08 September 2020: Original Paper
Prediction of Liver Prognosis from Pre-Transplant Renal Function Adjusted by Diuretics and Urinary Abnormalities in Adult-to-Adult Living Donor Liver Transplantation
Mineaki Kitamura1ABCDEFG*, Masaaki Hidaka2ABDE, Kumiko Muta1BC, Satoshi Miuma3BDE, Hisamitsu Miyaaki3CD, Mitsuhisa Takatsuki2D, Kazuhiko Nakao3D, Susumu Eguchi2DE, Hiroshi Mukae4D, Tomoya Nishino1ACDEDOI: 10.12659/AOT.924805
Ann Transplant 2020; 25:e924805
Abstract
BACKGROUND: Renal function is strongly associated with patient survival after liver transplantation. However, the estimated glomerular filtration rate (eGFR) after liver transplantation changes, especially in patients who receive diuretics or have urinary abnormalities. We aimed to elucidate how adjusting for these factors affecting eGFR predicted liver graft prognosis.
MATERIAL AND METHODS: This retrospective study included patients who underwent adult-to-adult living donor liver transplantation (LDLT) between 2000 and 2017. The factors affecting eGFR were assessed, and the association between eGFR and prognosis was investigated using Cox regression models after adjusting for factors affecting renal function.
RESULTS: We enrolled 244 patients. The median observation period was 4.6 years, and 88 patients reached graft loss or death with a functioning graft. One year after transplantation, 193 patients were living, and one-third of these showed improved eGFR; most of the patients with improved eGFR had taken diuretics before transplantation. A Cox regression model adjusted for the classical risk factors showed that donor age (P<0.001) and lower eGFR (P=0.02) were the independent risk factors associated with poor prognosis. After adjusting for diuretics and urinary abnormalities, eGFR was more strongly associated with liver graft prognosis (P=0.003).
CONCLUSIONS: Pre-transplant eGFR was associated with prognosis following LDLT and had a stronger effect on prognosis after adjusting for factors affecting eGFR.
Keywords: Diuretics, Glomerular Filtration Rate, Graft Survival, Liver Transplantation, Living Donors, End stage liver disease, Kidney
Background
Liver transplantation has allowed patients with end-stage liver failure to survive; therefore, long-term graft survival is important. Increasing evidence shows that several factors are associated with liver graft prognosis, including recipient age, donor age, prothrombin time, and serum creatinine [1]. To estimate the severity of disease, the model of end-stage liver disease (MELD) score was proposed, which includes serum creatinine, serum bilirubin, and prothrombin time. MELD scores are used to determine transplantation priority [2] and are associated with liver graft prognosis [3].
Hepatorenal syndrome (HRS) is observed in approximately 10% of liver transplantation recipients [4]; however, a larger proportion of transplant recipients develop ascites, one of the most common complications in patients with cirrhosis, affecting renal function [5]. Diuretics, such as spironolactone and furosemide, are prescribed to relieve ascites, but the use of these medications leads to the further deterioration of renal function due to decreased renal blood flow [5].
Some patients with liver cirrhosis have urinary abnormalities and may experience glomerulonephritis. Distinct from primary glomerulonephritis, secondary etiologies of glomerulonephritis such as hepatitis virus – associated glomerulonephritis and secondary IgA glomerulonephritis should be considered in cirrhosis patients [6,7].
The creatinine-based estimated glomerular filtration rate (eGFR) is the most commonly used method for evaluating kidney function in clinical practice. Although, studies have shown that cystatin C-based eGFR or GFR that is calculated based on clearance of either inulin or 125I-iothalamate are more accurate in evaluating renal function in liver transplant patients [8,9]. However, it is difficult to routinely evaluate renal function by these methods because of their cost, complexity, and time lag in obtaining results.
Since renal function has a great effect on patient survival and is expected to recover following liver transplantation, it is important to identify the factors associated with renal function that could be used to predict liver graft prognosis. However, since such factors are not yet directly associated with graft prognosis, few reports have focused on them.
We aimed to elucidate the significance of adjusting the factors affecting renal function in liver transplantation by investigating the association between kidney function and liver graft prognosis.
Material and methods
PATIENTS:
This was a retrospective observational study conducted at a single center. Patients who underwent adult-to-adult living donor liver transplantation (LDLT) aged ≥18 years at the Nagasaki University Hospital between January 1, 2000 and December 31, 2017 were included. The follow-up period was from January 1, 2000 to December 31, 2018. Patients aged <18 years and those from other countries were excluded because of the validity of the renal function evaluation method. We collected donor information and patient histories including the results of blood examinations performed immediately before LDLT. For patients on renal replacement therapy, creatinine levels recorded immediately before initiation of treatment were used. Results of urinary examinations within 1 month before LDLT were collected. Due to the retrospective design of this study, the Ethics Committee (Nagasaki University Hospital IRB) waived the need for informed consent.
SURGICAL PROCEDURES AND IMMUNOSUPPRESSION THERAPY:
Surgical procedures and immunosuppression protocols were as previously described [10]. Briefly, a left-lobe graft with the middle hepatic vein was selected if the graft volume/standard liver volume ratio (GV/SLV) was >30%. A right-lobe graft was used as an alternative if the left lobe was not suitable for donation. In ABO-incompatible LDLT cases, rituximab (375 mg/m2) was administered for induction 10–14 days before LDLT. Immunosuppression was achieved with tacrolimus, cyclosporine, mycophenolate mofetil, and steroids. Steroids were gradually tapered and discontinued within 3 months after LDLT.
RENAL FUNCTION:
The definition of HRS was based on the revised classification system of the International Ascites Club [11]. Chronic kidney disease (CKD) was defined as more than 3 months of continuous deterioration of renal function with an eGFR <60 mL/min/1.73 m2 [12]. Since the results of urine examinations performed at other medical facilities were not available in all cases, we focused on the eGFR only. The Modification of Diet in Renal Disease equation adapted for Japanese patients was used [13]. The percent change in eGFR (%eGFR) was calculated based on the eGFR reported before transplantation. Patients were expected to visit our hospital on a monthly or bi-monthly basis after discharge, and their renal functions were evaluated based on blood examination results.
STATISTICAL ANALYSIS:
Based on the study design, only data before or at transplantation were included. Categorical variables were expressed with numbers and percentages, and continuous values were expressed as means±standard deviations (SD); if data were not normally distributed, median values with interquartile ranges were calculated. The Wilcoxon rank-sum test was used to analyze continuous variables, and the chi-square test was used to analyze categorical variables.
The primary endpoints were graft loss and death with a functioning graft. For survival analyses, the Log-rank test was used, and a post hoc comparison was performed by the Bonferroni method for multiple comparisons. Survival and Cox multivariate regression analyses were performed. The covariates for the Cox multivariate regression analysis were selected based on the clinical significance for transplanted liver prognosis and renal function. Three models for Cox hazard regression analyses were developed: Model 1, adjusted for recipient age and sex, donor age, presence of pre-transplant diabetes and/or hypertension, GV/SLV, and eGFR; Model 2 (focusing more on liver function), in which hepatitis C virus (HCV) and bilirubin were added to the variables from Model 1; and Model 3 (focusing more on factors associated with renal function), in which the presence of pre-transplant diuretics and/or urinary abnormalities (urinary protein >1+, hematuria >1+ on qualitative tests) were added to the variables from Model 1. Statistical analyses were performed using the JMP 13 software (SAS Institute Inc, Cary, NC, USA).
Results
PATIENT BACKGROUND INFORMATION:
A total of 272 patients underwent liver transplantation. However, 28 patients were excluded because of young age, deceased donor (1 patient received liver and renal transplantation simultaneously), or overseas status (1 patient was from overseas). Therefore, 244 patients were analyzed (age at transplantation, 54.4±10.8 years; 141 men and 103 women; Figure 1).
The median observation period was 1689 days (interquartile range: 533–3346; Table 1). Of the 244 patients, 88 reached the endpoint and 192 maintained transplanted liver function for 1 year (Figure 1).
Patient history information is shown in Table 1. There were significant differences in donor age, pre-transplant hypertension, pre-existent CKD, HRS, pre-transplant albumin and creatinine, and proteinuria between patients who survived and those who lost the grafts. Among patients who had an eGFR <60 mL/min/1.73 m2 (n=93, 38%) just before transplantation, only 57 (24% of total patients) were categorized as having CKD.
RENAL FUNCTION CHANGES BEFORE AND AFTER LIVER TRANSPLANTATION:
We compared patients whose renal function improved with patients whose renal function deteriorated among those with transplanted liver function that was maintained for more than 1 year (n=192) (1 patient on maintenance hemodialysis was excluded). Mean eGFR decreased before and 1 year after transplantation in these patients from 76.1 mL/min/1.73 m2 to 65.0 mL/min/1.73 m2 (P<0.001), and the mean %eGFR change was −0.9 (Figure 2A, 2B). Renal function had improved 1 year after transplantation in 65 of the patients (about one-third) from 52.1±21.6 mL/min/1.73 m2 to 71.4±24.7 mL/min/1.73 m2; while deterioration was observed in the remaining 127 patients: mean eGFR decreased from 88.9±62.2 mL/min/1.73 m2 to 62.2±18.5 mL/min/1.73 m2 (Table 2).
Patients taking diuretics (63%) had significantly increased %eGFR compared to those not taking diuretics (37%; P=0.003), but the %eGFR of patients with urinary abnormalities (25%) did not differ significantly compared to that of those without urinary abnormalities (75%; P=0.06; Figure 2B).
We then compared patient factors between patients who had increased eGFR and those who did not. Among several parameters, pre-transplant CKD, pre-transplant hemoglobin level, and use of diuretics were significantly different between the 2 groups (Table 2).
MULTIVARIATE ANALYSES OF RENAL FUNCTION BEFORE AND 1 YEAR AFTER LIVER TRANSPLANTATION:
We constructed multivariate regression models based on patients who maintained transplanted liver function for more than 1 year. Patient age (P<0.001), body weight (P=0.02), use of diuretics (P=0.02), and pre-transplant hemoglobin level (P<0.001) were associated with pre-transplant renal function (Table 3).
With respect to renal function 1 year after liver transplantation, patient age (P<0.001), pre-transplant hemoglobin level (P=0.009), and pre-transplant albumin level (P=0.02) were associated with renal function (Table 3).
SURVIVAL ANALYSES ACCORDING TO RENAL FUNCTION:
As seen in Figure 3, the prognosis of patients who had an eGFR <30 mL/min/1.73 m2 was significantly lower than that of those who had an eGFR >60 mL/min/1.73 m2 (P<0.001). There was no significant difference between an eGFR >60 mL/min/1.73 m2 and an eGFR of 30–60 mL/min/1.73 m2.
MULTIVARIATE COX REGRESSION ANALYSES:
We performed Cox regression analyses using the 3 models to elucidate the factors associated with liver prognosis. Model 1 showed that donor age (per 10 years; hazard ratio (HR), 1.42; P<0.001) and lower eGFR (per 10 mL/min; HR, 0.90; P=0.01) were independent risk factors for poor prognosis. Model 2 also showed that renal function and donor age were associated with liver prognosis. Model 3 was adjusted for factors associated with pre-transplant renal function and yielded the most significant association between eGFR and liver prognosis among the 3 models (HR, 0.86; P=0.003; Table 4).
Discussion
We elucidated the association between eGFR and graft survival after LDLT and evaluated renal function change after transplantation. Notably, multiple Cox regression analyses showed that, after adjusting for diuretic use and urinary abnormalities, eGFR was more closely associated with liver graft prognosis.
Some patients with liver dysfunction can expect their renal function to improve after liver transplantation [14]. In the present study, about one-third of patients experienced an improvement in renal function 1 year after liver transplantation, and these patients tended to take prescribed diuretics. The commonly applied International Ascites Club definition of HRS uses serum creatinine cut-off levels of 2.5 mg/dL for type 1 HRS and 1.5 mg/dL for type 2 HRS [11]. The prevalence of HRS was approximately 10% in this study, similar to the results of a previous report [4]; however, more than half of the patients in the present study had ascites and were treated with diuretics, which worsened their renal function by decreasing renal blood flow.
Although this study focused on pre-transplant factors, the perception of post-transplant acute kidney injury (AKI) and CKD are crucial since pre- and post-renal functions are associated with liver graft prognosis. One of the most important etiologies for AKI was sepsis, and patients who experienced post-transplant AKI had poor prognosis [15]. The development of post-transplant CKD is one of the most important issues in liver transplantation, and it may be caused by calcineurin inhibitors and complications such as infection [15]. Patients in our study whose renal function did not improve after transplantation could have been affected by post-transplant factors.
A previous report suggested that the initial evaluation of renal function for nonrenal solid organ transplantation should include the assessment of prior kidney function, measurement of current kidney function, urinalysis, and imaging studies of the kidneys [16]. Since eGFR based on serum creatinine levels will be overestimated in patients with end-stage liver disease due to sarcopenia, some reports have proposed that using cystatin C for assessment would be more useful for predicting prognosis [8]. However, multiple measurements of cystatin C are not feasible in clinical practice.
In this study, we collected information on renal function within 3 months before transplantation to distinguish between deterioration due to CKD and AKI. However, patients who met the CKD criteria also tended to have improved renal function after liver transplantation. Urinary abnormalities should also be evaluated in liver transplant patients as some patients may experience hepatic glomerulosclerosis [11], and loss of IgA clearance from the hepatic portal system may lead to secondary glomerulonephritis, the clinical characteristics of which are similar to those of IgA nephropathy [7]. Moreover, hepatitis B virus and HCV infection can lead to secondary glomerulonephritis [6]. In addition, malignancies can affect renal function via proteinuria or other factors; thus, liver transplantation has been performed to treat hepatocellular carcinoma [17]. In this study, %eGFR in patients with urinary abnormalities before and after liver transplantation differed from that of those without urinary abnormalities, but the difference was not statistically significant (
Although the mechanisms of the association between renal function and transplant liver prognosis remain unclear, there are some possible explanations. Deterioration of renal function would reflect arteriosclerosis in liver transplant patients. Arteriosclerosis can affect the vascular anastomoses during liver transplantation, and the prevalence of cardiovascular complications in patients with CKD is known to be higher than other complications, which may affect prognosis [18]. It is well known that immune system function is attenuated by uremic toxin; therefore, patients with renal impairment are likely to be at risk of infections such as sepsis and pneumonia [19]. Moreover, renal function is known to be associated with malnutrition, inflammation, and arteriosclerosis, all of which may contribute to a poorer prognosis [20].
We focused on renal function in this study; however, several factors should be considered when assessing transplanted liver function. For example, pre-transplant prothrombin time and lower pre-transplant platelet levels were associated with early graft dysfunction [21]. Donor factors are important for LDLT, and donor complications should be taken into account [22]. In this study, donor age was also strongly associated with liver prognosis. Older donors may be acceptable in some circumstances [23], but there were significant differences in recipient survival between older and younger donors [24].
In our previous study, the outcomes of recipients from donors older than 50 years were inferior to those of recipients from younger donors [10]. The precise mechanisms of how donor age affects recipient prognosis should be investigated; it is likely, however, that age-related liver morphological change, endothelial dysfunction, and a lower number of Kupffer cells may be related to delayed liver regeneration and increased risk of infection [10]. Considering the association between donor age and recipient prognosis, recipients with poor kidney function should not be matched with older donors so that the risk of poor prognosis is not increased.
There are several limitations of this study. First, as the study was designed to predict prognosis based on pre-transplant parameters, the post-transplant factors were not taken into consideration, such as trough levels of calcineurin inhibitors and infections. Second, this study was conducted at a single center, with only Japanese patients. Moreover, the study period was long, and, therefore, the findings may not apply to different populations. Third, continuous urinalysis was not performed because a post-transplant urinary examination was not possible in all cases. Fourth, combinations of diuretics were prescribed; for example, spironolactone and furosemide tended to be administered simultaneously. Therefore, the specific effects of different diuretics could not be evaluated. Fifth, the decision-making process regarding treatment for hepatorenal syndrome lacked consistency because almost all the patients were transferred from other facilities, and the condition for liver transplantation varied depending on the case.
This study also had several strengths. First, we limited the analysis to LDLTs to address the scarcity of studies in this area. The association between renal function and liver prognosis in deceased donor liver transplantation has been well investigated [3], but few researchers have investigated the association between renal function and liver prognosis in LDLT. Second, the average observation periods were longer than those in previous studies. Third, we analyzed factors that affect kidney function, which could allow future assessments to more precisely adjust for the identified prerenal factors. Our results showed that, when adjusted for diuretics and urinary abnormalities, eGFR had a larger effect on liver prognosis, suggesting that renal function itself affects liver prognosis.
Conclusions
Pre-transplant renal function predicted transplanted liver function quite well. Renal function was affected by several factors and could be improved by liver transplantation. Therefore, renal function should be evaluated after adjusting for reversible factors associated with renal function, such as the use of diuretics.
Figures
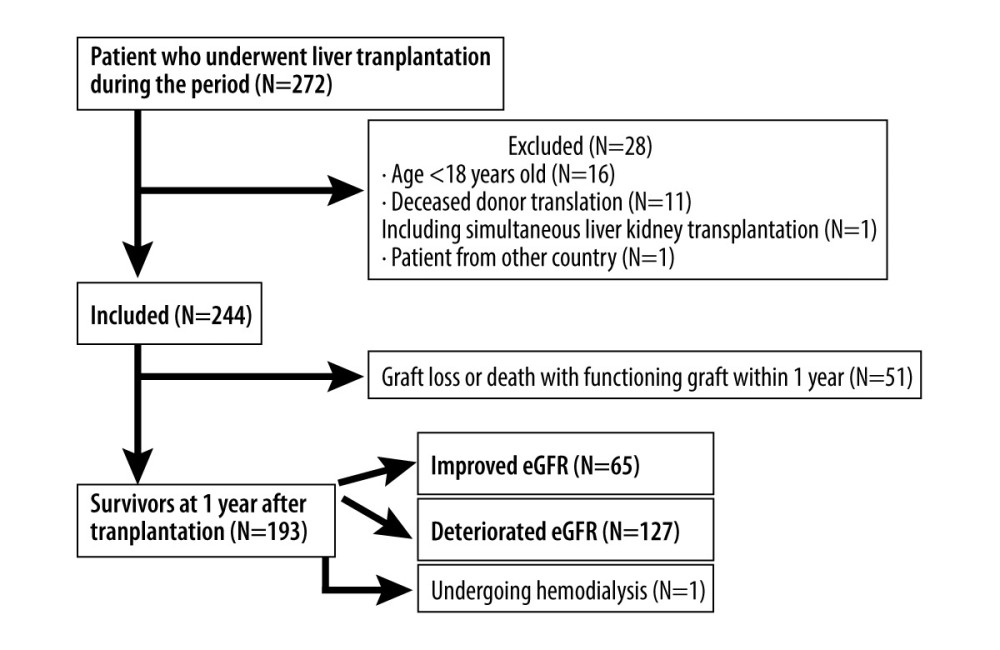 Figure 1. Patient flow chart
Figure 1. Patient flow chart 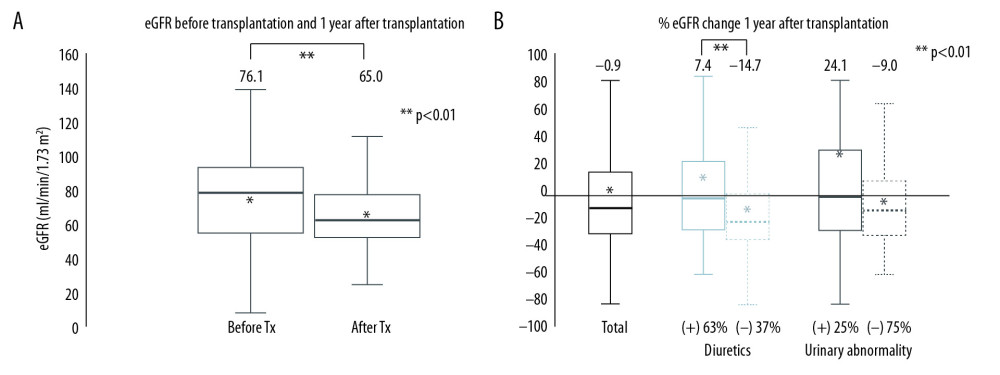 Figure 2. (A) estimated glomerular filtration rate (eGFR) change before and 1 year after living donor liver transplantation. (B) Percent change in GFR (%eGFR) (before and 1 year after transplantation): total patients with or without diuretics, and patients with or without urinary abnormalities. The proportions of each group are shown at the bottom of the figure. ** P<0.01.
Figure 2. (A) estimated glomerular filtration rate (eGFR) change before and 1 year after living donor liver transplantation. (B) Percent change in GFR (%eGFR) (before and 1 year after transplantation): total patients with or without diuretics, and patients with or without urinary abnormalities. The proportions of each group are shown at the bottom of the figure. ** P<0.01. 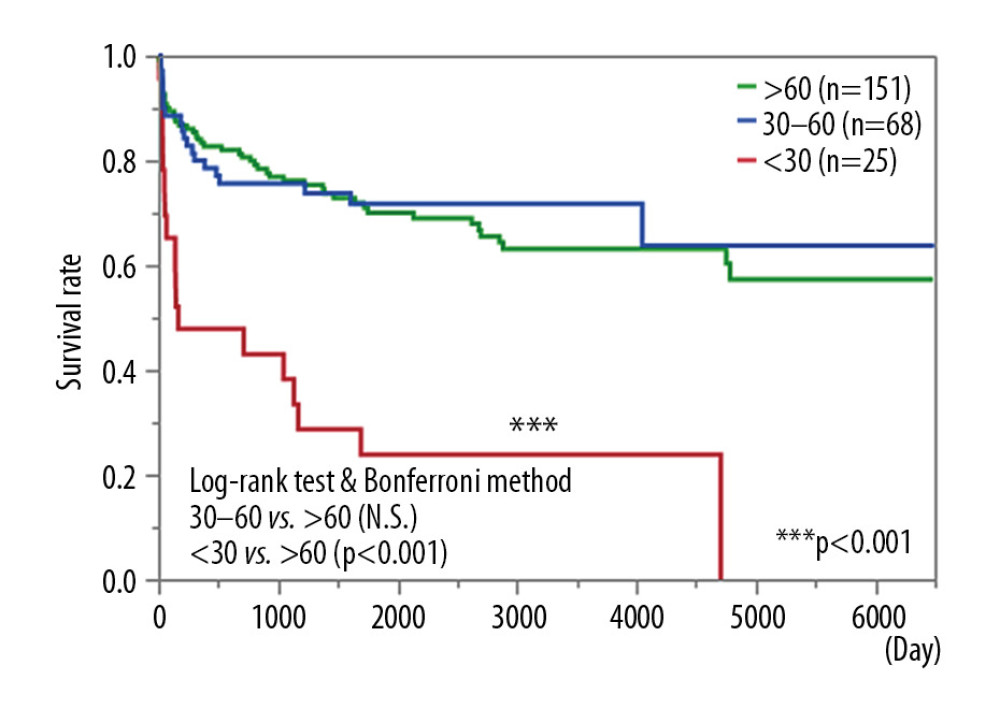 Figure 3. Survival curve of patients according to estimated glomerular filtration rate (eGFR): eGFR >60 mL/min/1.73 m2; 30≤ eGFR <60 mL/min/1.73 m2; eGFR <30 mL/min/1.73 m2 or on renal replacement therapy. *** P<0.001.
Figure 3. Survival curve of patients according to estimated glomerular filtration rate (eGFR): eGFR >60 mL/min/1.73 m2; 30≤ eGFR <60 mL/min/1.73 m2; eGFR <30 mL/min/1.73 m2 or on renal replacement therapy. *** P<0.001. Tables
Table 1. Demographic data of liver transplantation recipients according to graft survival.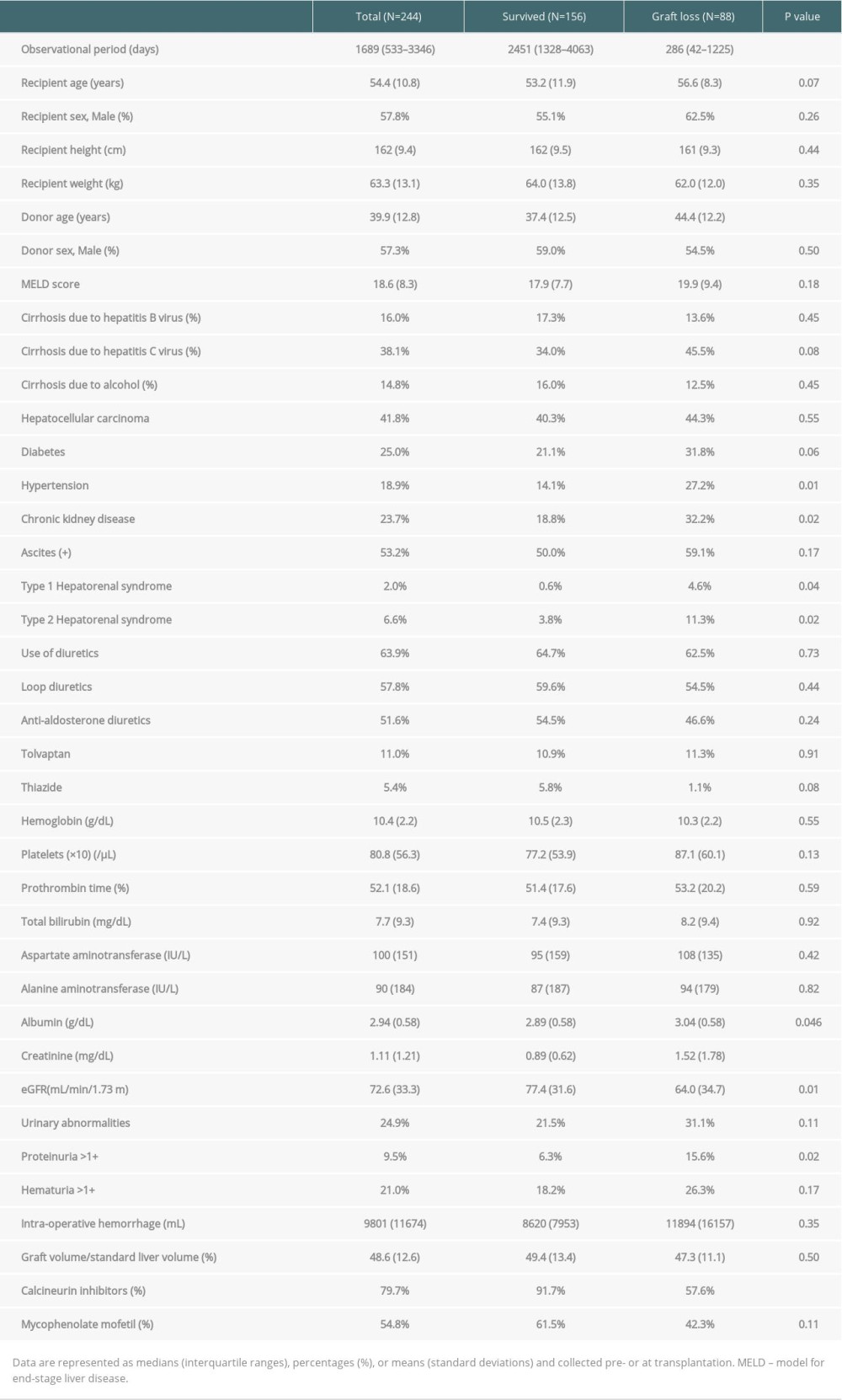 Table 2. Patient characteristics according to change in renal function.
Table 2. Patient characteristics according to change in renal function.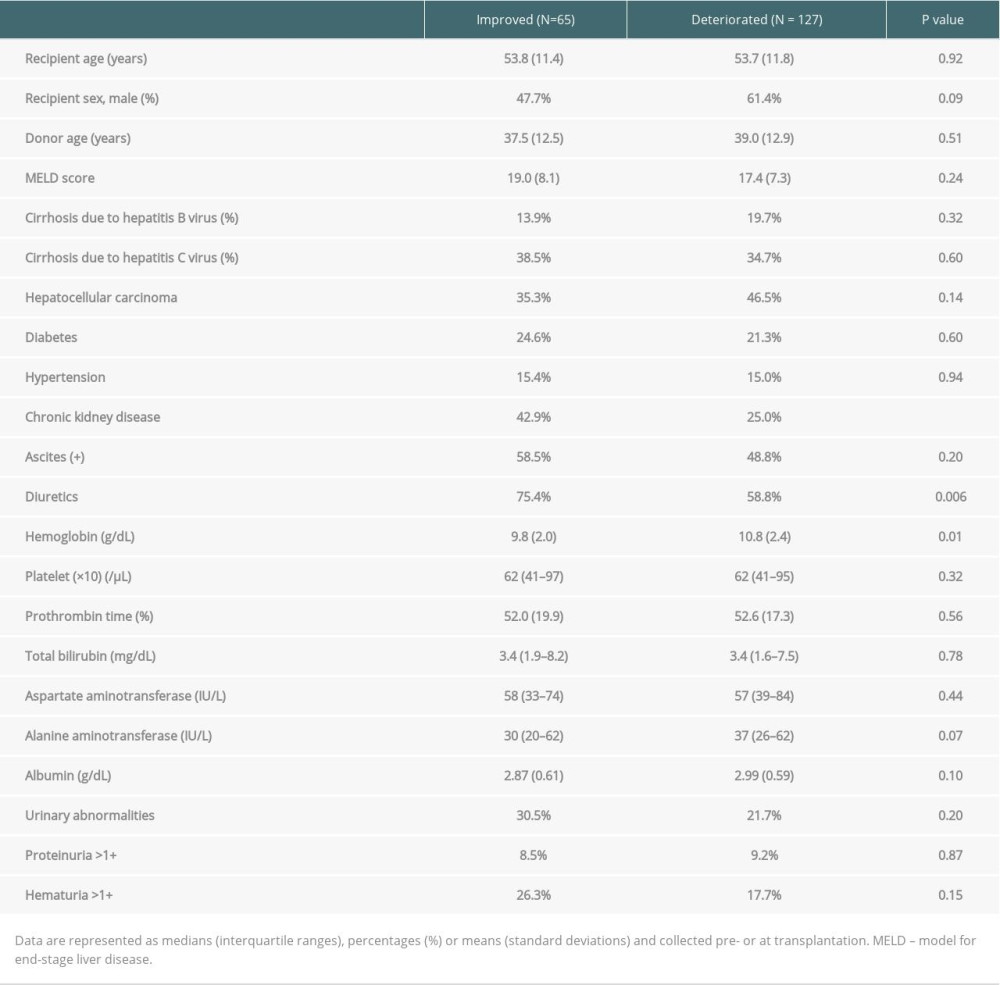 Table 3. Multiple linear regression analysis models of eGFR immediately before and one year after transplantation.
Table 3. Multiple linear regression analysis models of eGFR immediately before and one year after transplantation.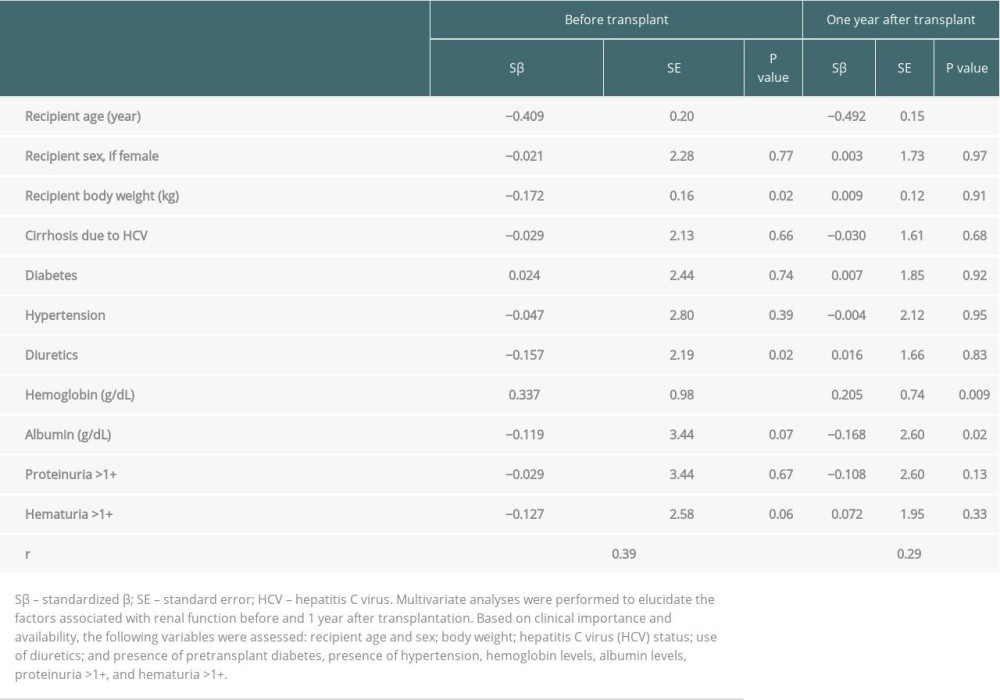 Table 4. Multiple Cox regression analysis of liver transplant prognostic factors.
Table 4. Multiple Cox regression analysis of liver transplant prognostic factors.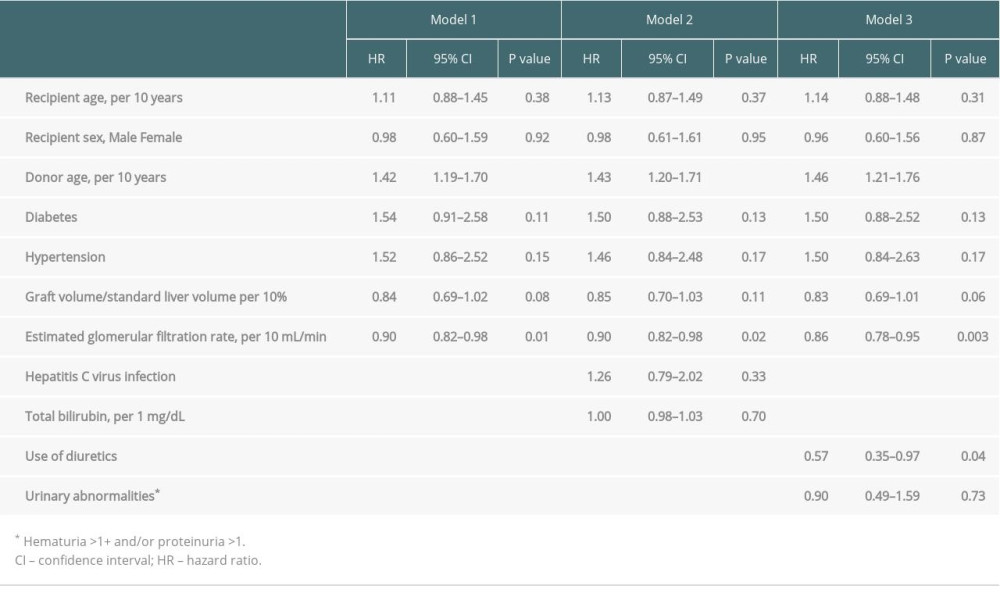
References
1. D’Amico G, Garcia-Tsao G, Pagliaro L, Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies: J Hepatol, 2006; 44; 217-31
2. Sharma P, Schaubel DE, Guidinger MK, Impact of MELD-based allocation on end-stage renal disease after liver transplantation: Am J Transplant, 2011; 11; 2372-78
3. Gonwa TA, McBride MA, Anderson K, Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: Where will MELD lead us?: Am J Transplant, 2006; 6; 2651-59
4. Okamura Y, Hata K, Inamoto O, Influence of hepatorenal syndrome on outcome of living donor liver transplantation: A single-center experience in 357 patients: Hepatol Res, 2017; 47; 425-34
5. Ginès P, Schrier RW, AKI – renal failure in cirrhosis: N Engl J Med, 2009; 13361; 1279-90
6. Kupin WL, Viral-associated GN hepatitis B and other viral infections: Clin J Am Soc Nephrol, 2017; 12; 1529-33
7. Saha MK, Julian BA, Novak J, Rizk DV, Secondary IgA nephropathy: Kidney Int, 2018; 94; 1-8
8. De Souza V, Hadj-Aissa A, Dolomanova O, Creatinine-versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis: Hepatology, 2014; 59; 1522-31
9. Uguen T, Jezequel C, Ropert M, Pretransplant renal function according to CKD-EPI cystatin C equation is a prognostic factor of death after liver transplantation: Liver Int, 2016; 36; 547-54
10. Hidaka M, Eguchi S, Takatsuki M, The Kupffer cell number affects the outcome of living donor liver transplantation from elderly donors: Transplant Direct, 2016; 2; e94
11. Wong F, Nadim MK, Kellum JA, Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis: Gut, 2011; 60; 702-9
12. , Chapter 1: Definition and classification of CKD: Kidney Int Suppl, 2013; 3; 19-62
13. Imai E, Horio M, Nitta K, Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease: Clin Exp Nephrol, 2007; 11; 41-50
14. Sharma P, Goodrich NP, Zhang M, Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone: Clin J Am Soc Nephrol, 2013; 8; 1135-42
15. Inoue Y, Soyama A, Takatsuki M, Does the development of chronic kidney disease and acute kidney injury affect the prognosis after living donor liver transplantation?: Clin Transplant, 2016; 30; 518-27
16. Bloom RD, Reese PP, Chronic kidney disease after nonrenal solid-organ transplantation: J Am Soc Nephrol, 2007; 18; 3031-41
17. Vitale A, Boccagni P, Brolese A, Progression of hepatocellular carcinoma before liver transplantation: dropout or liver transplantation?: Transplant Proc, 2009; 41; 1264-67
18. Raval Z, Harinstein ME, Skaro AI, Cardiovascular risk assessment of the liver transplant candidate: J Am Coll Cardiol, 2011; 58; 223-31
19. Kato S, Chmielewski M, Honda H, Aspects of immune dysfunction in end-stage renal disease: Clin J Am Soc Nephrol, 2008; 3; 1526-33
20. Stenvinkel P, Heimbürger O, Lindholm B, Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome): Nephrol Dial Transplant, 2000; 15; 953-60
21. Gruttadauria S, Di Francesco F, Vizzini GB, Early graft dysfunction following adult-to-adult living-related liver transplantation: Predictive factors and outcomes: World J Gastroenterol, 2009; 15; 4556-60
22. Gruttadauria S, Pagano D, Cintorino D, Right hepatic lobe living donation: A 12 years single Italian center experience: World J Gastroenterol, 2013; 19; 6353-59
23. Nardo B, Masetti M, Urbani L, Liver transplantation from donors aged 80 years and over: Pushing the limit: Am J Transplant, 2004; 4; 1139-47
24. Umeshita K, Inomata Y, Furukawa H, Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society: Hepatol Res, 2016; 46; 1171-86
Figures
 Figure 1. Patient flow chart
Figure 1. Patient flow chart Figure 2. (A) estimated glomerular filtration rate (eGFR) change before and 1 year after living donor liver transplantation. (B) Percent change in GFR (%eGFR) (before and 1 year after transplantation): total patients with or without diuretics, and patients with or without urinary abnormalities. The proportions of each group are shown at the bottom of the figure. ** P<0.01.
Figure 2. (A) estimated glomerular filtration rate (eGFR) change before and 1 year after living donor liver transplantation. (B) Percent change in GFR (%eGFR) (before and 1 year after transplantation): total patients with or without diuretics, and patients with or without urinary abnormalities. The proportions of each group are shown at the bottom of the figure. ** P<0.01. Figure 3. Survival curve of patients according to estimated glomerular filtration rate (eGFR): eGFR >60 mL/min/1.73 m2; 30≤ eGFR <60 mL/min/1.73 m2; eGFR <30 mL/min/1.73 m2 or on renal replacement therapy. *** P<0.001.
Figure 3. Survival curve of patients according to estimated glomerular filtration rate (eGFR): eGFR >60 mL/min/1.73 m2; 30≤ eGFR <60 mL/min/1.73 m2; eGFR <30 mL/min/1.73 m2 or on renal replacement therapy. *** P<0.001. Tables
 Table 1. Demographic data of liver transplantation recipients according to graft survival.
Table 1. Demographic data of liver transplantation recipients according to graft survival. Table 2. Patient characteristics according to change in renal function.
Table 2. Patient characteristics according to change in renal function. Table 3. Multiple linear regression analysis models of eGFR immediately before and one year after transplantation.
Table 3. Multiple linear regression analysis models of eGFR immediately before and one year after transplantation. Table 4. Multiple Cox regression analysis of liver transplant prognostic factors.
Table 4. Multiple Cox regression analysis of liver transplant prognostic factors. Table 1. Demographic data of liver transplantation recipients according to graft survival.
Table 1. Demographic data of liver transplantation recipients according to graft survival. Table 2. Patient characteristics according to change in renal function.
Table 2. Patient characteristics according to change in renal function. Table 3. Multiple linear regression analysis models of eGFR immediately before and one year after transplantation.
Table 3. Multiple linear regression analysis models of eGFR immediately before and one year after transplantation. Table 4. Multiple Cox regression analysis of liver transplant prognostic factors.
Table 4. Multiple Cox regression analysis of liver transplant prognostic factors. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








