, the IVC should be reconstructed when RHIVC resection is combined with multi-organ resection because the operation is lengthy and causes a large volume of blood loss.
In our patient, the remnant liver had unique hemodynamic properties and underwent unusual surgical procedures. Because of the unique collateral pathways, the patient did not exhibit Budd-Chiari syndrome symptoms. Only a small number of similar cases have been reported so far, and those with hepatic AE are particularly rare. Hepatic venography is crucial for identifying and evaluating the condition of the IVC and hepatic vein in hepatic AE. Moreover, it is clear that intrahepatic pathological collaterals play a significant role in maintaining systemic hemodynamic stability in certain situations. To achieve blood return, care should be taken to protect these collateral pathways during the procedure, maintain their integrity, and make them a normal pathway following surgery.
Conclusions
This case demonstrates the unique venous access, hemodynamic alterations, and surgical decision-making that follow the invasion of important hepatic vessels by alveolar echinococcosis lesions. HAE exhibits unique collateral vessels, which are uncommon in other diseases. Additionally, this kind of therapy offers fresh perspectives for the surgical treatment of end-stage HAE.
Figures
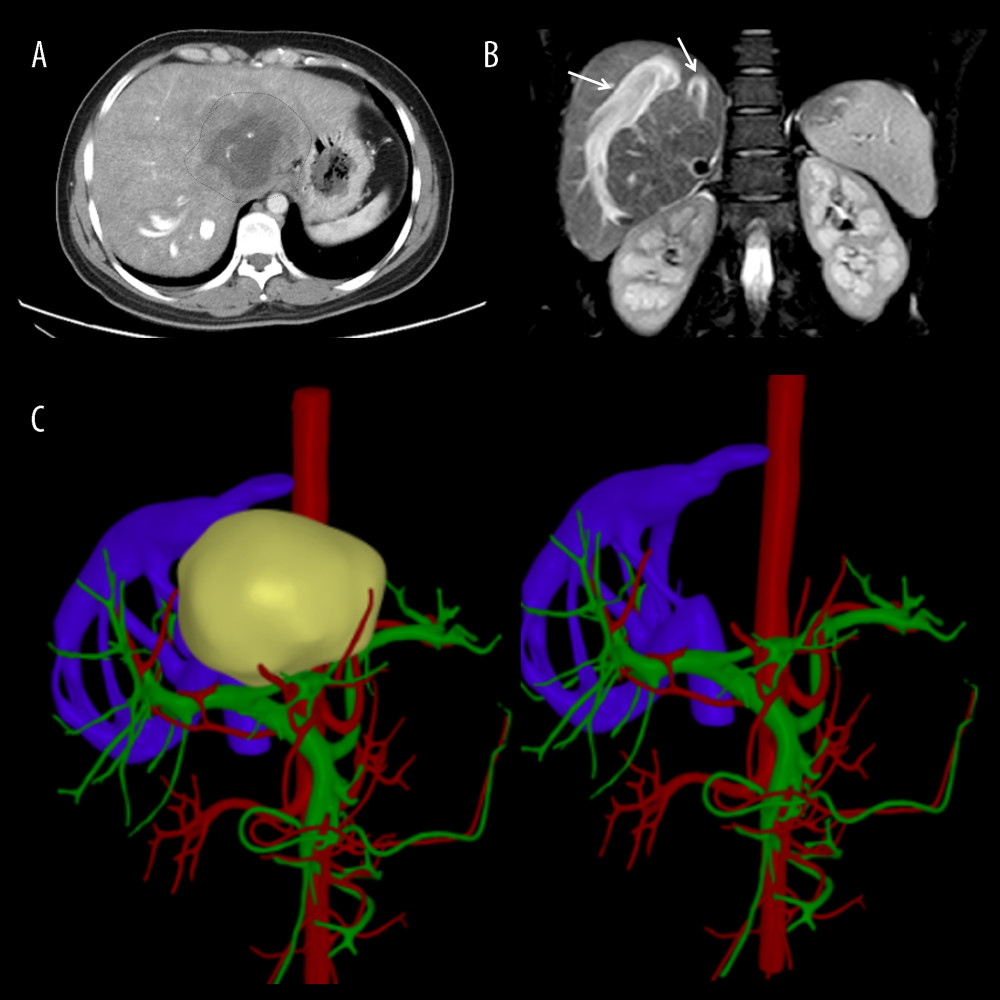 Figure 1. Representative perioperative images(A) Abdominal CT showed an AE lesion and mild hepatic congestion in the right lobe of the liver. (B) Abdominal MRI demonstrated dilation of RHV and SHV (arrow). (C) A three-dimensional model of liver was established before surgery, RHIVC was completely invaded and the RHV and SHV expanded and thickened to form multiple collateral circulation. Figures 1A and 1B were created using Adobe Photoshop 2022 V23.5. Figure 1C was created using 3D Studio Max 2020 from Autodesk.
Figure 1. Representative perioperative images(A) Abdominal CT showed an AE lesion and mild hepatic congestion in the right lobe of the liver. (B) Abdominal MRI demonstrated dilation of RHV and SHV (arrow). (C) A three-dimensional model of liver was established before surgery, RHIVC was completely invaded and the RHV and SHV expanded and thickened to form multiple collateral circulation. Figures 1A and 1B were created using Adobe Photoshop 2022 V23.5. Figure 1C was created using 3D Studio Max 2020 from Autodesk. 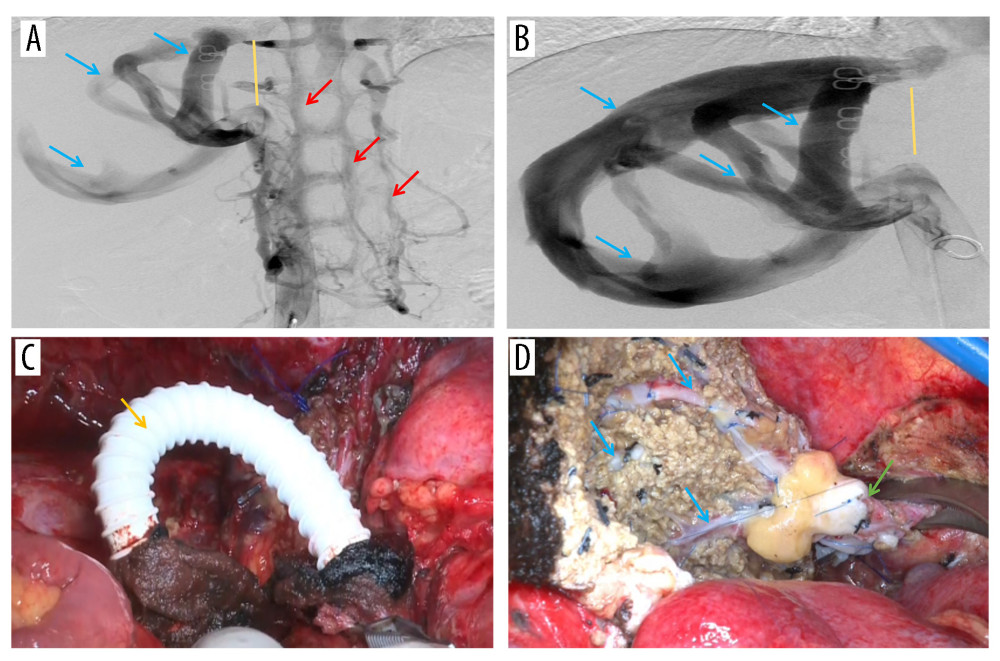 Figure 2. Representative perioperative and intraoperative images(A, B) IVC angiography showed invaded IVC (yellow line), dilated RHV and SHV (blue arrow), and formed vertebral venous plexus (red arrow). (C) The IVC was not reconstructed and temporary portacaval shunt was performed (yellow arrow). (D) Dilated collateral veins (blue arrow) were seen on the wound of the right lobe of liver and the suprahepatic vena cava anastomosed with the RHV (green arrow). Figures 2A–2D were created using Adobe Photoshop 2022 V23.5.
Figure 2. Representative perioperative and intraoperative images(A, B) IVC angiography showed invaded IVC (yellow line), dilated RHV and SHV (blue arrow), and formed vertebral venous plexus (red arrow). (C) The IVC was not reconstructed and temporary portacaval shunt was performed (yellow arrow). (D) Dilated collateral veins (blue arrow) were seen on the wound of the right lobe of liver and the suprahepatic vena cava anastomosed with the RHV (green arrow). Figures 2A–2D were created using Adobe Photoshop 2022 V23.5. 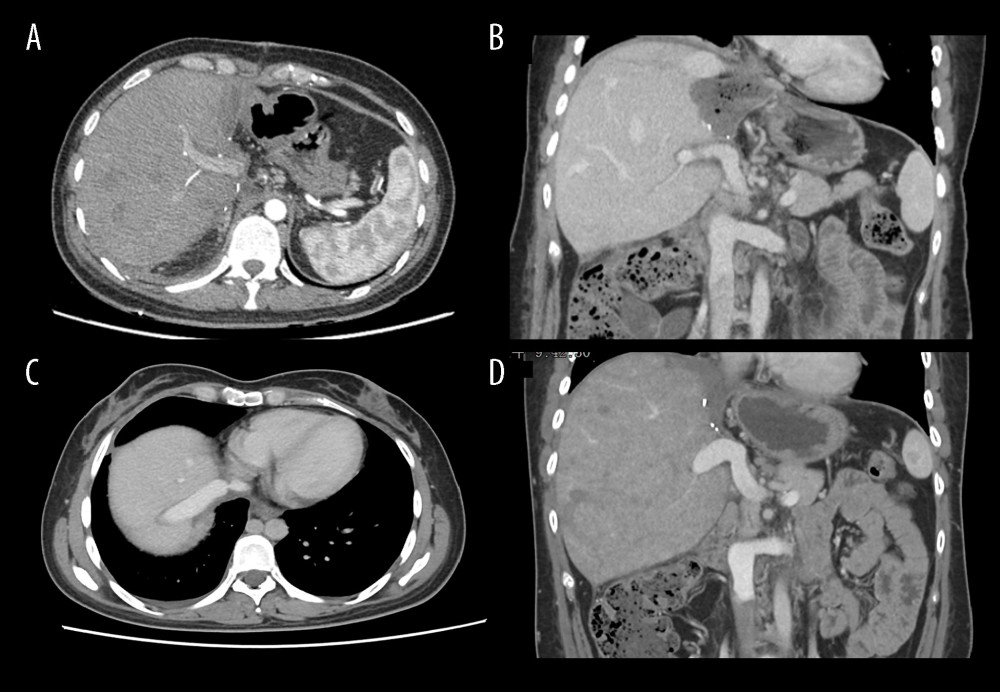 Figure 3. Abdominal CT before and 6 months after discharge(A, B) Abdominal CT before discharge showed that all vessels were unobstructed. (C, D) Follow-up abdominal CT 6 months after discharge showed no liver abnormalities, and hepatic inflow and outflow channels were unobstructed. Figures 3A–3D were created using Procreate V5.3.5 from Savage Interactive Pty, Ltd.
Figure 3. Abdominal CT before and 6 months after discharge(A, B) Abdominal CT before discharge showed that all vessels were unobstructed. (C, D) Follow-up abdominal CT 6 months after discharge showed no liver abnormalities, and hepatic inflow and outflow channels were unobstructed. Figures 3A–3D were created using Procreate V5.3.5 from Savage Interactive Pty, Ltd. 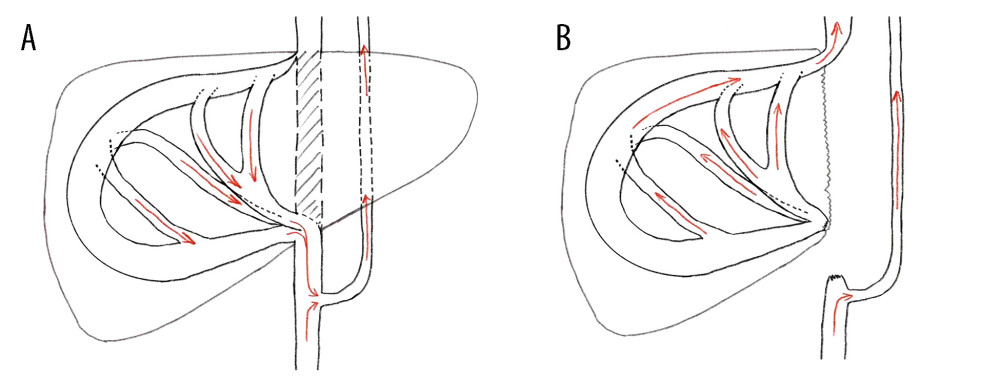 Figure 4. Characteristics of intrahepatic blood flow in the healthy side before and after operation(A) Before surgery, the outlet of RHV was also invaded and almost occluded, and the intrahepatic blood flowed back into the infrahepatic vena cava through the SHV after circling through the hepatic vein network in the right liver, and then flowed back to the right atrium through the vertebral venous plexus. (B) The SHV, RHV, and PV were well connected, so the SHV was sutured and closed. Postoperative blood flow was infrahepatic, and intrahepatic blood was flowed flowing directly into the right atrium through the paravertebral venous plexus. Intrahepatic blood was flowing out of the liver through the reconstructed RHV and flowing into the right atrium through the suprahepatic vena cava. Figures 4A and 4B were created using Adobe Photoshop 2022 V23.5.
Figure 4. Characteristics of intrahepatic blood flow in the healthy side before and after operation(A) Before surgery, the outlet of RHV was also invaded and almost occluded, and the intrahepatic blood flowed back into the infrahepatic vena cava through the SHV after circling through the hepatic vein network in the right liver, and then flowed back to the right atrium through the vertebral venous plexus. (B) The SHV, RHV, and PV were well connected, so the SHV was sutured and closed. Postoperative blood flow was infrahepatic, and intrahepatic blood was flowed flowing directly into the right atrium through the paravertebral venous plexus. Intrahepatic blood was flowing out of the liver through the reconstructed RHV and flowing into the right atrium through the suprahepatic vena cava. Figures 4A and 4B were created using Adobe Photoshop 2022 V23.5. References
1. Deplazes P, Rinaldi L, Alvarez Rojas CA, Global distribution of alveolar and cystic echinococcosis: Adv Parasitol, 2017; 95; 315-493
2. Wen H, Vuitton L, Tuxun T, Echinococcosis: Advances in the 21st Century: Clin Microbiol Rev, 2019; 32(2); e00075-18
3. Cho KJ, Geisinger KR, Shields JJ, Forrest ME, Collateral channels and histopathology in hepatic vein occlusion: Am J Roentgenol, 1982; 139(4); 703-9
4. Kamba M, Ochi S, Ochi H, Asymptomatic membranous obstruction of the inferior vena cava forming intrahepatic collateral pathways: J Gastroenterol, 1995; 30(6); 783-85
5. Salomonowitz E, Castaneda-Zuniga WR, Bass JL, Transhepatic collateral pathway due to vena caval obstruction: Am J Roentgenol, 1984; 142(6); 1210-12
6. Takayasu K, Moriyama N, Muramatsu Y, Intrahepatic venous collaterals forming via the inferior right hepatic vein in 3 patients with obstruction of the inferior vena cava: Radiology, 1985; 154(2); 323-28
7. Du Q, Wang Y, Zhang M, Author Correction: A new treatment strategy for end-stage hepatic alveolar echinococcosis: IVC resection without reconstruction: Sci Rep, 2020; 10(1); 17125
8. Yang X, Wang T, Kong J, Resection of retrohepatic inferior vena cava without reconstruction in ex vivo liver resection and autotransplantation: A retrospective study: BMC Surgery, 2020; 20(1); 56
Figures
 Figure 1. Representative perioperative images(A) Abdominal CT showed an AE lesion and mild hepatic congestion in the right lobe of the liver. (B) Abdominal MRI demonstrated dilation of RHV and SHV (arrow). (C) A three-dimensional model of liver was established before surgery, RHIVC was completely invaded and the RHV and SHV expanded and thickened to form multiple collateral circulation. Figures 1A and 1B were created using Adobe Photoshop 2022 V23.5. Figure 1C was created using 3D Studio Max 2020 from Autodesk.
Figure 1. Representative perioperative images(A) Abdominal CT showed an AE lesion and mild hepatic congestion in the right lobe of the liver. (B) Abdominal MRI demonstrated dilation of RHV and SHV (arrow). (C) A three-dimensional model of liver was established before surgery, RHIVC was completely invaded and the RHV and SHV expanded and thickened to form multiple collateral circulation. Figures 1A and 1B were created using Adobe Photoshop 2022 V23.5. Figure 1C was created using 3D Studio Max 2020 from Autodesk. Figure 2. Representative perioperative and intraoperative images(A, B) IVC angiography showed invaded IVC (yellow line), dilated RHV and SHV (blue arrow), and formed vertebral venous plexus (red arrow). (C) The IVC was not reconstructed and temporary portacaval shunt was performed (yellow arrow). (D) Dilated collateral veins (blue arrow) were seen on the wound of the right lobe of liver and the suprahepatic vena cava anastomosed with the RHV (green arrow). Figures 2A–2D were created using Adobe Photoshop 2022 V23.5.
Figure 2. Representative perioperative and intraoperative images(A, B) IVC angiography showed invaded IVC (yellow line), dilated RHV and SHV (blue arrow), and formed vertebral venous plexus (red arrow). (C) The IVC was not reconstructed and temporary portacaval shunt was performed (yellow arrow). (D) Dilated collateral veins (blue arrow) were seen on the wound of the right lobe of liver and the suprahepatic vena cava anastomosed with the RHV (green arrow). Figures 2A–2D were created using Adobe Photoshop 2022 V23.5. Figure 3. Abdominal CT before and 6 months after discharge(A, B) Abdominal CT before discharge showed that all vessels were unobstructed. (C, D) Follow-up abdominal CT 6 months after discharge showed no liver abnormalities, and hepatic inflow and outflow channels were unobstructed. Figures 3A–3D were created using Procreate V5.3.5 from Savage Interactive Pty, Ltd.
Figure 3. Abdominal CT before and 6 months after discharge(A, B) Abdominal CT before discharge showed that all vessels were unobstructed. (C, D) Follow-up abdominal CT 6 months after discharge showed no liver abnormalities, and hepatic inflow and outflow channels were unobstructed. Figures 3A–3D were created using Procreate V5.3.5 from Savage Interactive Pty, Ltd. Figure 4. Characteristics of intrahepatic blood flow in the healthy side before and after operation(A) Before surgery, the outlet of RHV was also invaded and almost occluded, and the intrahepatic blood flowed back into the infrahepatic vena cava through the SHV after circling through the hepatic vein network in the right liver, and then flowed back to the right atrium through the vertebral venous plexus. (B) The SHV, RHV, and PV were well connected, so the SHV was sutured and closed. Postoperative blood flow was infrahepatic, and intrahepatic blood was flowed flowing directly into the right atrium through the paravertebral venous plexus. Intrahepatic blood was flowing out of the liver through the reconstructed RHV and flowing into the right atrium through the suprahepatic vena cava. Figures 4A and 4B were created using Adobe Photoshop 2022 V23.5.
Figure 4. Characteristics of intrahepatic blood flow in the healthy side before and after operation(A) Before surgery, the outlet of RHV was also invaded and almost occluded, and the intrahepatic blood flowed back into the infrahepatic vena cava through the SHV after circling through the hepatic vein network in the right liver, and then flowed back to the right atrium through the vertebral venous plexus. (B) The SHV, RHV, and PV were well connected, so the SHV was sutured and closed. Postoperative blood flow was infrahepatic, and intrahepatic blood was flowed flowing directly into the right atrium through the paravertebral venous plexus. Intrahepatic blood was flowing out of the liver through the reconstructed RHV and flowing into the right atrium through the suprahepatic vena cava. Figures 4A and 4B were created using Adobe Photoshop 2022 V23.5. In Press
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
03 Apr 2024 : Review article
Alternative Therapies in Transplantology as a Promising Perspective in MedicineAnn Transplant In Press; DOI: 10.12659/AOT.943387
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860