16 April 2024: Case Report
Treatment of Cavernous Transformation of Portal Vein Caused by Hepatic Cystic Echinococcosis Using Ex Vivo Liver Resection and Autotransplantation
Ayidu Reyimu1ABCDEF, Jiang Tiemin23ABCDEF, Shao Yingmei23ABCDEF, Zhu Xuepeng1ABCDEF, Guo Wenjiang12ABCDEF, Du Yuhao1ABCDE, Dilixiati Halimulati1ABC, Wusiman Ababokeli1AB, Aji Tuerganaili23AB*, Wen Hao23ABDOI: 10.12659/AOT.942358
Ann Transplant 2024; 29:e942358
Abstract
BACKGROUND: Hepatic cystic echinococcosis (HCE) is a frequently overlooked parasitic liver disease, for which the commonly recommended treatment is radical resection. However, this approach is often associated with severe comorbidities such as HBV/HCV, cirrhosis, and hepatic carcinoma, among others.
CASE REPORT: In this report, we present a case successfully managed by ex vivo liver resection and autologous liver transplantation (ELRA). In the described case, ex vivo resection was not feasible due to recurrent lesions and infections invading the portal vein, which resulted in portal vein cavernous transformation.
CONCLUSIONS: Through this paper, we aim to detail the treatment process, showcasing the feasibility and advantages of ELRA. Additionally, we propose a novel approach for the treatment of this disease, while emphasizing the importance of radical resection surgery to prevent long-term complications.
Keywords: Portal Vein, cavernous transformation of portal vein
Introduction
Hepatic cystic echinococcosis (HCE) is a frequently overlooked parasitic liver disease, typically presenting without clear clinical symptoms. It is often only detected when complications arise, with instances of portal vein cavernous transformation due to portal vein invasion being exceedingly rare [1,2]. The presence of multiple tortuous vessels and increased hilar region pressure following portal vein trunk occlusion can lead to uncontrollable bleeding during surgery. This scenario is widely regarded as high risk and exceptionally challenging [3–5]. As a result, management primarily involves interventional or endoscopic treatment for complications, such as upper-gastrointestinal bleeding. However, ex vivo liver resection and autologous liver transplantation (ELRA) offers the benefit of controlled intraoperative bleeding, as the liver is resected and revascularized in the ex vivo state [6]. We present a case successfully treated through ELRA, where in vivo resection was not possible due to recurrent lesion with infection invading the portal vein, leading to portal vein cavernous transformation. The patient eventually achieved lesion annihilation and portal vein reconstruction, demonstrating improved outcomes.
Case Report
SURGERY PLANNING:
The patient, previously diagnosed with hepatic cystic echinococcosis (HCE), was found to have a new lesion infiltrating the portal venous and hepatic arterial systems. This was particularly evident in the grade II branches of the portal vein, which exhibited multiple tortuous vessels in the first hilar region. A radical resection of the lesion and complex revascularization were necessitated. However, in vivo liver resection posed an extremely high risk of bleeding, rendering it challenging to execute. Consequently, the decision was made to proceed with ex vivo liver resection and autotransplantation (ELRA).
EXPLORATION AND LESION VISUALIZATION:
The patient underwent a Mercedes-Benz incision. The intraoperative examination revealed significant intra-abdominal adhesions, absence of ascites, and a calcified, 10×10-cm porta hepatis with tight adhesions. Varices were observed in the hepatic hilum. The caudate lobe of the liver was palpable, presenting as a solid, 7×7-cm mass that was firm and adhered to the surrounding tissues. Chronic inflammatory changes were noted, likely due to recurrent infection and calcification within the cystic cavity of the worm. The adhesions were systematically released, revealing the caudate lobe of the liver and hepatic hilum, with focal infiltration consistent with preoperative imaging. The liver exhibited extensive inflammatory adhesions with surrounding tissues, with the lower edge severely adhered to the duodenum. An internal fistula was observed between the residual cavity of the HCE and the duodenum, which was intraoperatively repaired. A portion of the infectious lesion in the right hepatic apex protruded into the diaphragm, potentially causing recurrent thoracic pustulosis in the past.
LIVER ISOLATION AND ESTABLISHMENT OF TEMPORARY VASCULAR DIVERSION:
The supra-hepatic and retro-hepatic vena cava were separately isolated. Following this, the porta hepatis was meticulously isolated, revealing multiple tortuous and dilated vessels within the portal vein. The proper hepatic artery and the common bile duct were successfully isolated without complications. However, the main trunk of the portal vein, along with its left and right branches, exhibited eroded lesions that could not be resolved. Once the normal portal vein trunk was identified at the superior margin of the pancreas, the hepatic artery, portal vein, and inferior vena cava were independently blocked. This allowed for complete resection of the liver. Subsequently, the inferior vena cava was replaced with an artificial vessel and the portal vein was anastomosed with this synthetic vessel. This created a portal-inferior vena cava blood flow to temporarily restore circulation. Simultaneously, the resected liver was promptly perfused in HTK fluid at a temperature range of 0–4°C on a sterile table.
EX VIVO LIVER RESECTION AND RE-VASCULARIZATION:
Following sufficient perfusion, the diseased liver was resected in an ex vivo state. The lesion was meticulously removed using CUSA, and the communicating venules of the left and right liver were ligated. The left hepatic artery was identified along the proper hepatic artery, extending upwards. The lesion and tortuous vessels at the first porta hepatica were excised, ensuring the protection of the hepatic artery, which exposed the normal portal vein leading into the liver. Due to the insufficient length of the portal vein, we decided to reconstruct it using an artificial vessel. The root of the left hepatic vein had been severely invaded, necessitating excision of the hepatic vein. The patient’s hepatic round ligament was suitably trimmed to restore the integrity of the hepatic vein, as observed intraoperatively. The posterior inferior hepatic vena cava was also severely invaded. Considering the artificial vessel’s more suitable length and caliber, it was decided to replace the inferior hepatic vena cava with an artificial vessel after resection. Finally, a Roux-en-Y hepatojejunostomy was performed to drain the left hepatobiliary duct.
IMPLANTATION OF THE LIVER: The liver, after being trimmed (extrahepatic left, 690 g), was implanted. This involved end-to-side anastomosis of the left hepatic vein to the artificial inferior vena cava, end-to-end anastomosis of the left hepatic artery to the proper hepatic artery, and bridging of the portal vein with an artificial vessel (as illustrated in Figure 2). After blood flow was re-established, an ultrasound examination was conducted and showed all vessels within the normal range of blood flow. The operation lasted a total of 20 hours, with an anhepatic period of 7 hours and 34 minutes. During the surgery, a total of 1000 ml of blood was transfused, which included 4 units of red blood cell suspension, 400 ml of autologous blood, and 1050 ml of devirulent plasma.
POSTOPERATIVE MANAGEMENT: Postoperative care included routine administration of anti-inflammatory and hepatoprotective medications, appropriate analgesics, rehydration, and symptomatic treatment. To prevent thrombosis, bridging anticoagulation with low-molecular-weight heparin (rivaroxaban) was administered. The patient’s liver function test (LFT) showed gradual recovery, and she was successfully discharged 12 days after surgery without any liver insufficiency or vascular and biliary-related complications. One month after discharge, the patient was followed up. The size and quality of the residual liver and vascular status were assessed using liver function tests, serology, ultrasound, and CT scans. No recurrence or metastasis was observed. The patient was scheduled for follow-up appointments every 3–6 months after discharge to assess any changes in their condition. A CT scan conducted during the 10-month follow-up revealed no vascular complications. Both the portal vein and the artificial vessel were flowing smoothly without obstructions. No peripheral varicose vessels were observed (Figure 4). Pathology results indicated hepatic cystic encystment. Within the lesion, there was evidence of microbiliary hyperplasia and inflammatory cell infiltration in a large area of necrotic tissue.
Discussion
Hepatic echinococcosis is a somewhat overlooked parasitic disease that is prevalent in agricultural and pastoral regions, with cystic echinococcosis being the most common form [7,8]. The primary treatment approach is radical surgery, during which the capsule of the liver hydatid is stripped. However, if this procedure is not performed initially, there is a high risk of recurrence and complications such as biliary leakage, bleeding, and, in fewer cases, long-term complications like biliary obstruction and vascular occlusion. This case report describes a recurrence after surgery, combined with a residual cavity infection and chronic inflammation that invaded the portal vein trunk, leading to portal vein cavernous degeneration. This condition is typically a secondary effect of portal vein thrombosis. It is extremely rare for portal vein cavernous degeneration to occur due to inclusion disease [9,10]. Consequently, there are few references regarding previous reports of ex vivo liver resection and autotransplantation for the treatment of hepatic cystic echinococcosis. This is the first report of such a technique being used for this disease’s treatment. Treating this condition is very challenging due to the many tortuous, high-pressure vessels in the hilar region of the portal vein, which can lead to uncontrollable intraoperative hemorrhage. Currently, the treatment for portal vein cavernous degeneration is limited to managing the symptoms of complications such as gastrointestinal bleeding [11], but this approach does not address the root cause of the condition.
ELRA is a surgical procedure designed to treat liver lesions or injuries that cannot be addressed in vivo. This process involves excision of the liver, complete resection of the lesion, and reconstruction of critical vessels under cryogenic conditions in vitro, before the liver is transplanted back into the cavity [12,13]. ELRA has successfully overcome the time constraints of liver ischemia, the technical limitations of in vivo vascular reconstruction, and the risk of uncontrollable bleeding, making it particularly advantageous in cases of severe intrahepatic vessel invasion that cannot be resected in vivo [14,15]. For the patient in question, the lesion had invaded the bifurcation of the portal vein in segments II and III, and there were multiple tortuous vessels surrounding the hilar region. The surgery required not only a radical resection of the lesion but also a reconstruction of the portal vein [16]. Performing this resection in vivo could potentially lead to uncontrollable bleeding, prolonged hepatic thermal ischemia time, and difficulties in safely isolating the left hepatic artery due to multiple constraints. Consequently, we determined that ELRA may be the safest and most effective approach.
The emergence of such a complex complication in this instance is directly attributable to the incomplete resection of the lesion years prior. Despite the low incidence of similar long-term complications, they pose severe consequences for the patient and could be circumvented with meticulous intraoperative management. Hence, we underscore the importance of the initial procedure.
Conclusions
In this case, we successfully performed resection of the lesion, repair of defective vessels, and reconstruction of the portal vein ex vivo, utilizing the technique of ex vivo hepatectomy and autologous liver transplantation. This approach facilitated both the radical removal of the lesion and the treatment of the portal vein’s cavernous degeneration. Our experience suggests that ex vivo liver resection coupled with autologous liver transplantation is a safe and feasible treatment for such patients, offering significant advantages in controlling the risk of bleeding and thermal ischemia time. Furthermore, we underscore the criticality of the initial operation for HCE in preventing similar long-term complications.
Figures
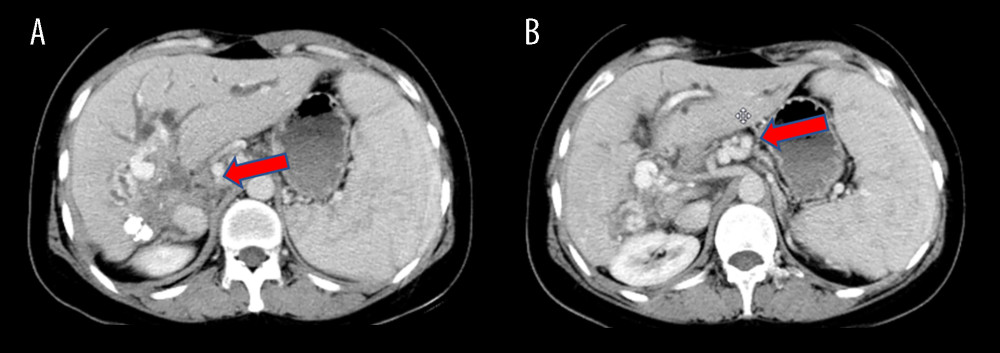 Figure 1. Preoperative CT scans of the patient(A) The lesion, situated in the hilar region, exhibits severe invasion into the portal vein, bile duct, and hepatic artery. (B) Portal vein occlusion has resulted in cavernous degeneration of the portal vein. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe).
Figure 1. Preoperative CT scans of the patient(A) The lesion, situated in the hilar region, exhibits severe invasion into the portal vein, bile duct, and hepatic artery. (B) Portal vein occlusion has resulted in cavernous degeneration of the portal vein. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe). 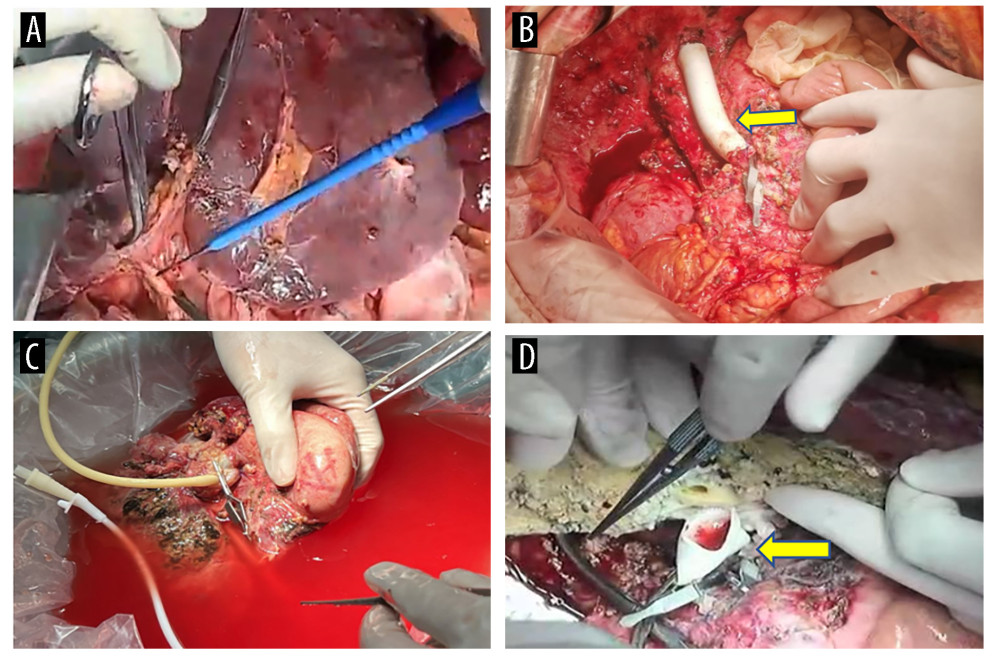 Figure 2. Surgical process(A) The liver prior to isolation. (B) A temporary venous diversion of the portal-hepatic vein was established post liver isolation. (C) The liver was perfused and revised in an ex vivo state. (D) The portal vein was reconstructed with artificial vessels. The arrow indicates the portal vein anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk).
Figure 2. Surgical process(A) The liver prior to isolation. (B) A temporary venous diversion of the portal-hepatic vein was established post liver isolation. (C) The liver was perfused and revised in an ex vivo state. (D) The portal vein was reconstructed with artificial vessels. The arrow indicates the portal vein anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk). 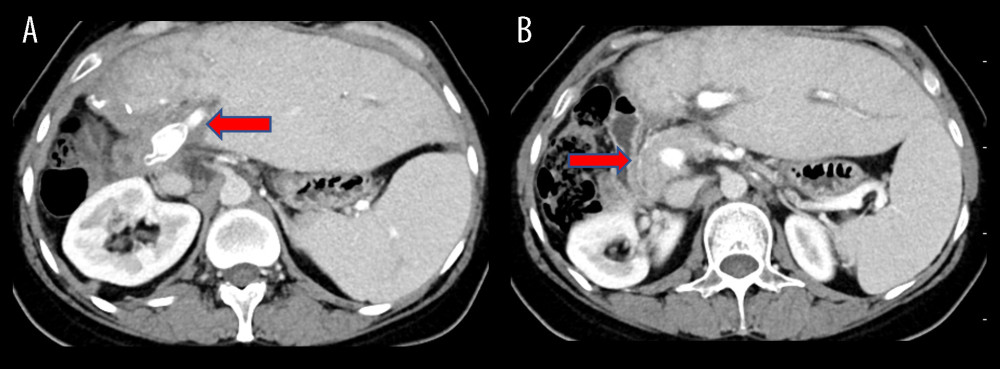 Figure 3. Surgical process(A) Completion of portal vein anastomosis. The arrow indicates post-portal vein anastomosis. (B) Surgery conclusion following hepatic duct-jejunum anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk).
Figure 3. Surgical process(A) Completion of portal vein anastomosis. The arrow indicates post-portal vein anastomosis. (B) Surgery conclusion following hepatic duct-jejunum anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk). 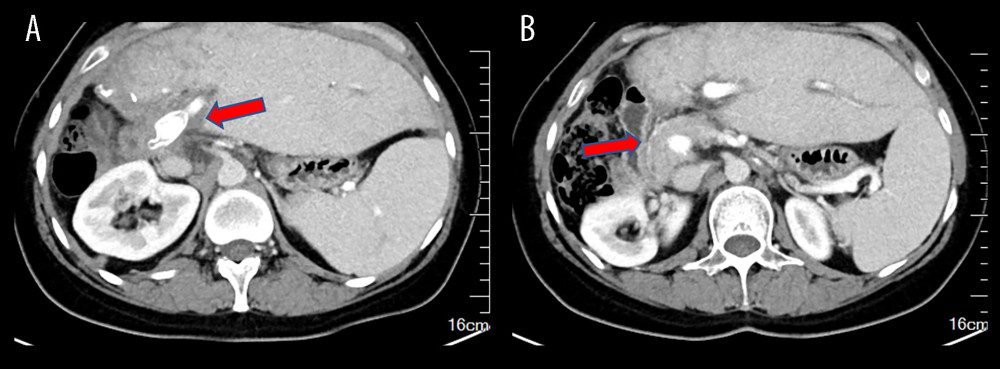 Figure 4. Ten-month postoperative CT scans show unobstructed portal vein flow(A) Intrahepatic portal vein flow is unobstructed. The arrow indicates an unobstructed artificial vessel flow. (B) The portal vein trunk is unobstructed with peripheral varicose vessels. The arrow indicates the portal vein trunk. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe).
Figure 4. Ten-month postoperative CT scans show unobstructed portal vein flow(A) Intrahepatic portal vein flow is unobstructed. The arrow indicates an unobstructed artificial vessel flow. (B) The portal vein trunk is unobstructed with peripheral varicose vessels. The arrow indicates the portal vein trunk. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe). References
1. Stojković M, Weber TF, Junghanss T, Clinical management of cystic echinococcosis: State of the art and perspectives: Curr Opin Infect Dis, 2018; 31(5); 383-92
2. Kern P, Menezes da Silva A, Okhan A, The echinococcoses: Diagnosis, clinical management and burden of disease: Adv Parasitol, 2017; 96; 259-69
3. Collado Aliaga J, Romero-Alegría A, Alonso-Sardón M, Portal hypertension as a complication of cystic echinococcosis: A 20-year cohort analysis: Am J Trop Med Hyg, 2021; 105(3); 692-97
4. San Juan López C, Lázaro Sáez M, Barrientos Delgadoet A, Portal hypertension as a complication of hepatic hydatidosis: Gastroenterol Hepatol, 2018; 41(10); 648-49
5. Yang C, He J, Yang X, Surgical approaches for definitive treatment of hepatic alveolar echinococcosis: Results of a survey in 178 patients: Parasitology, 2019; 146(11); 1414-20
6. Jiang T, Ran B, Guo Q, Use of the ligamentum teres hepatis for outflow reconstruction during ex vivo liver resection and autotransplantation in patients with hepatic alveolar echinococcosis: A case series of 24 patients: Surgery, 2021; 170(3); 822-30
7. Wen H, Vuitton L, Tuxun T: Clin Microbiol Rev, 2019; 32(2); e00075-18
8. Bhutani N, Kajal P, Hepatic echinococcosis: A review: Ann Med Surg (Lond), 2018; 36; 99-105
9. Hassine HB, Hydatid disease of the liver with portal vein invasion and cavernous transformation: A case report and literature review: IDCases, 2021; 23; e01006
10. López Grove R, Aineseder M, Spina JC, Hydatid liver disease with portal vein invasion, cavernous transformation and portal biliopathy: Rev Esp Enferm Dig, 2022; 114(11); 681
11. Wei B, Huang Z, Tang C, Optimal treatment for patients with cavernous transformation of the portal vein: Front Med (Lausanne), 2022; 9; 853138
12. Hwang R, Liou P, Kato T, Ex vivo liver resection and autotransplantation: An emerging option in selected indications: J Hepatol, 2018; 69(5); 1002-3
13. Baimas-George M, Thompson KJ, Watson MD, The technical aspects of ex vivo hepatectomy with autotransplantation: A systematic review and meta-analysis: Langenbecks Arch Surg, 2021; 406(7); 2177-200
14. Zawistowski M, Nowaczyk J, Jakubczyk M, Domagała P, Outcomes of ex vivo liver resection and autotransplantation: A systematic review and meta-analysis: Surgery, 2020; 168(4); 631-42
15. Yang C, Yang H-J, Deng S-P, Zhang Y, Current status of ex-vivo liver resection and autologous liver transplantation for end-stage hepatic alveolar echinococcosis: Ann Palliat Med, 2020; 9(4); 2271-78
16. Singh N, Washburn L, Black S, Schenk A, Techniques for management of portal vein thrombosis during liver transplantation: Case Rep Transplant, 2020; 2020; 8875196
Figures
 Figure 1. Preoperative CT scans of the patient(A) The lesion, situated in the hilar region, exhibits severe invasion into the portal vein, bile duct, and hepatic artery. (B) Portal vein occlusion has resulted in cavernous degeneration of the portal vein. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe).
Figure 1. Preoperative CT scans of the patient(A) The lesion, situated in the hilar region, exhibits severe invasion into the portal vein, bile duct, and hepatic artery. (B) Portal vein occlusion has resulted in cavernous degeneration of the portal vein. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe). Figure 2. Surgical process(A) The liver prior to isolation. (B) A temporary venous diversion of the portal-hepatic vein was established post liver isolation. (C) The liver was perfused and revised in an ex vivo state. (D) The portal vein was reconstructed with artificial vessels. The arrow indicates the portal vein anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk).
Figure 2. Surgical process(A) The liver prior to isolation. (B) A temporary venous diversion of the portal-hepatic vein was established post liver isolation. (C) The liver was perfused and revised in an ex vivo state. (D) The portal vein was reconstructed with artificial vessels. The arrow indicates the portal vein anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk). Figure 3. Surgical process(A) Completion of portal vein anastomosis. The arrow indicates post-portal vein anastomosis. (B) Surgery conclusion following hepatic duct-jejunum anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk).
Figure 3. Surgical process(A) Completion of portal vein anastomosis. The arrow indicates post-portal vein anastomosis. (B) Surgery conclusion following hepatic duct-jejunum anastomosis. The figure was created using 3D Studio Max (Version: 3Dmax 2020; Manufacturer: Autodesk). Figure 4. Ten-month postoperative CT scans show unobstructed portal vein flow(A) Intrahepatic portal vein flow is unobstructed. The arrow indicates an unobstructed artificial vessel flow. (B) The portal vein trunk is unobstructed with peripheral varicose vessels. The arrow indicates the portal vein trunk. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe).
Figure 4. Ten-month postoperative CT scans show unobstructed portal vein flow(A) Intrahepatic portal vein flow is unobstructed. The arrow indicates an unobstructed artificial vessel flow. (B) The portal vein trunk is unobstructed with peripheral varicose vessels. The arrow indicates the portal vein trunk. The figure was created using Adobe Photoshop 2022 (Version: Photoshop 2022 V23.5; Manufacturer: Adobe). In Press
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
03 Apr 2024 : Review article
Alternative Therapies in Transplantology as a Promising Perspective in MedicineAnn Transplant In Press; DOI: 10.12659/AOT.943387
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








