26 March 2024: Database Analysis
Outcomes of Renal Transplantation in ANCA-Associated Vasculitis: A Systematic Review and Meta-Analysis
Xiaoqin Long1AB, Xiaobing Yang1ABF, Shudong Yao2ABCD, Jia Wu3ABCDE*DOI: 10.12659/AOT.943433
Ann Transplant 2024; 29:e943433
Abstract
BACKGROUND: Antineutrophil cytoplasmic antibody-associated vasculitis is characterized by small-vessel inflammation and ANCA-positive serology that often lead to end-stage kidney disease. This study investigated the outcomes of renal transplantation in patients with antineutrophil cytoplasmic antibody-associated vasculitis.
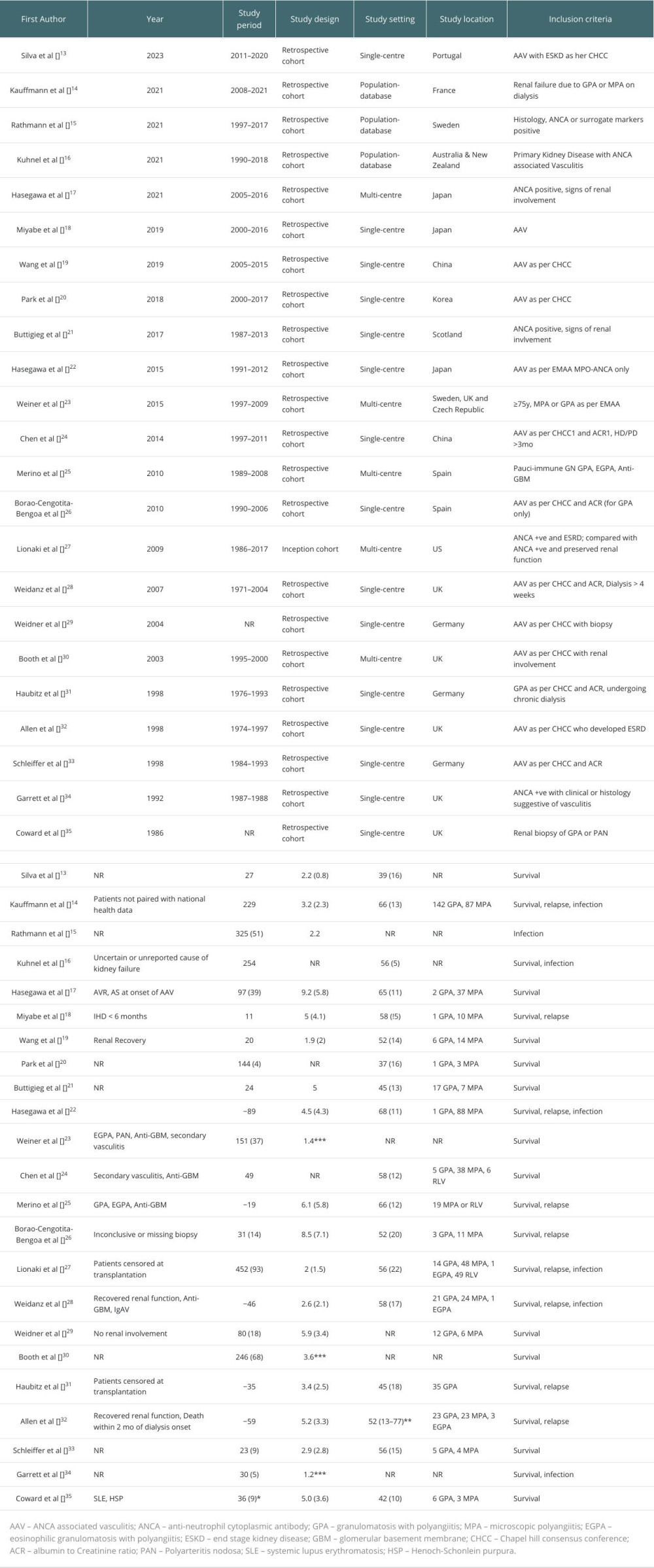
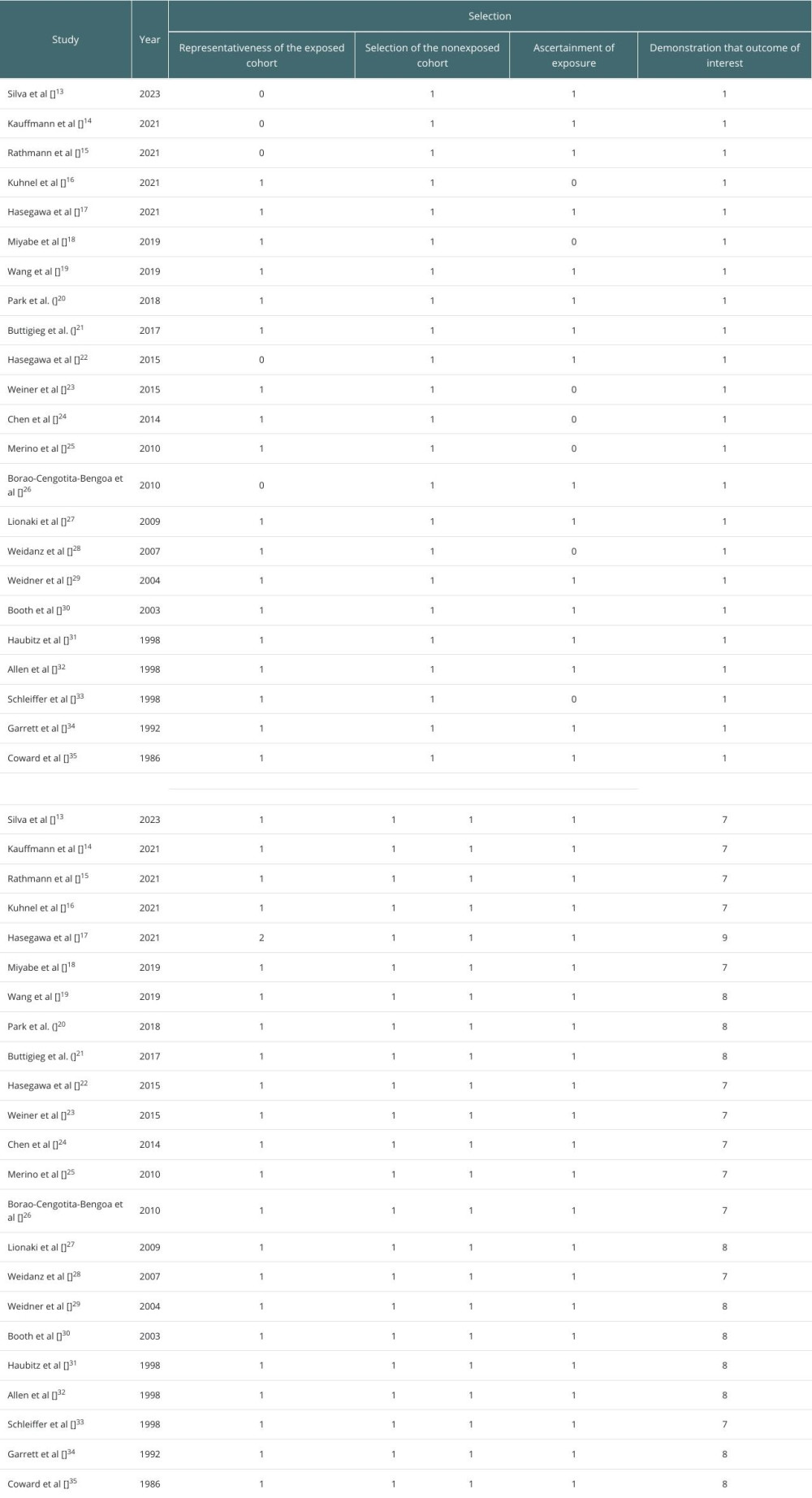
MATERIAL AND METHODS: A comprehensive search of PubMed, Scopus, and Embase databases was done to retrieve studies that reported on the outcomes of renal transplantation in these patients. Data on mortality, survival, infection, and relapse rates were analyzed. The quality of the included studies was evaluated using the Newcastle-Ottawa Scale for cohort studies.
RESULTS: Twenty-three retrospective cohort studies were included in this review. Antineutrophil cytoplasmic antibody-associated vasculitis was associated with high post-transplantation mortality rates, with a pooled rate ratio of 11.99 per 100 patient-years, but relatively favorable survival rate (hazard rate of 0.80). After renal transplantation, these patients had elevated infection rates (pooled rate ratio of 52.70 per 100 patient-years), and high risk of relapse (pooled rate ratio of 6.96), emphasizing the importance of vigilant post-transplantation monitoring.
CONCLUSIONS: End-stage kidney disease patients with vasculitis, undergoing renal transplantation, are at elevated risk of mortality and postoperative infection compared to patients without antineutrophil cytoplasmic antibody-associated vasculitis. The risk of relapse is also high in these patients. However, renal transplantation offers a survival advantage for vasculitis patients who survive the early post-transplantation period.
Keywords: Meta-Analysis, Renal Transplantation Unit Solution, systematic review
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of rare, complex autoimmune conditions [1–4]. This heterogeneous spectrum of diseases includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis, and renal-limited ANCA vasculitis [5,6]. Renal damage is a hallmark feature of the disease, with 50–80% of patients rapidly progressing to glomerulonephritis [7]. Notably, end-stage kidney disease (ESKD) develops in 20–40% of AAV patients [8,9].
While kidney transplantation remains the optimal renal replacement therapy for eligible patients with AAV and ESKD, it is associated with unique complications in this population. Factors such as histological features, patient age, glomerular filtration rate, and PR3-ANCA status at the time of kidney transplantation have all been identified as poor prognostic indicators [10–12]. Despite a relatively low rate of AAV relapse after kidney transplantation (about 0.02 events per patient-year), the absence of clear guidelines regarding the timing and indications for kidney transplantation, as well as the risk factors for post-transplantation AAV relapse, have raised concerns about the potential adverse effects of disease recurrence on both graft and patient survival.
While individual studies [13–16] have explored the outcomes of ANCA-associated renal transplantation, no comprehensive meta-analysis of these outcomes is available to date.
This systematic review summarizes the existing data and provides insight into the factors influencing transplantation outcomes in AAV patients with ESKD, including primary outcomes like graft and patient survival, and secondary outcomes like disease recurrence, and post-transplantation complications.
Material and Methods
RESEARCH QUESTION:
What are the outcomes of renal transplantation in patients with ANCA-associated vasculitis?
Population/Participants (P): Patients with ESKD undergoing kidney transplantation.
Exposure (E): Patients with AAV.
Comparison (C): Patients with no AAV.
Outcomes (O): Primary outcomes like graft and patient survival and secondary outcomes like disease recurrence, and post-transplantation complications.
SEARCH STRATEGY:
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were strictly followed, and the protocol was registered at PROSPERO with registration number CRD42023464690. Comprehensive searches of relevant electronic databases, including PubMed, Embase, and Scopus, were performed to identify studies published up to 30 September 2023. Combinations of the following keywords and Medical Subject Headings (MeSH) terms were used: “ANCA-associated vasculitis”; “AAV”; “renal transplantation”; “kidney transplant”; “outcomes”; “graft survival”; “disease recurrence”. Search string was as follows: (ANCA-Associated Vasculitis/therapy [MeSH Terms] OR “ANCA-associated vasculitis” OR “AAV” OR “Granulomatosis with Polyangiitis” OR “GPA” OR “Microscopic Polyangiitis” OR “MPA”) AND (Kidney Transplantation [MeSH Terms] OR “Renal transplantation” OR “Kidney transplant” OR “Kidney transplantation” OR “Kidney graft”) AND (Treatment Outcome [MeSH Terms] OR “Outcomes” OR “Graft survival” OR “Patient survival” OR “Disease recurrence” OR “Complications” OR “Risk factors”).
Bibliographies of included articles and applicable reviews were also screened to identify missed studies. A manual screening of issues of relevant nephrology journals was done for any potentially eligible studies that were missed.
Inclusion criteria:
Exclusion Criteria:
DATA EXTRACTION:
Initial screening of titles and abstracts for eligibility was independently done by 2 reviewers. Full-text articles were then read, and any discrepancies or disagreements were resolved by discussion. A standardized form for data extraction included study characteristics (author, publication year, study design), patient demographics (age, sex), AAV subtype, and relevant outcome data like survival, relapse rate, infection, and mortality.
QUALITY ASSESSMENT:
The quality and risk of bias were assessed by the Newcastle-Ottawa Scale for observational studies. Two reviewers independently assessed each study, and resolved differences by discussion.
STATISTICAL ANALYSIS:
A meta-analysis was conducted using statistics software (RevMan 5.4v, Cochrane Collaboration, UK). For studies with comparable outcome measures, pooled estimates, including hazard ratios (HRs), odds ratios (ORs), or mean differences (MDs) with 95% confidence interval (CI) were calculated and plotted as forest plots. The “Inverse Variance (IV), Random” method was used to calculate the effect estimates for each outcome. Heterogeneity among studies was assessed using the I2 statistic, and a random-effects model was used if substantial heterogeneity was detected. I2 >70% indicated high heterogeneity and I2 <50% indicated low heterogeneity. The publication bias was assessed using the visualization of funnel plots.
Results
META-ANALYSIS:
Of 23 selected studies, 18 were eligible for the meta-analysis, and were found to report similar outcomes, to systematically analyze and quantify the outcomes of renal transplantation in AAV patients, with a focus on mortality, survival, infection rates, and relapse rates. The rest of the studies were analyzed qualitatively.
AAV patients who underwent renal transplantation had an approximately 12-fold higher mortality rate (RR of 11.99). The 95% CI of 9.09–15.82 was indicative of the level of uncertainty associated with the estimate (Figure 2).
The cumulative survival estimate of HR 0.80 [0.43, 1.50] indicated that the mortality risk in AAV patients was comparable to that of patients without AAV (Figure 3).
AAV was linked to a significant, 53-fold increase in the rate of infections (RR of 52.70). The 95% CI ranged from 21.74 to 127.74, indicating a wide range of uncertainty (Figure 4).
The relapse rate (RR of 6.96) indicated a significantly higher relapse rate in AAV patients undergoing renal transplantation. The 95% CI ranged from 5.62 to 8.62, showing a relatively narrow range of uncertainty (Figure 5).
Discussion
This study analyzed the outcomes of renal transplantation in patients with AAV, and showed that AAV was associated with a high post-transplantation mortality rate, increased infection risk, and a high relapse rate. However, AAV patients who survived renal transplantation had overall favorable long-term outcomes.
The meta-analysis revealed a high mortality rate in AAV patients, with a pooled rate ratio of 11.99 per 100 patient-years. This rate underscores the considerable risk faced by AAV patients following the surgery. Several factors may contribute to this increased mortality risk. First, the extensive immunosuppression required to prevent graft rejection can render AAV patients more susceptible to infections, malignancies, and other transplantation-related complications [36,37]. Second, AAV is characterized by small-vessel inflammation, thus potentially presenting ongoing post-transplantation challenges [38]. Nevertheless, our results showed that AAV patients have a favorable survival rate, with a hazard ratio (HR) of 0.80. This suggests that while AAV patients face a higher mortality risk, those who do survive may experience relatively satisfactory long-term outcomes. Our findings underscore the delicate balance between managing AAV and preventing graft rejection, requiring close post-transplant monitoring and management.
The high post-transplantation infection rates in AAV patients are a matter of concern. Immunosuppressive regimens in renal transplantation patients make them highly susceptible to infections [36,39]. The high rate of infections observed in our study further emphasizes the importance of stringent infection control measures in the post-transplantation care of AAV patients.
Our study has some limitations. The diversity in patient populations across the studies was a source of heterogeneity. Variability was observed in terms of patient demographics, clinical characteristics, and comorbidities. Differences in the inclusion criteria, such as the specific types of AAV considered (GPA, MPA, or others), and the definitions of end-stage kidney disease (ESKD) may have influenced patient selection. The geographic locations of the studies also introduced potential regional disparities in patient characteristics and clinical practices. Clinical practices were not standardized across the studies, including the choice of immunosuppressive regimens, post-transplantation monitoring protocols, and management of infections and relapses. Different institutions and regions may have distinct protocols and preferences, introducing variability in patient care, which can directly impact outcomes like infection rates, relapse rates, and patient survival. While a random-effects model may address some of the heterogeneity by providing more conservative estimates [40–42], the presence of heterogeneity should not be overlooked. Further studies, incorporating standardized protocols and larger sample sizes, are needed to provide more precise insights into the outcomes of renal transplantation in AAV patients.
The findings of this systematic review have several clinical implications. They emphasize the necessity for continuous research and the development of standardized protocols for patient selection, immunosuppressive regimens, and post-transplantation monitoring. A multidisciplinary approach involving nephrologists, rheumatologists, and transplant specialists is crucial in achieving better outcomes for AAV patients after transplantation.
Conclusions
In conclusion, renal transplantation remains a vital therapeutic option for AAV patients with ESKD. The high post-transplantation mortality rate, increased infection risk, and an increased relapse rate, reported by our study may guide clinicians in optimizing care of AAV patients undergoing renal transplantation. A comprehensive understanding of the complexities of managing AAV-associated renal transplantation is crucial for providing informed care, mitigating risks, and improving patient outcomes in this population of ESKD patients.
Figures
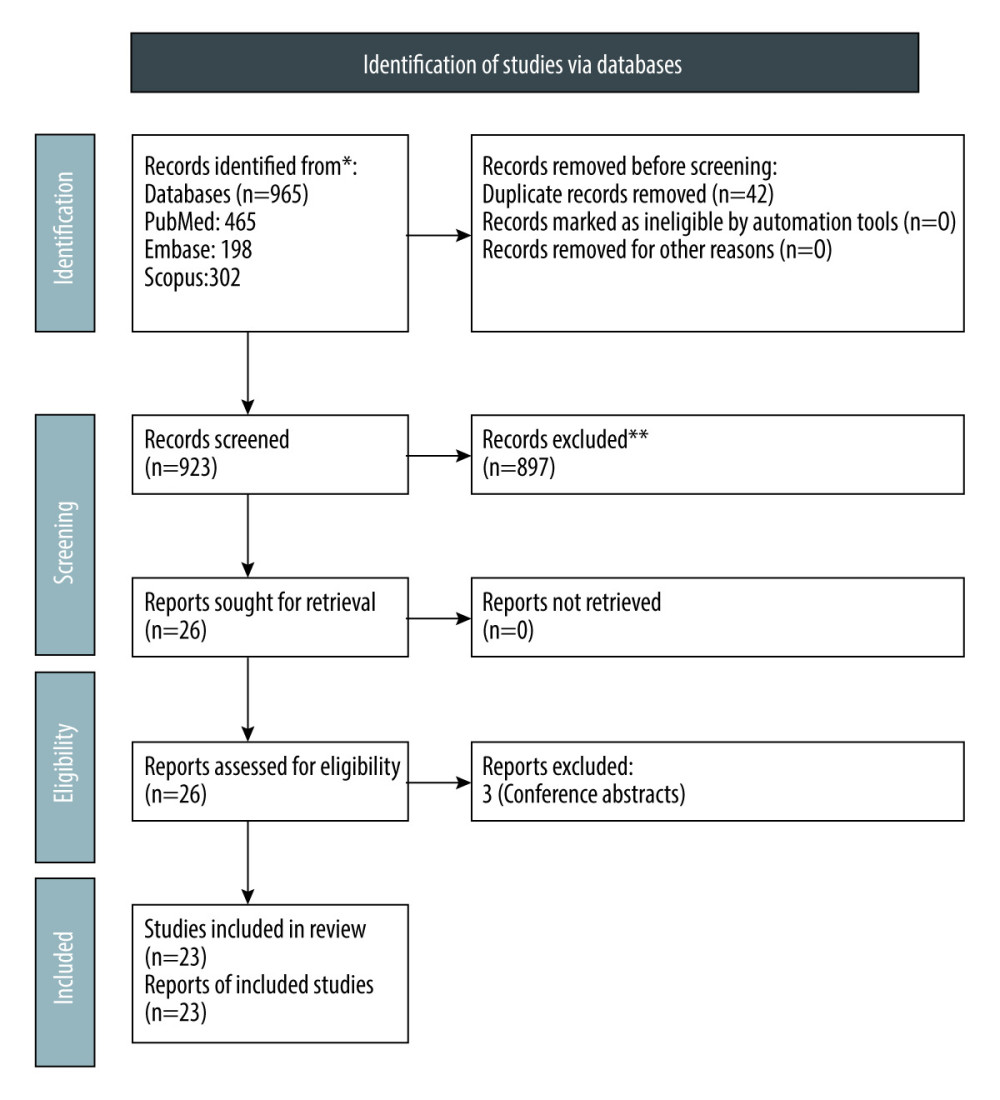 Figure 1. PRISMA flow chart for study selection.
Figure 1. PRISMA flow chart for study selection. 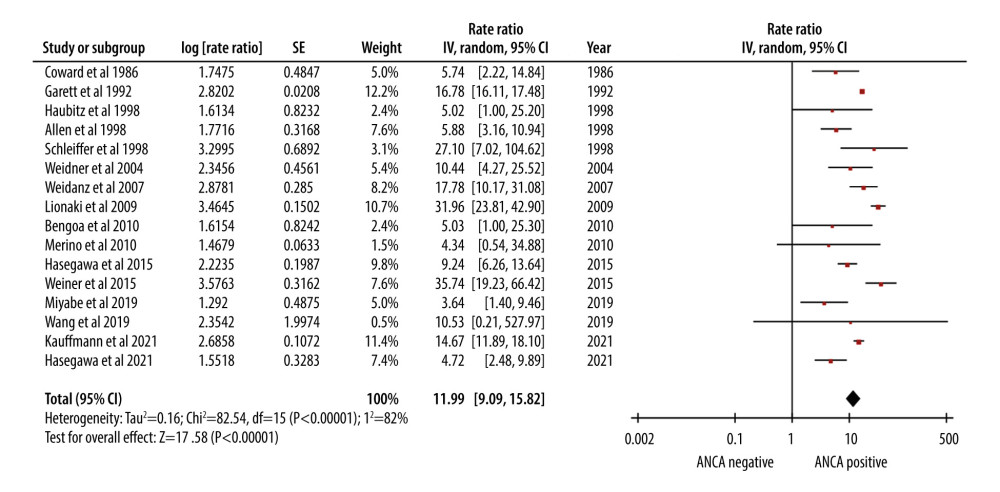 Figure 2. Forest plot showing the rate of mortality per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 2. Forest plot showing the rate of mortality per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation. 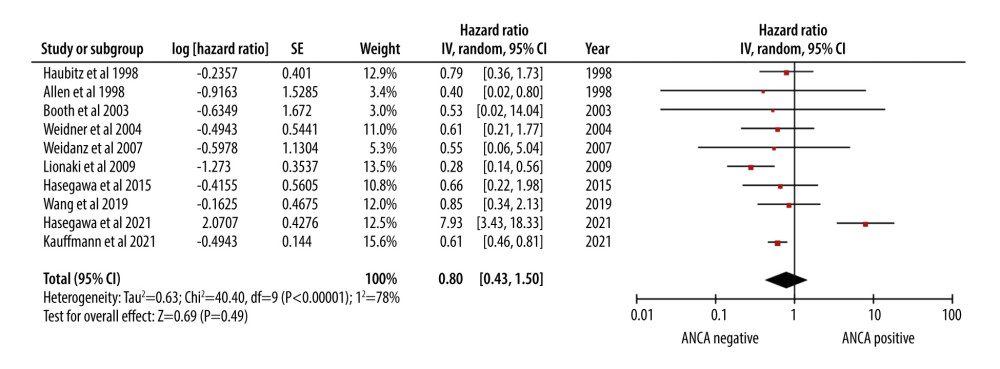 Figure 3. Forest plot showing the cumulative survival rate after 5 years of follow-up in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 3. Forest plot showing the cumulative survival rate after 5 years of follow-up in ANCA-associated vasculitis patients undergoing renal transplantation. 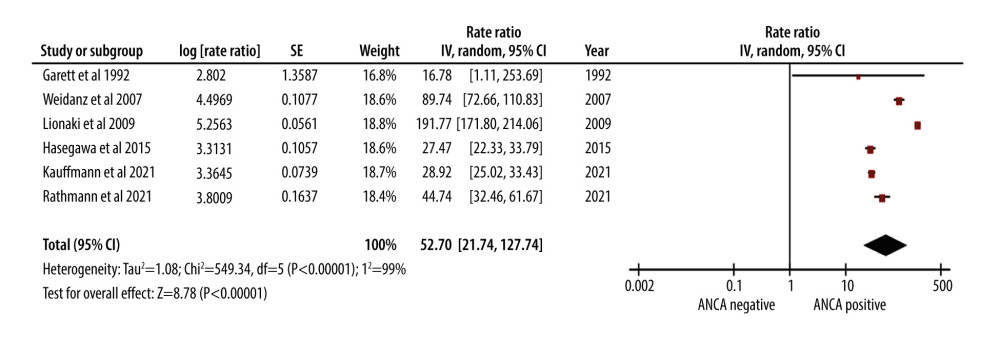 Figure 4. Forest plot showing rate of infection per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 4. Forest plot showing rate of infection per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation. 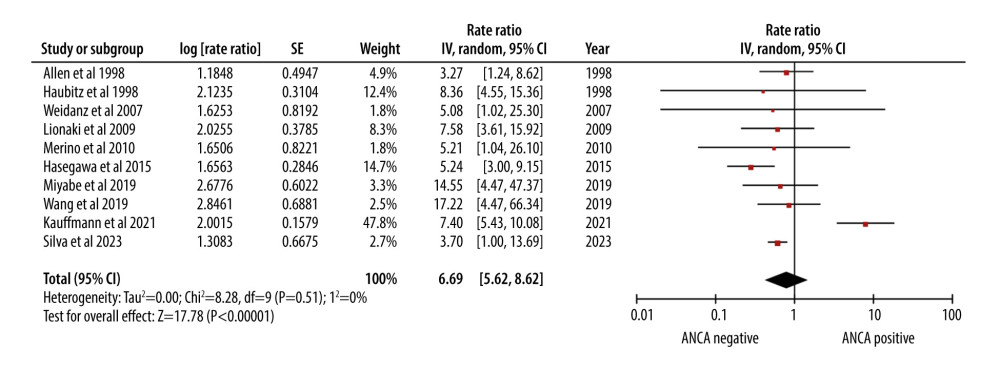 Figure 5. Forest plot showing relapse rate per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 5. Forest plot showing relapse rate per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation. References
1. Villacorta J, Martinez-Valenzuela L, Martin-Capon I, Bordignon-Draibe J, Antineutrophil cytoplasmic antibody-associated vasculitis: Toward an individualized approach: Nephron, 2021; 146; 121-37
2. Hunter RW, Welsh N, Farrah TE, ANCA associated vasculitis: BMJ, 2020; 369; m1070
3. Yates M, Watts R, ANCA-associated vasculitis: Clin Med, 2017; 17; 60-64
4. Marvisi C, Elena G, Umberto MC, EULAR guidelines on ANCA-associated vasculitis in the real life: Rheumatol, 2021; 2; e281
5. Casal Moura M, Branco C, Martins-Martinho J, A glance into the future of anti-neutrophil cytoplasmic antibody-associated vasculitis: Ther Adv Musculoskelet Dis, 2022; 14; 1759720X221125979
6. Molnár A, Studinger P, Ledó N, Diagnostic and therapeutic approach in ANCA-Associated glomerulonephritis: A review on management strategies: Front Med (Lausanne), 2022; 9; 884188
7. Berti A, Cornec-Le Gall E, Cornec D, Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort: Nephrol Dial Transplant, 2019; 34; 1508-17
8. Almaani S, Fussner LA, Brodsky S, ANCA-associated vasculitis: An update: J Clin Med, 2021; 10; 1446
9. Binda V, Favi E, Calatroni M, Moroni G, Anti-neutrophil cytoplasmic antibody-associated vasculitis in kidney transplantation: Medicina (Mex), 2021; 57; 1325
10. Liao Q-Q, Ren Y-F, Zhu K-W, Long-term prognostic factors in patients with antineutrophil cytoplasmic antibody-associated vasculitis: A 15-year multicenter retrospective study: Front Immunol, 2022; 13; 913667
11. Lee T, Gasim A, Derebail VK, Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure: Clin J Am Soc Nephrol, 2014; 9; 905-13
12. de Lind van Wijngaarden RAF, Hauer HA, Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement: J Am Soc Nephrol, 2006; 17; 2264
13. Silva RM, Leal R, Marques MG, Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis: a single-center 10-year experience: Transplant Proc, 2023; 55; 1396-99
14. Kauffmann M, Bobot M, Robert T, Disease activity and adverse events in patients with ANCA-associated vasculitides undergoing long-term dialysis: Clin J Am Soc Nephrol, 2021; 16; 1665-75
15. Rathmann J, Jayne D, Segelmark M, Incidence and predictors of severe infections in ANCA-associated vasculitis: A population-based cohort study: Rheumatology, 2021; 60; 2745-54
16. Kuhnel L, Hawley CM, Johnson DW, Allograft failure in kidney transplant recipients who developed kidney failure secondary to ANCA-associated vasculitis: Clin Transplant, 2021; 35; e14235
17. Hasegawa M, Iwasaki J, Sugiyama S, Development of aortic valve stenosis in myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis with renal involvement. Cheungpasitporn W, editor: PLoS One, 2021; 16; e0245869
18. Miyabe Y, Karasawa K, Takabe T, Long-term follow-up characteristics of patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) receiving chronic hemodialysis at a single center: Clin Exp Nephrol, 2020; 24; 136-42
19. Wang J-W, A retrospective study on combined treatment of continuous ambulatory peritoneal dialysis and immunosuppressive therapy of anca-associated vasculitis complicating renal failure: Acta Medica Mediterr, 2019; 3; 1531-37
20. Park ES, Ahn SS, Jung SM, Renal outcome of kidney-transplantation in Korean recipients with ANCA-associated vasculitis: Clin Exp Rheumatol, 2018; 36(Suppl 111); 115-20
21. Buttigieg J, Henderson L, Kidder D, Outcome of kidney transplant in antineutrophil cytoplasmic antibody-associated vasculitis: Exp Clin, 2017; 15; 509-15
22. Hasegawa M, Hattori K, Sugiyama S, A retrospective study on the outcomes of MPO-ANCA-associated vasculitis in dialysis-dependent patients: Mod Rheumatol, 2016; 26; 110-14
23. Weiner M, Goh SM, Mohammad AJ, Outcome and treatment of elderly patients with ANCA-associated vasculitis: Clin J Am Soc Nephrol, 2015; 10; 1128-35
24. Chen Y-X, Zhang W, Chen X-N, Clinical analysis of ANCA-associated renal vasculitis patients with chronic dialysis: Clin Exp Rheumatol, 2014; 32; S5-10
25. Merino JL, Galeano C, Espejo B, A retrospective study on outcome of microscopic polyangiitis in chronic renal replacement therapy: Nephrol Dial Transplant, 2011; 26; 1360-66
26. Borao-Cengotita-Bengoa M, Corral-Gudino L, Del Pino-Montes J, Lerma-Márquez JL, Long-term follow-up of microscopic polyangiitis, 17-year experience at a single center: Eur J Intern Med, 2010; 21; 542-47
27. Lionaki S, Hogan SL, Jennette CE, The clinical course of ANCA small-vessel vasculitis on chronic dialysis: Kidney Int, 2009; 76; 644-51
28. Weidanz F, Day CJ, Hewins P, Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis: Am J Kidney Dis, 2007; 50; 36-46
29. Weidner S, Geuss S, Hafezi-Rachti S, ANCA-associated vasculitis with renal involvement: An outcome analysis: Nephrol Dial Transplant, 2004; 19; 1403-11
30. Booth AD, Almond MK, Burns A, Outcome of ANCA-associated renal vasculitis: A 5-year retrospective study: Am J Kidney Dis, 2003; 41; 776-84
31. Haubitz M, Survival and vasculitis activity in patients with end-stage renal disease due to Wegener’s granulomatosis: Nephrol Dial Transplant, 1998; 13; 1713-18
32. Allen A, Pusey C, Gaskin G, Outcome of renal replacement therapy in antineutrophil cytoplasmic antibody-associated systemic vasculitis: J Am Soc Nephrol, 1998; 9; 1258-63
33. Schleiffer T, Burkhard B, Klooker P, Brass H, Clinical course and symptomatic prediagnostic period of patients with Wegener’s granulomatosis and microscopic polyangiitis: Ren Fail, 1998; 20; 519-32
34. Garrett PJ, Dewhurst AG, Morgan LS, Renal disease associated with circulating antineutrophil cytoplasm activity: Q J Med, 1992; 85; 731-49
35. Coward RA, Hamdy NAT, Shortland JS, Brown CB, Renal micropolyarteritis: A treatable condition: Nephrol Dial Transplant, 1986; 1; 31-37
36. Duncan MD, Wilkes DS, Transplant-related Immunosuppression: Proc Am Thorac Soc, 2005; 2; 449-55
37. Villard J, Immunity after organ transplantation: Swiss Med Wkly, 2006; 136; 71-77
38. Sangolli PM, Lakshmi DV, Vasculitis: A checklist to approach and treatment update for dermatologists: Indian Dermatol Online J, 2019; 10; 617-26
39. Roberts MB, Fishman JA, Immunosuppressive agents and infectious risk in transplantation: Managing the “net state of immunosuppression.”: Clin Infect Dis, 2020; 73; e1302-17
40. Mishra S, Misra S, Panda S, Role of probiotics in adjunct to non-surgical periodontal therapy in patients with chronic periodontitis: A systematic review and meta-analysis: J Biol Regul Homeost Agents, 2021; 35; 67-78
41. Rai A, Misra SR, Panda S, Nystatin effectiveness in oral candidiasis treatment: A systematic review & meta-analysis of clinical trials: Life, 2022; 12; 1677
42. Chaudhury S, Panda S, Mohanty N, Can immunoexpression of cancer stem cell markers prognosticate tongue squamous cell carcinoma? A systematic review and meta-analysis: J Clin Med, 2023; 12; 2753
Figures
 Figure 1. PRISMA flow chart for study selection.
Figure 1. PRISMA flow chart for study selection. Figure 2. Forest plot showing the rate of mortality per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 2. Forest plot showing the rate of mortality per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation. Figure 3. Forest plot showing the cumulative survival rate after 5 years of follow-up in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 3. Forest plot showing the cumulative survival rate after 5 years of follow-up in ANCA-associated vasculitis patients undergoing renal transplantation. Figure 4. Forest plot showing rate of infection per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 4. Forest plot showing rate of infection per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation. Figure 5. Forest plot showing relapse rate per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation.
Figure 5. Forest plot showing relapse rate per 100 patient-years in ANCA-associated vasculitis patients undergoing renal transplantation. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860