27 February 2024: Case Report
Successful Sequential Liver and Isolated Intestine Transplantation for Mitochondrial Neurogastrointestinal Encephalopathy Syndrome: A Case Report
Chandrashekhar A. KubalDOI: 10.12659/AOT.941881
Ann Transplant 2024; 29:e941881
Abstract
BACKGROUND: Mitochondrial neurogastrointestinal encephalopathy syndrome (MNGIE) is an autosomal recessive disease caused by thymidine phosphorylase deficiency leading to progressive gastrointestinal dysmotility, cachexia, ptosis, ophthalmoparesis, peripheral neuropathy and leukoencephalopathy. Although liver transplantation corrects thymidine phosphorylase deficiency, intestinal deficiency of the enzyme persists. Retrospective chart review was carried out to obtain clinical, biochemical, and pathological details.
CASE REPORT: We present a case of liver and subsequent intestine transplant in a 28-year-old man with MNGIE syndrome with gastrointestinal dysmotility, inability to walk, leukoencephalopathy, ptosis, cachexia, and elevated serum thymidine. To halt progression of neurologic deficit, he first received a left-lobe partial liver transplantation. Although his motor deficit improved, gastrointestinal dysmotility persisted, requiring total parenteral nutrition. After exhaustive intestinal rehabilitation, he was listed for intestine transplantation. Two-and-half years after liver transplantation, he received an intestine transplant. At 4 years after LT and 20 months after the intestine transplant, he remains off parenteral nutrition and is slowly gaining weight.
CONCLUSIONS: This is the first reported case of mitochondrial neurogastrointestinal encephalomyopathy to undergo successful sequential liver and intestine transplantation.
Keywords: Liver Transplantation, Parenteral Nutrition, Total, Thymidine Phosphorylase, Transplantation, Visceral Myopathy Familial External Ophthalmoplegia
Background
MNGIE is a rare autosomal recessive disease that primarily affects the gastrointestinal (GI) and nervous systems; it is caused by mutations in the TYMP gene which encodes thymidine phosphorylase (TP) [1]. Morbidity involves progressive GI dysmotility leading to cachexia, ptosis, opthalmoplegia, leukoencephalopathy, and peripheral neuropathy. [2] Gastrointestinal dysmotility is usually the most debilitating and life-limiting aspect of MNGIE. The prognosis of MNGIE is remains poor and inevitably fatal, with an average life expectancy of 37.5 years [3]. Gastrointestinal dysmotility is one of the most common features of MNGIE, eventually leading to malnutrition and cachexia [3,4]. Intestinal smooth muscle dysfunction caused by mitochondrial defects, and enteric nervous system dysfunction lead to GI pathogenesis [5]. As the disease progresses, the gastrointestinal symptoms worsen, with patients dying from severe malnutrition and gastrointestinal complications [6]. Unfortunately, affected patients often experience diagnostic delay. Allogeneic haemopoietic stem cell transplantation (HSCT) has been tried, with significant morbidity and mortality [7]. Liver transplantation (LT) has been successful and is considered to be safer than HSCT [8]. The first case of LT for MNGIE was reported in 2016 from the University of Bologna, Italy [9]. Since then, 7 cases of LT of MNGIE have been reported, 3 cases from Italy [8] and the other 4 cases from the United States [10]. LT immediately corrects TP levels, halting the progression or even improvement of some of the clinical features, and is associated with lower mortality compared to HSCT. Recently, advances have been made in the field of hematopoietic gene therapy for MNGIE, but initial studies suggest that gene therapy could be effective in the future [10]. Thus, for now, clinical application of these strategies remains experimental. Improvement in clinical features depends on cellular damage already incurred. This brings up the role of intestine transplantation [IT] in cases with damage to the GI system. IT in MNGIE has not been previously described and in conjunction with LT has potential to improve quality of life and prolong survival. Here, we describe the first case of MNGIE with sequential liver and intestine transplantation in a patient with significant intestinal damage.
Case Report
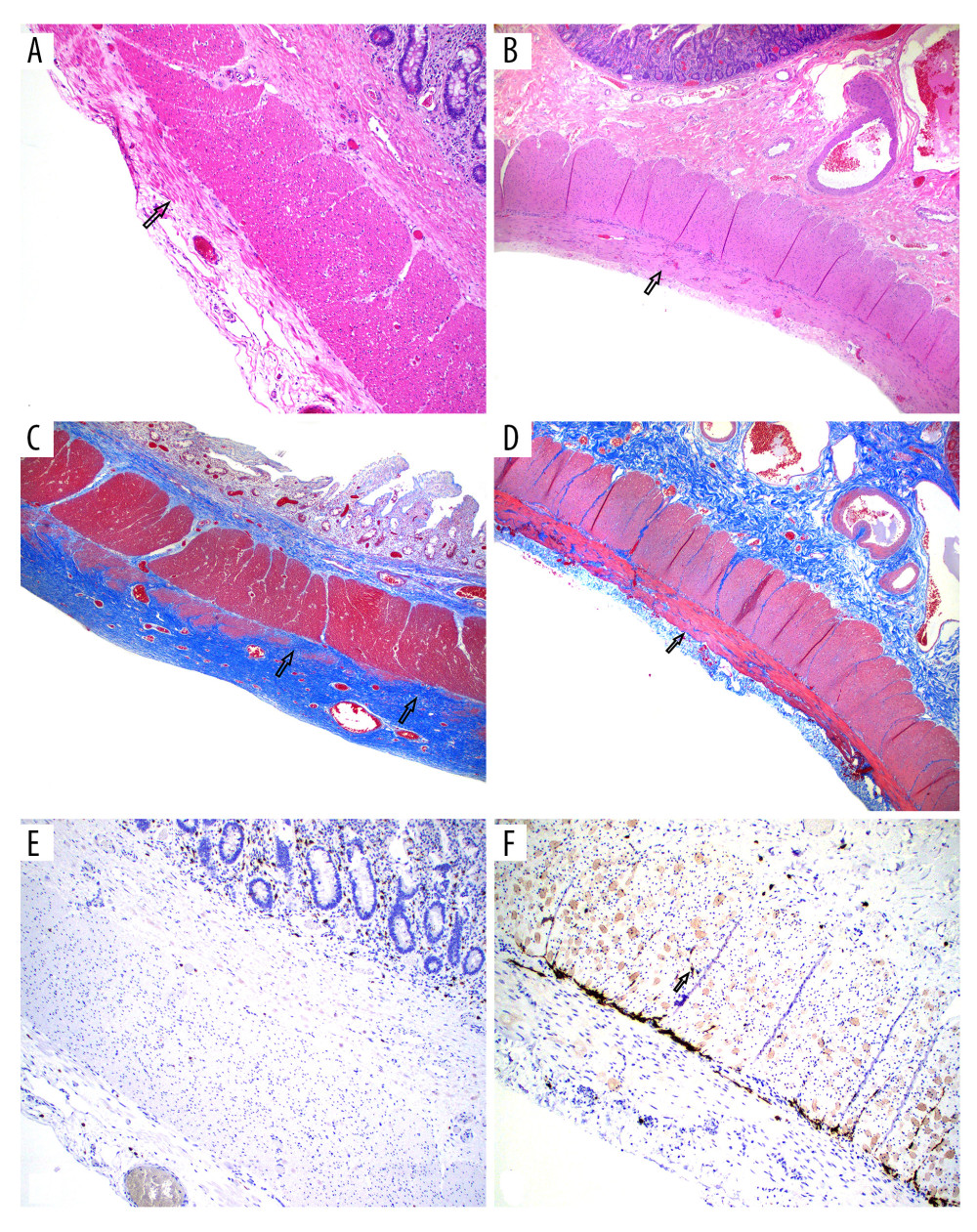
A 28-year-old man with neurologic symptoms, elevated serum thymidine, and biallelic missense pathogenic variants in
Discussion
In this case report we demonstrate the utility of IT in restoration of intestinal function and nutritional independence. It has been shown that HSCT or LT, if performed late in the course of disease, may not restore GI function. The Milan Group reported no improvement in GI symptoms after LT [8]. Therefore, liver–intestine or MVT can potentially address the pathogenesis of MNGIE and restore GI function. Halter et al showed the mortality rate after HSCT reached to 62% [7]. LT has been shown to have lasting benefit in this condition. Since the GI tract is also significantly affected in this syndrome, most patients remain nutritionally challenged. In fact, many patients receiving HSCT for this condition had significant morbidity and mortality related to GI complications [12]. Furthermore, histologic studies have shown that in patients receiving HSCT, gut tissue changes do not revert to normal. Thus, in patients with significant GI damage, IT is a reasonable therapeutic option, especially when immunosuppression is already given for LT. Furthermore, in patients with significant GI dysfunction requiring parenteral nutritional support at presentation, a liver–intestine transplant/multivisceral transplant (MVT) may be an even more appropriate option, as the liver allograft provides immunoprotection to the intestine graft from the same donor [11].
Intestinal transplantation is performed in select centers in the world and majority in the U.S. [13] In the U.S. providing access to a deceased donor liver remains a challenge in patients without liver disease except for certain approved conditions. The current deceased donor liver allocation policy provides no feasible pathway for transplantation for MNGIE, whether LT or MVT. While living donor liver transplantation is an attractive option, living donor IT is uncommon, and the parents of an affected individual are obligate heterozygotes. Within organ allocation system, a pathway for exceptional cases such as this exists, however for our patient, an appeal for additional MELD points to access liver allograft was unsuccessful. For this reason, we performed a left-lobe graft where right lobe graft was allocated to an index patient with higher MELD score. One of the reasons for the denial of our MELD exception request was lack of evidence that LT benefits patients with MNGIE. We believe that awareness of therapies for MNGIE is important as early referrals are crucial for successful management of these patients.
Conclusions
Liver–intestine transplantation can be considered for late referrals of MNGIE presenting with irreversible damage to small intestine requiring parenteral nutrition.
References
1. Filosto M, Piccinelli SC, Caria F, Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE-MTDPS1): J Clin Med, 2018; 7(11); 389
2. Yadak R, Breur M, Bugiani M, Gastrointestinal dysmotility in MNGIE: From thymidine phosphorylase enzyme deficiency to altered interstitial cells of Cajal: Orphanet J Rare Dis, 2019; 14(1); 33
3. Nishino I, Spinazzola A, Papadimitriou A, Mitochondrial neurogastrointestinal encephalomyopathy: An autosomal recessive disorder due to thymidine phosphorylase mutations: Ann Neurol, 2000; 47(6); 792-800
4. Garone C, Tadesse S, Hirano M, Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy: Brain, 2011; 134(Pt 11); 3326-32
5. Verma A, Piccoli DA, Bonilla E, A novel mitochondrial G8313A mutation associated with prominent initial gastrointestinal symptoms and progressive encephaloneuropathy: Pediatr Res, 1997; 42(4); 448-54
6. Pacitti D, Levene M, Garone C, Mitochondrial neurogastrointestinal encephalomyopathy: into the fourth decade, what we have learned so far: Front Genet, 2018; 9; 669
7. Halter JP, Michael W, Schüpbach M, Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy: Brain, 2015; 138(Pt 10); 2847-58
8. D’Angelo R, Boschetti E, Amore G, Liver transplantation in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Clinical long-term follow-up and pathogenic implications: J Neurol, 2020; 267(12); 3702-10
9. De Giorgio R, Poironi L, Rinaldi R, Liver transplantation for mitochondrial neurogastrointestinal encephalomyopathy: Ann Neurol, 2016; 80(3); 448-55
10. Kripps KA, Nakayuenyongsuk W, Shayota BJ, Successful liver transplantation in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Mol Genet Metab, 2020; 30(1); 58-64
11. Selvaggi G, Gaynor JJ, Moon J, Analysis of acute cellular rejection episodes in recipients of primary intestinal transplantation: A single center, 11-year experience: Am J Transplant, 2007; 7(5); 1249-57
12. Zaidman I, Elhasid R, Gefen A, Hematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalopathy: A single-center experience underscoring the multiple factors involved in the prognosis: Pediatr Blood Cancer, 2021; 68(5); e28926
13. Grant D, Abu-Elmagd K, Mazariegos G, Intestinal transplant registry report: Global activity and trends: Am J Transplant, 2015; 15(1); 210-19
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860