02 April 2024: Original Paper
Association of Coronary Calcium Score on Cardiac PET During Pre-Kidney Transplant Assessment with Persistent Hyperparathyroidism: A Retrospective Study
Ziad Arabi1234ABCDEF*, Mazin Ibrahim Musab El Sarrag4DEF, Tarek ArabiDOI: 10.12659/AOT.943532
Ann Transplant 2024; 29:e943532
Abstract
BACKGROUND: Secondary hyperparathyroidism and coronary calcifications are common complications in chronic kidney disease. However, the relation between coronary calcium score (CCS) and persistent hyperparathyroidism (pHPT) after kidney transplantation (KT) remains unknown.
MATERIAL AND METHODS: This was a single-center retrospective study of KT candidates from January 2017 to May 2020. We collected patients’ demographics, cardiovascular (CV) risk factors, and the findings of pre-KT CV imaging. We also collected parathyroid hormone (PTH) values before KT, at 1-6 months, 6-12 months, and 12-24 months after KT. We defined pHPT as PTH ≥25.5 pmol/L after 12 months post-KT.
RESULTS: A total of 111 KT recipients (KTRs) with a mean age of 50.4 years were included, of which 62.2% were men and 77.5% were living-donor KTRs. Dialysis modality used before KT was peritoneal dialysis in 9.9% and hemodialysis in 82.9%. Dialysis vintage was 3±2.9 years. The prevalence of pHPT was 24.3% (n=27), and the prevalence of severe coronary calcifications (CCS >400 Agatston units) was 19.8% (n=22). PTH values at baseline, 1-6 months, 6-12 months, and 12-24 months were not different among between CCS >400 or CCS <400 groups. However, pHPT after KT was significantly more prevalent in KTRs with severe CCS (37% vs 14.3%, p=0.014). Severe CCS was associated with less improvement of PTH values after KT (r=0.288, p=0.020). Otherwise, the findings of cardiac PET and coronary angiogram were not significantly different between pHPT and non-pHPT patients. CCS >400 was independently associated with pHPT after transplant (aOR=18.8, P=0.012).
CONCLUSIONS: Severe CCS on pre-KT cardiac assessment is associated with pHPT after KT.
Keywords: Hypercalcemia, Hyperparathyroidism, Kidney Transplantation, vascular calcification
Introduction
Vascular calcifications are common in chronic kidney disease (CKD) and are associated with increased morbidity and mortality [1]. Patients on dialysis have 2- to 5-fold more coronary calcification than age-matched individuals with angiographically proven coronary artery disease (CAD) [2]. Several mechanisms can adversely affect vascular calcification in CKD, including altered PTH secretion, use of calcium-based binders, and excessive vitamin D therapy [3]. These changes induce vascular smooth muscle cells to change into a chondrocyte- or osteoblast-like cell [4,5], leading to calcifications into the coronaries, peripheral artery, and cardiac valves [4,6]. Furthermore, proteins such as α-Klotho, fibroblast growth factor (FGF)-23, and fetuin A play a critical role in vascular calcification in CKD. In CKD, disrupted phosphate metabolism due to impaired kidney function leads to elevated FGF23 levels, which can promote vascular calcification [7]. α-Klotho, often reduced in CKD, exacerbates this by failing to effectively regulate FGF23 and phosphate levels [8]. Fetuin A acts as a systemic inhibitor of calcification, but its deficiency in CKD contributes to enhanced vascular calcification [9]. Depositions can be either intimal or medial, and show a progressive tendency [4,10,11] The progression of coronary calcifications slows but does not completely halt after kidney transplant (KT), and a higher baseline CCS is associated with a higher risk of progression [12]. CCS provides additional prognostic indicator beyond traditional CV risk factors [1,13,14].
Secondary hyperparathyroidism is also a common complication of CKD. Parathyroid hormone (PTH) levels increase early as kidney function starts to decline until patients reach end-stage kidney disease. After KT, PTH levels decline in most patients [15]. However, only 23% of patients will reach PTH levels within normal range. Furthermore, 20–50% of patients will have persistently elevated PTH values 2 times higher than the normal levels at 12 months after KT, also known as persistent hyperparathyroidism (pHPT) [16–18]. pHPT is typically defined as persistently elevated PTH over 2 times above the upper normal limit in a renal graft that is functioning well (eGFR ≥30 ml/min) during the first 6–12 months after KT [12,19]. High calcium phosphate product, older age, and dialysis vintage before KT are associated with an increased risk of pHPT [15,20]. A significant improvement in secondary hyperparathyroidism (HPT) after KT favorably affects the progression of CCS [12].
There are only limited data on the relationship between CCS and HPT in KTRs [11,21]. Although a significant improvement in secondary HPT after KT favorably affects the progression of CAC [12], the relationship between pHPT and CCS, to the best of our knowledge, has not been studied. In this study we examined the association between CCS, and PTH changes after KT, and more specifically pHPT, at our center. We hope this study helps shed light on the relationship between CCS and pHPT.
Material and Methods
STATISTICAL ANALYSIS:
We used SPSS 26 (IBM, Armonk, NY, United States) for the analysis. Categorical variables were presented as frequencies and percentages and compared using the chi-squared or Fisher’s exact tests, as appropriate. Continuous variables were presented as mean±SD. The Shapiro-Wilk test was used to assess the normality of continuous variables and guide the selection of parametric or nonparametric tests for the comparison of variables. The variables were compared using Welch’s t-test, Student t-test, and Mann-Whitney-U test. Spearman’s rank correlation was applied to evaluate the association between PTH values and calcium score. All clinically relevant independent variables from univariate logistic regression analysis were included in a multivariate logistic regression model to examine the association between risk factors and study outcome. All reported values are two-sided and a
Results
A total of 111 KTRs were included (Table 1). The average age was 50.4±13 years, 62.2% were men, and 77.5% were living-donor KTRs. Preemptive KT was performed in 7.2%. The dialysis modality used pre-KT was peritoneal dialysis (PD) in 9.9% and hemodialysis (HD) in 82.9% (using an arteriovenous fistula in 41.5% and tunneled hemodialysis catheter in 58.5%). Dialysis vintage (the time on dialysis) was 3±3 years (4.8±3.3 years for deceased donor KT vs 2.4±2.6 years for living-donor KT).
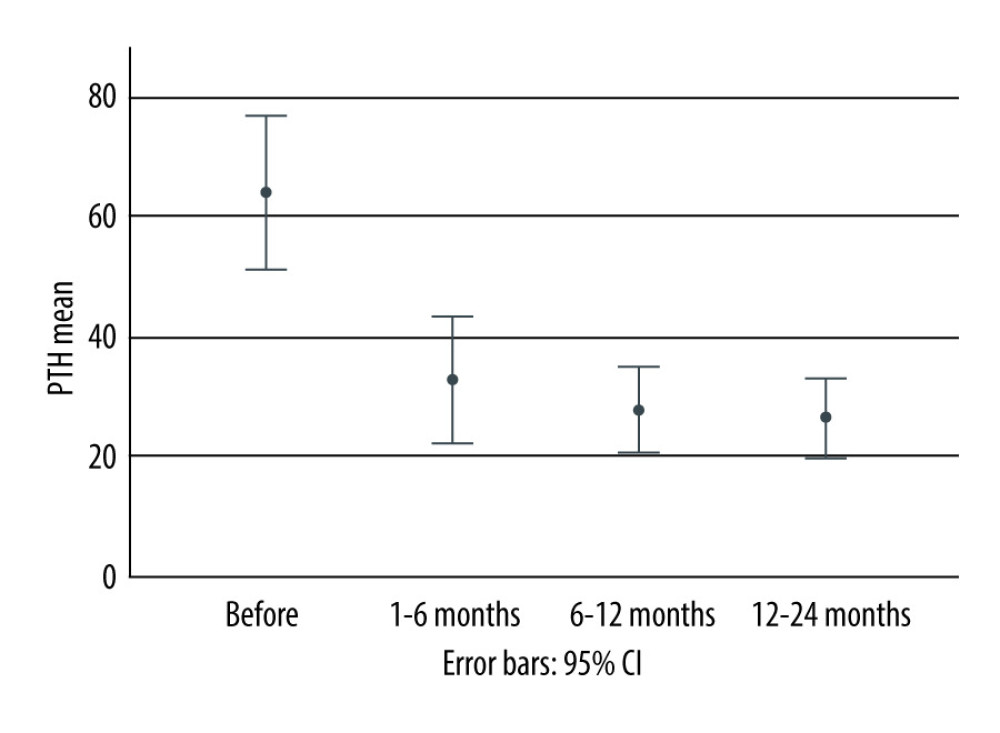
Mean PTH at baseline (before KT) was 64.1±57 pmol/L, 32.7±36.4 pmol/L at 1–6 months after KT, 27.6±26.1 pmol/L at 6–12 months, and 26.2±32.5 pmol/L at 12–24 months. Accordingly, PTH declined significantly from baseline to 12 months by −45.2±53.6 pmol/L (Figure 1). The prevalence of pHPT was 24.3% (n=27).
The mean dialysis vintage was significantly higher in the pHPT group (4±3.2 years) than in the non-persistent group (2.7±2.7years) (
Other factors, such as patient demographics, baseline cardiovascular risk factors, types of kidney replacement therapy, types of KT, and metabolic and cardiovascular changes after KT were not significantly different between the 2 groups (Table 2). Additionally, calcium did not significantly change from baseline to 12–24 months after KT in either group, whereas phosphorous dropped significantly in both groups after KT (
Severe CCS (≥400) was also significantly more prevalent in the pHPT than the non-persistent group (37% vs 14.3%,
The prevelence of CCS > 400 was sigificatly higher in the pHPT group (37% vs 14.3%,
Additionaly, CCS was positively correlated with PTH change in the patient population (coefficient 0.288,
Multivariate analysis revealed a strong association between pHPT and severe coronary calcifications (CCS >400) (aOR=18.804,
Discussion
The present study examined the association of pHPT in KTRs with a variety of patient demographic and clinical variables, including several components of pre-KT cardiac assessment, such as CCS. Of the 111 KTRs, pHPT was evident in 24.3% of patients and high CCS (>400 Agatston units) was present in 19.8%.
Studies have reported a variable prevalence of pHPT based on the definition used, recipient age, dialysis vintage, rate of pre-emptive KTs, pre-transplant PTH levels, and rate of calcimimetics use [29–31]. Consistent with previous studies [29,31], our study showed that patients in the pHPT group had significantly longer dialysis vintage [30,32].
The prevalence of high CCS (CCS >400 Agatston units) in the present study is similar to that of previous reports, which reach 33% [33,34]. Older age, male sex, diabetes, and longer dialysis vintage have been linked to higher CCS in ESKD [11]. However, the relationship between coronary calcifications and secondary hyperparathyroidism after KT is less clear. Two previous studies [6,11] have found no association between CCS and pre-KT PTH values; however, Megahed et al [21] reported a possible association. To the best of our knowledge, this is the first study to examine the relationship between CCS and PTH values after KT.
The relationship between serum calcium and phosphorus with CCS has also been reported inconsistently [4,6,11,21]. Our study showed no association between CCS and isolated pre- and post-KT PTH values, but there was a strong association between severe CCS and pHPT. Additionally, the current study showed that a significant improvement in secondary HPT after KT was favorably correlated with lower baseline CCS. These findings are in line with the findings of Cianciolo et al, who showed that a significant improvement in secondary HPT after KT significantly reduces the progression of coronary calcifications after KT [12].
CCS is associated with a stepwise increase in cardiac events among the general population. However, many studies have suggested that only severe CCS is involved in this relationship [27,28]. In KT candidates, CCS ≥400 provides a sensitivity of 70% for diagnosing obstructive CAD in a proximal segment [28]. The same CCS threshold is also associated with increased risk of CV disease and all-cause mortality in CKD patients [35]. Our study also suggests a similar threshold governing the relationship between CCS and pHPT.
The finding of strong relationships between CCS and pHPT but the lack of such relation with isolated PTH values is also very intriguing. It is possible that severe CCS is a consequence of worse metabolic and inflammatory background before KT, leading to higher rate of pHPT [36–38]. On the other hand, studies have reported that arterial calcifications develop at a different rate in dialysis patients, despite similar exposure to these risk factors and regardless of the dialysis vintage [4]. Studies have also suggested the presence of protective factors in blood vessels and in the circulation [4], a possible genetic predisposition [39], and potentially a specific variant of vitamin D receptor, Klotho, or FGF-23 [4,39]. Further research is required to study whether pHPT is a subcategory of secondary hyperparathyroidism with a different pathophysiology leading to more elevated CCS, or whether higher CCS is merely a consequence of worse metabolic and inflammatory background before KT resulting in a higher rate of pHPT.
The current study showed no significant relation between pHPT and the severity of CAD when assessed by cardiac nuclear stress tests and coronary angiograms. This is likely due to the nature of coronary calcifications in end-stage kidney disease (ESKD) and the propensity for developing medial rather than intimal arterial calcifications, leading to a lower sensitivity and specificity CCS [4,40–44].
Our study has several limitations. First, its retrospective and single-center nature may limit its generalizability. Secondly, CCS was calculated as part of the pre-KT workup but not at the end of the study (12 months after KT). However, this is unlikely to change the validity of our results since KT only slows but does not halt or regress the progression of coronary calcifications for at least the first 2 years after KT [34,45–47].
Despite these limitations, this study included a relatively large sample of KTRs and is one of the few studies to examine the association between CCS and hyperparathyroidism in KTRs. To the best of our knowledge, the association between severe CCS and pHPT is a novel finding. A prospective study with longer follow-up is required to examine the potential causal relation between pHPT and CCS and any potential implications for prevention and treatment.
Conclusions
Severe CCS is associated with pHPT at 12 months after KT, but not PTH serum levels. Further research is needed to explore the complex association between pHPT and coronary calcium deposition in KTRs.
Tables
Table 1. Characteristics of kidney transplant recipients studied in our population.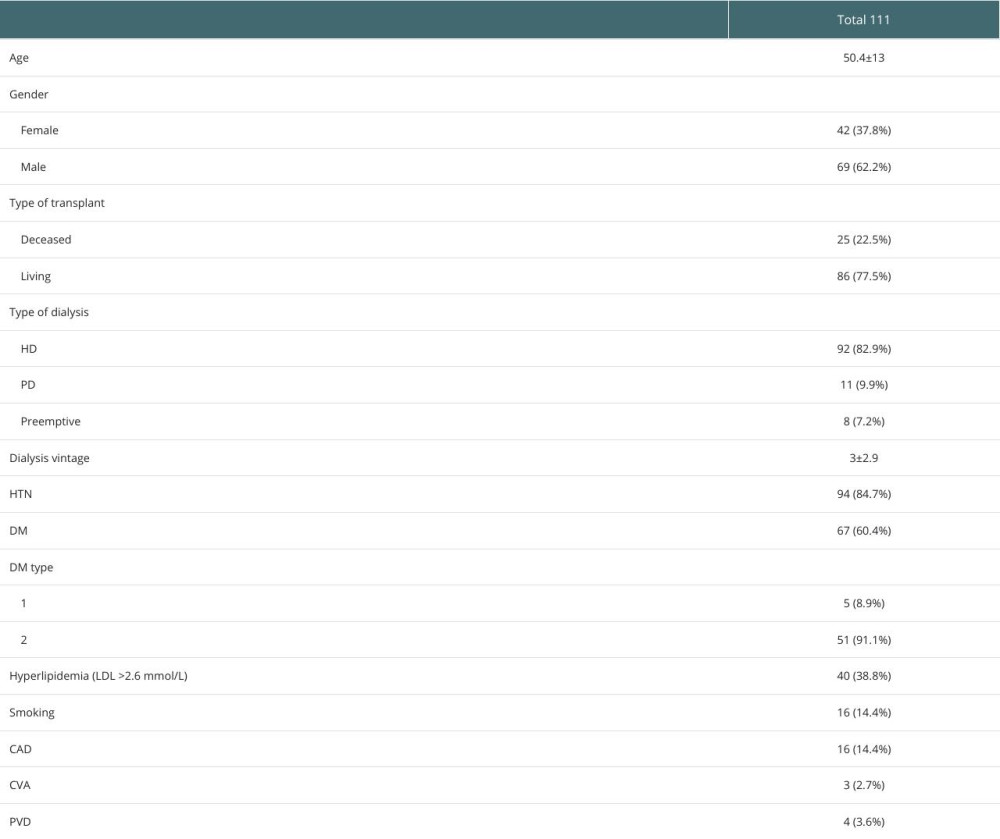 Table 2. Characteristics of KTRs with pHPT vs non-pHPT.
Table 2. Characteristics of KTRs with pHPT vs non-pHPT.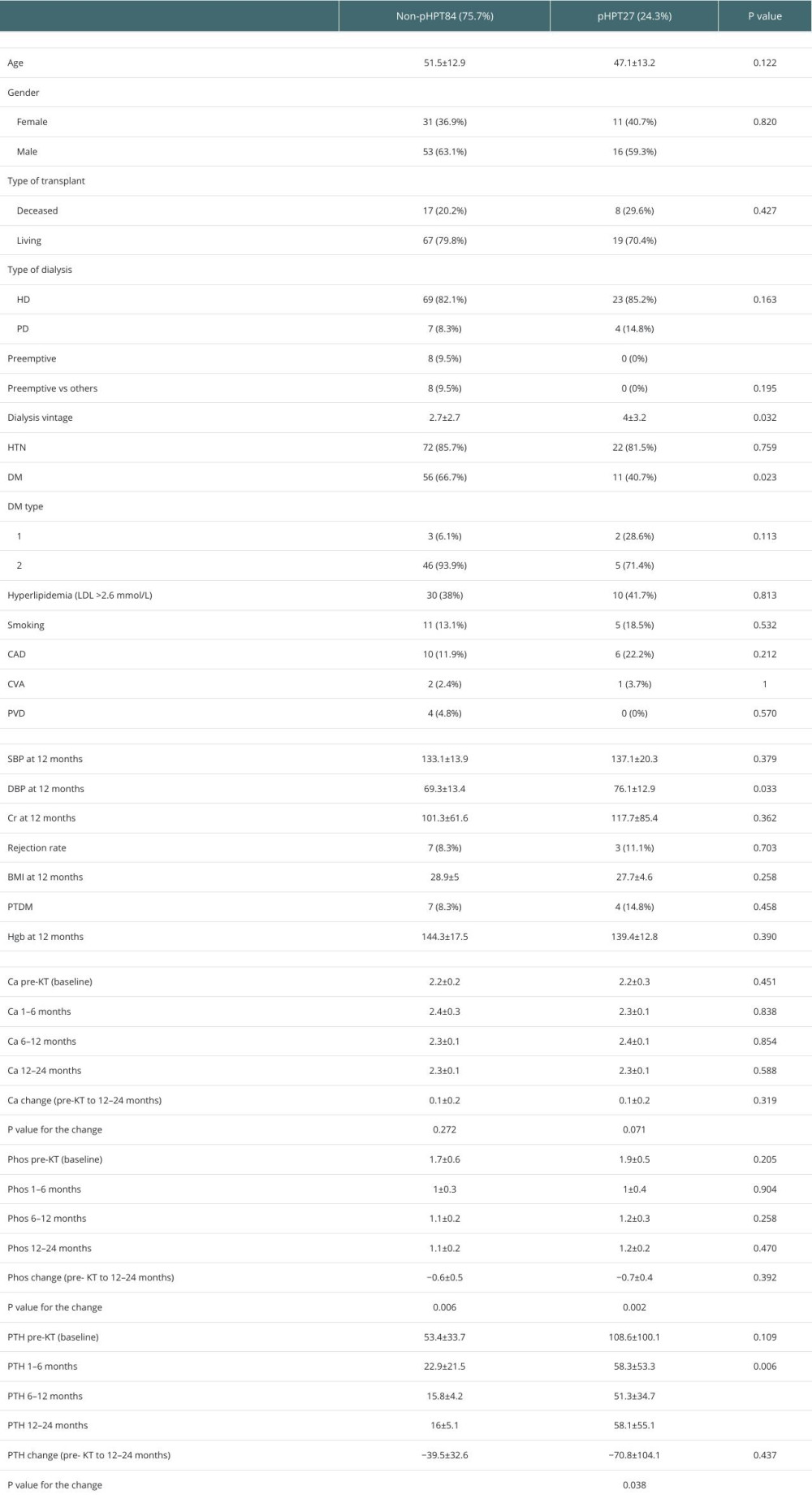 Table 3. Findings of pre-KT cardiac and vascular imaging in pHPT versus non-pHPT KTRs.
Table 3. Findings of pre-KT cardiac and vascular imaging in pHPT versus non-pHPT KTRs.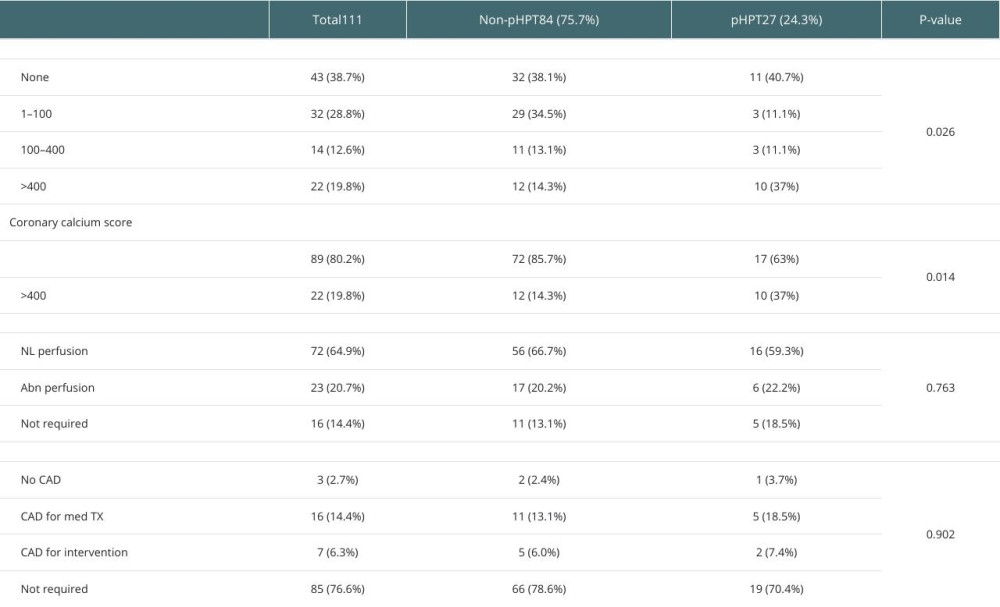 Table 4. Comparison of PTH changes post-KT according to CCS (CCS <400 vs CCS ≥400).
Table 4. Comparison of PTH changes post-KT according to CCS (CCS <400 vs CCS ≥400).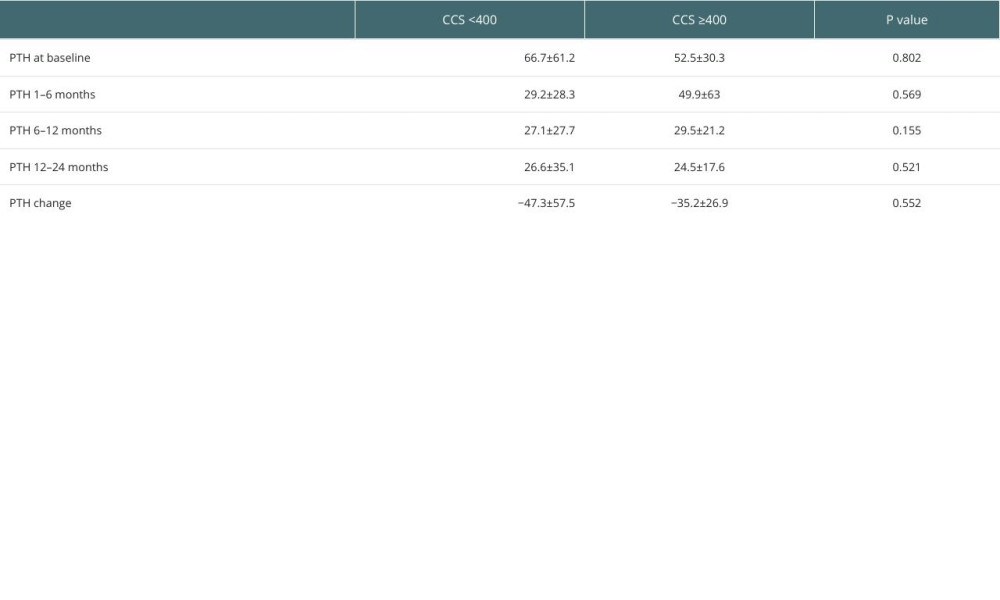 Table 5. Multivariate analyses of the associated factors with persistent hyperparathyroidism including coronary calcium scoring.
Table 5. Multivariate analyses of the associated factors with persistent hyperparathyroidism including coronary calcium scoring.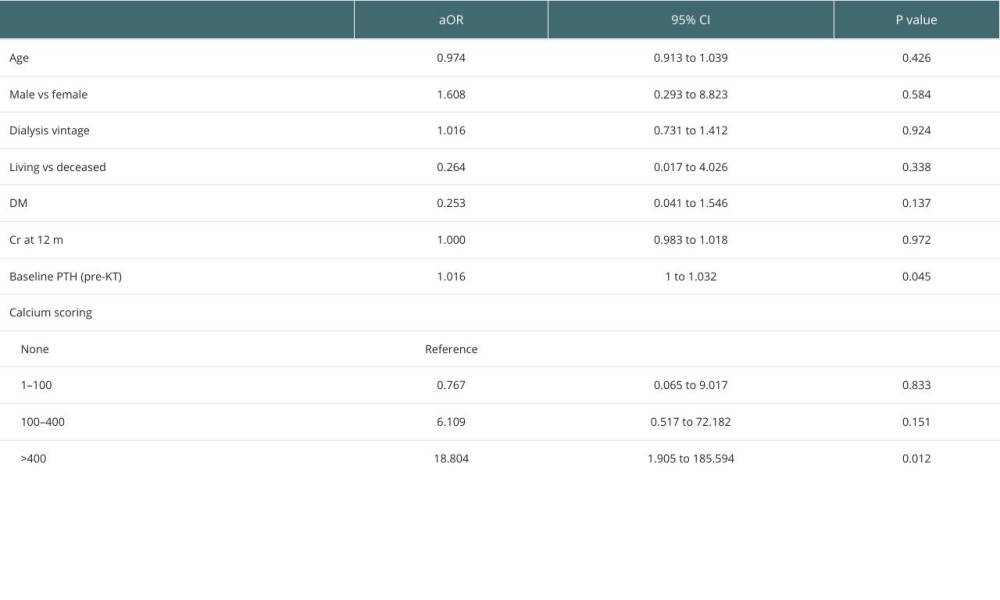
References
1. Block GA, Raggi P, Bellasi A, Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients: Kidney Int, 2007; 71(5); 438-41
2. Braun J, Oldendorf M, Moshage W, Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients: Am J Kidney Dis, 1996; 27(3); 394-401
3. Moe SM, Vascular calcification and renal osteodystrophy relationship in chronic kidney disease: Eur J Clin Invest, 2006; 36(s2); 51-62
4. Moe SM, Chen NX, Mechanisms of vascular calcification in chronic kidney disease: J Am Soc Nephrol, 2008; 19(2); 213-16
5. Reynolds JL, Joannides AJ, Skepper JN, Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD: J Am Soc Nephrol, 2004; 15(11); 2857-67
6. Braun J, Oldendorf M, Moshage W, Electron beam computed tomography in the evaluation of cardiac calcifications in chronic dialysis patients: Am J Kidney Dis, 1996; 27(3); 394-401
7. Sun T, Yu X, FGF23 Actions in CKD-MBD and other organs during CKD: Curr Med Chem, 2023; 30(7); 841-56
8. Lu X, Hu MC, Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease: Kidney Diseases, 2017; 3(1); 15-23
9. Westenfeld R, Jahnen-Dechent W, Ketteler M, Vascular calcification and fetuin-A deficiency in chronic kidney disease: Trends Cardiovasc Med, 2007; 17(4); 124-28
10. Bural GG, Torigian DA, Sözmen M, Comparison of atherosclerotic inflammation and calcification in subjects with end stage renal disease (ESRD) on hemodialysis to normal controls utilizing (18)F-FDG PET/CT: Hell J Nucl Med, 2018; 21(3); 169-74
11. Raggi P, Boulay A, Chasan-Taber S, Cardiac calcification in adult hemodialysis patients: A link between end-stage renal disease and cardiovascular disease?: J Am Coll Cardiol, 2002; 39(4); 695-701
12. Cianciolo G, Capelli I, Angelini ML, Importance of vascular calcification in kidney transplant recipients: Am J Nephrol, 2014; 39(5); 418-26
13. Winther S, Svensson M, Jørgensen HS, Prognostic value of risk factors, calcium score, coronary CTA, myocardial perfusion imaging, and invasive coronary angiography in kidney transplantation candidates: JACC Cardiovasc Imaging, 2018; 11(6); 842-54
14. Winther S, Svensson M, Jørgensen HS, Prognostic value of risk factors, calcium score, coronary CTA, myocardial perfusion imaging, and invasive coronary angiography in kidney transplantation candidates: JACC Cardiovasc Imaging, 2018; 11(6); 842-54
15. Jahromi A, Roozbeh J, Raiss-Jalali G-A, Risk factors of post renal transplant hyperparathyroidism: Saudi J Kidney Dis Transpl, 2009; 20(4); 573-76
16. Torres A, Rodríguez AP, Concepción MT, Parathyroid function in long-term renal transplant patients: Importance of pre-transplant PTH concentrations: Nephrol Dial Transplant, 1998; 13(Suppl 3); 94-97
17. Rivelli G, Lima ML, Mazzali M, Treatment of persistent hyperparathyroidism after renal transplant: Single center experience: Transplantation, 2018; 102; S641
18. Delos Santos R, Rossi A, Coyne D, Maw TT, Management of post-transplant hyperparathyroidism and bone disease: Drugs, 2019; 79(5); 501-13
19. Cruzado JM, Moreno P, Torregrosa JV, A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism: J Am Soc Nephrol, 2016; 27(8); 2487-94
20. Nakai K, Fujii H, Ishimura T, Incidence and risk factors of persistent hyperparathyroidism after kidney transplantation: Transplant Proc, 2017; 49(1); 53-56
21. Megahed Mo, Mahmoud MI, El-Deeb SA, Fouda MA, Coronary artery calcification in renal transplant recipients: Zagazig University Medical Journal, 2022; 28(5); 982-90
22. Arabi Z, Theaby A, Sibai A, Consensus guidelines of cardiovascular risk assessment in kidney transplantation in Saudi Arabia: Review of current practice, evidence, and recommendations: Saudi J Kidney Dis Transpl, 2020; 31(3); 655-75
23. Lentine KL, Costa SP, Weir MR, Cardiac disease evaluation and management among kidney and liver transplantation candidates: A scientific statement from the American Heart Association and the American College of Cardiology Foundation: endorsed by the American Society of Transplant Surgeons, American Society of Transplantation, and National Kidney Foundation: Circulation, 2012; 126(5); 617-63
24. Knez A, Becker C, Becker A, Determination of coronary calcium with multi-slice spiral computed tomography: A comparative study with electron-beam CT: Int J Cardiovasc Imaging, 2002; 18(4); 295-303
25. , What do the CT coronary artery calcium scores mean?: Myheartnet https://myheart.net/articles/ct-coronary-artery-calcium-heart-scan-the-facts/
26. : Cardiac CT for calcium scoring https://wwwradiologyinfoorg/en/info/ct_calscoring
27. Schenker MP, Dorbala S, Hong EC, Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study: Circulation, 2008; 117(13); 1693-700
28. Winther S, Svensson M, Jørgensen HS, Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates: JACC Cardiovasc Imaging, 2015; 8(5); 553-62
29. Sutton W, Canner JK, Shank JB, The impact of patient age on practice patterns and outcomes for primary hyperparathyroidism: Am J Surg, 2022; 224(1 Pt B); 400-7
30. Kirnap NG, Kirnap M, Sayin B, Risk factors and treatment options for persistent hyperparathyroidism after kidney transplantation: Transplant Proc, 2020; 52(1); 157-61
31. Yamamoto T, Tominaga Y, Okada M, Characteristics of persistent hyperparathyroidism after renal transplantation: World J Surg, 2016; 40(3); 600-6
32. Okada M, Sato T, Hasegawa Y, Persistent hyperparathyroidism after preemptive kidney transplantation: Clin Exp Nephrol, 2023; 27(10); 882-89
33. Alfieri C, Forzenigo L, Tripodi F, Long-term evaluation of coronary artery calcifications in kidney transplanted patients: A follow up of 5 years: Sci Rep, 2019; 9(1); 6869
34. Bubenicek P, Kautznerova D, Sotornik I, Coronary calcium score in renal transplant recipients: Nephron Clin Pract, 2009; 112(1); c1-8
35. Xiang X, He J, Zhang W, Coronary artery calcification in patients with advanced chronic kidney disease: BMC Cardiovasc Disord, 2022; 22(1); 453
36. Miedziaszczyk M, Lacka K, Tomczak O, Systematic review of the treatment of persistent hyperparathyroidism following kidney transplantation: Biomedicines, 2023; 11(1); 25
37. Raggi P, Chertow GM, Torres PU, The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis: Nephrol Dial Transplant, 2011; 26(4); 1327-39
38. Cianciolo G, Capelli I, Angelini ML, Importance of vascular calcification in kidney transplant recipients: Am J Nephrol, 2014; 39(5); 418-26
39. Messa P, Sindici C, Cannella G, Persistent secondary hyperparathyroidism after renal transplantation: Kidney Int, 1998; 54(5); 1704-13
40. London GM, Guérin AP, Marchais SJ, Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality: Nephrol Dial Transplant, 2003; 18(9); 1731-40
41. Nucifora G, Bax JJ, van Werkhoven JM, Coronary artery calcium scoring in cardiovascular risk assessment: Cardiovascular Therapeutics, 2011; 29(6); e43-e53
42. Schwarz U, Buzello M, Ritz E, Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure: Nephrol Dial Transplant, 2000; 15(2); 218-23
43. Haberl R, Becker A, Leber A, Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: Results of 1,764 patients: J Am Coll Cardiol, 2001; 37(2); 451-57
44. Nicoll R, Wiklund U, Zhao Y, Gender and age effects on risk factor-based prediction of coronary artery calcium in symptomatic patients: A Euro-CCAD study: Atherosclerosis, 2016; 252; 32-39
45. Oschatz E, Benesch T, Kodras K, Changes of coronary calcification after kidney transplantation: Am J Kidney Dis, 2006; 48(2); 307-13
46. Mazzaferro S, Pasquali M, Taggi F, Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation: Clin J Am Soc Nephrol, 2009; 4(3); 685-90
47. Schankel K, Robinson J, Bloom RD, Determinants of coronary artery calcification progression in renal transplant recipients: Am J Transplant, 2007; 7(9); 2158-64
Tables
 Table 1. Characteristics of kidney transplant recipients studied in our population.
Table 1. Characteristics of kidney transplant recipients studied in our population. Table 2. Characteristics of KTRs with pHPT vs non-pHPT.
Table 2. Characteristics of KTRs with pHPT vs non-pHPT. Table 3. Findings of pre-KT cardiac and vascular imaging in pHPT versus non-pHPT KTRs.
Table 3. Findings of pre-KT cardiac and vascular imaging in pHPT versus non-pHPT KTRs. Table 4. Comparison of PTH changes post-KT according to CCS (CCS <400 vs CCS ≥400).
Table 4. Comparison of PTH changes post-KT according to CCS (CCS <400 vs CCS ≥400). Table 5. Multivariate analyses of the associated factors with persistent hyperparathyroidism including coronary calcium scoring.
Table 5. Multivariate analyses of the associated factors with persistent hyperparathyroidism including coronary calcium scoring. Table 1. Characteristics of kidney transplant recipients studied in our population.
Table 1. Characteristics of kidney transplant recipients studied in our population. Table 2. Characteristics of KTRs with pHPT vs non-pHPT.
Table 2. Characteristics of KTRs with pHPT vs non-pHPT. Table 3. Findings of pre-KT cardiac and vascular imaging in pHPT versus non-pHPT KTRs.
Table 3. Findings of pre-KT cardiac and vascular imaging in pHPT versus non-pHPT KTRs. Table 4. Comparison of PTH changes post-KT according to CCS (CCS <400 vs CCS ≥400).
Table 4. Comparison of PTH changes post-KT according to CCS (CCS <400 vs CCS ≥400). Table 5. Multivariate analyses of the associated factors with persistent hyperparathyroidism including coronary calcium scoring.
Table 5. Multivariate analyses of the associated factors with persistent hyperparathyroidism including coronary calcium scoring. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860