04 August 2020: Original Paper
Inferior Vena Cava Constriction After Liver Transplantation Is a Severe Complication Requiring Individually Adapted Treatment: Report of a Single-Center Experience
Jan-Paul Gundlach1CDEF*, Rainer Günther2B, Marcus Both3BD, Jens Trentmann3BD, Jost Philipp Schäfer3BCD, Jochen T. Cremer4B, Christoph Röcken5BDE, Thomas Becker1DEG, Felix Braun1ABD, Alexander Bernsmeier1CDEDOI: 10.12659/AOT.925194
Ann Transplant 2020; 25:e925194
Abstract
BACKGROUND: Reports on vena cava occlusion after liver transplantation (LT) are rare, but this finding represents a severe complication in the early postoperative period. In the context of the complex presentation of a patient after LT, symptoms are often misinterpreted and can be subtle.
MATERIAL AND METHODS: In our cohort of 138 LTs performed between 2014 and 2017 at our University’s Transplantation Department, 117 transplantations were valid for further analysis after exclusion of pediatric transplantations and transplants with primary non-function grafts. In 101 cases (73%), patients received a deceased-donor full-size organ. Living-donor LT was performed in 8 patients (6.4%) and 8 patients (6.4%) received a split graft. We report on 6 patients who had inferior vena cava (IVC) occlusion and summarize the treatment choices.
RESULTS: In our series, patients with positive findings (age 38–70 years) received an orthotopic full-size deceased-donor graft with end-to-end IVC anastomosis. In the subsequent period, imaging revealing IVC occlusion was done on a follow-up basis (n=2), due to dyspnea (n=1), and for progressive ascites (n=2). In 3 cases, a thrombus was found. We give detailed information on our treatment options from interventional treatment to transcardial thrombus removal and anastomosis augmentation.
CONCLUSIONS: IVC constriction and subsequent thrombosis are severe complications after LT that require individually adapted treatment in specialized centers. Since patients often present with subclinical symptoms, vascular diagnosis should be performed early to detect caval anastomosis pathologies. Despite regular ultrasonography, we favor CT and cavography for subsequent quantification. We also review the literature on IVC occlusion after LT.
Keywords: Anastomosis, Surgical, Angioplasty, Balloon, Liver Transplantation, Thrombosis, Vena Cava, Inferior, Constriction, Pathologic, Vascular Diseases, Venous Thrombosis
Background
Although vascular difficulties following LT are infrequent, they are serious complications with a high incidence of both graft loss and mortality [1]. Their incidence remains around 7% after LT in various series [2,3]. While inferior vena cava (IVC) obstructions are expectable complications after LT, they are rare, as is their scientific assessment. Apart from bleeding complications in the early postoperative setting, stenosis and thrombosis are the main problems. End-to-end caval-cavostomy is a widely accepted standard technique for caval anastomosis. The later-introduced piggyback technique (partial clamping of the cava) has become an accepted alternative and is mandatory in living-donor liver transplantation (LDLT) [4]. In the context of the complex presentation of a patient after LT, symptoms are often misinterpreted and can be subtle – ascites [5,6], early graft dysfunction, reduced portal venous (PV) flow, decreased renal function [7], lower venous congestion, and allograft dysfunction can result. IVC stenoses can be divided into early and late stenoses after LT. Acute stenoses, which are mostly caused by technical complications, occur due to constriction by a swollen anastomosis (e.g., edema), extravascular compression (e.g., hematoma), or kinking by a rotated organ [1,8]. Secondary stenosis can be caused by neointimal hyperplasia, fibrosis [9], thrombosis, or extravascular compression from edema, hematoma, or localized ascites [10,11]. The causes of thrombosis are unclear, although, stasis due to mechanical restriction seems predominant. In the literature, reports on thrombosis are infrequent (Table 1). IVC complications remain a rare finding, diagnosed in less than 1% of the transplanted cases (Table 1) [2, 6]. In the present article, we discuss our insights on a variety of therapeutic options and present our own data on IVC complications after LT, with special emphasis on thrombotic problems.
Material and Methods
PERIOPERATIVE SETUP:
Caval anastomoses were sewn with an Optilene© 4/0 suture (B. Braun AG, Melsungen, Germany) in everting end-to-end technique for full-size grafts or via performing a unification venoplasty for reconstruction of the inferior right hepatic vein in case of right-lobe transplantation.
All patients received standard immunosuppression with tacrolimus taper scheme (tacrolimus through 4–6 ng/ml); cortisone (early postoperative dosage of 20 mg prednisolone daily) and Basiliximab 20 mg at day 0 and day 4 after transplant. Additionally, all patients received anti-infective treatment; standard antibiosis consisted of cefotaxime and metronidazole, with amphotericin B and cotrimoxazole prophylaxis as well as valganciclovir in the case of a CMV-positive donor and CMV-negative recipient.
IMAGING PROCEDURES:
Postoperatively, Doppler ultrasound was performed 3 times daily in the early postoperative period and at any point of the periodically conducted consultation in our outpatient clinic, followed by CT scanning in case of suspicious findings to avoid interobserver variation. IVC constrictions were quantified using cavography. Patients were seen quarterly in the first year after LT. The mean follow-up duration was 21.5 (±15.3) months, with a maximum of 49 months.
Results
We analyzed 117 transplantations for occurrence of vascular complications. Patient age ranged from 17 to 77 years, including 32 women and 85 men. Major indications for LT were alcohol-induced cirrhosis (42.7%), primary sclerosing cholangitis (13.6%), hepatocellular carcinoma (44.4%), chronic viral hepatitis (27.2%), and cryptogenic cirrhosis (4.5%). The patients were listed to Eurotransplant and received an organ within 14 days to 11 years after listing (mean 348±672 days). Mean cold ischemic time was 554.8 min. Re-transplantation within the same hospital stay was done in 8 cases (6.4%), and in 5 other cases (4.0%) within the time of the study, with a total of 16 (12.8%) re-transplantations, respectively. Full-size LT was carried out in 101 cases (73.2%). Living-donor liver transplantation (LDLT) was performed in 8 patients (6.4%, all right-lobe) and 8 patients received a split graft (3 patients: right-lobe, 5 patients: extended right-lobe). The bile duct was connected in end-to-end technique in 105 cases (76.1%) and a biliodigestive anastomosis was performed in 12 cases (10.3%). After 1 and 2 years, 80.3% (94/117) and 74.4% (87/117), respectively, of the patients were alive. Twelve patients (10.3%) died during the hospital stay.
We present detailed information on our patients with IVC abnormalities. Table 2 summarizes the clinical characteristics of our patients with a positive IVC occlusion. In the thorough LT follow-up carried out from 2014 until 2019, we found 3 cases of IVC thrombosis (Table 2, cases # 1–3; Figure 1) as well as 3 cases of manifest or occult (n=1) caval stenosis (Table 2, cases # 4–6). All transplantations were full-size grafts with duct-to-duct biliary anastomoses. Transplantation was successfully performed in all cases, although 2 cases (33%) needed re-transplantation within the same hospital stay due to primary non-function of the transplant. We analyzed whether time of transplantation or surgeon’s experience had an influence on the occurrence of undetected stenosis or thrombosis. The procedural quality of LT in Germany is ensured by the independent Institute for Quality Assurance and Transparency in Healthcare (IQTiG). In the recent quality report published in September 2019, in-hospital mortality was reported to be 11.2%, 1-year survival was 82.3%, and 2-year survival with known or unknown status (worst-case analysis) was 72.8% [23]. Accordingly, our 1- and 2-year survival rates (80.3% and 74.4%, respectively) are well above the lower limit.
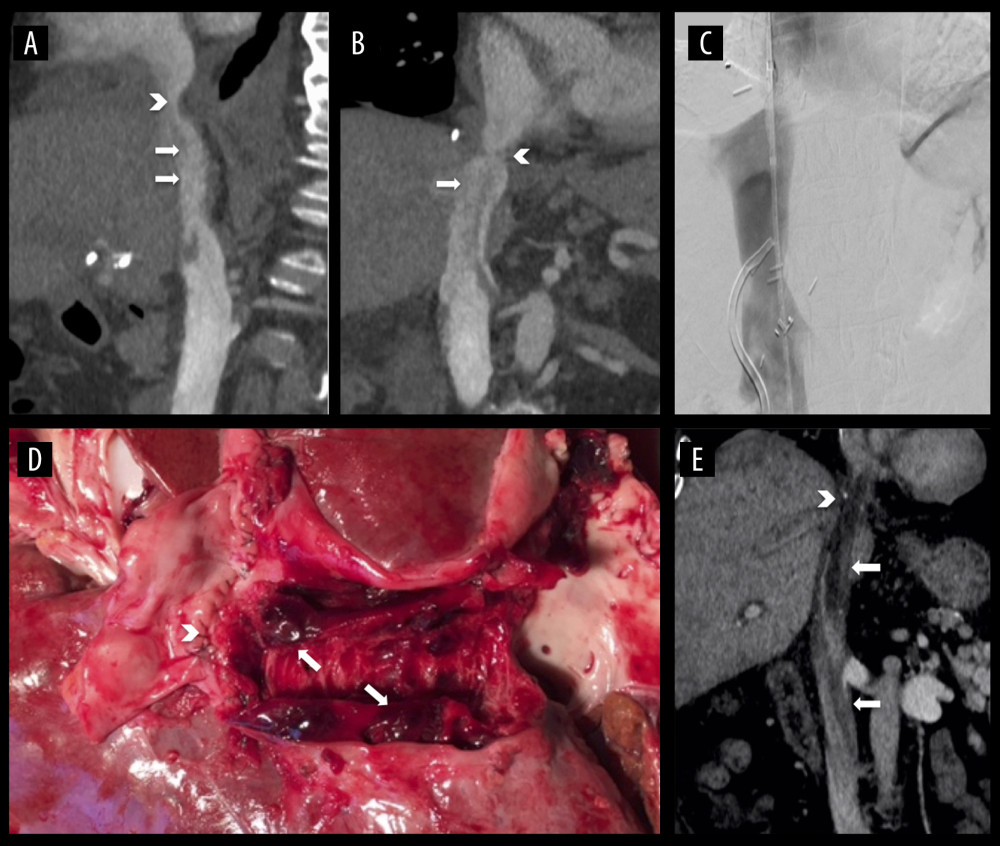
The first patient (Tables 2 and 3, #1), age 68 years, was listed due to post-alcoholic liver cirrhosis and received a full-size LT with a MELD score of 29. During the in-hospital postoperative period 6 weeks after transplantation, following persisting ascites, ultrasound revealed a thrombus in the IVC, which was confirmed by cavography (Figure 1A–1C). The thrombus was located in the IVC until the pelvic circulation and was caused by a fibrotic stenosis of the suprahepatic IVC anastomosis. Combined surgical and interventional trans-thoracic thrombectomy under balloon protection was performed and a simultaneous arterio-venous fistula was created to improve caval perfusion. After an angiographic control 2 weeks later demonstrating no remaining thrombosis, an anastomosis dilatation via percutaneous transluminal angioplasty (PTA) was conducted. By the use of regularly-performed imaging procedures, no pathologies have been detected to date.
The second patient (Tables 2 and 3, #2; Figure 1D), aged 61 years, received LT due to autoimmune hepatitis and non-alcoholic steatohepatitis. The early postoperative course was uneventful, but on the day of planned discharge, 16 days after transplantation, the patient suffered a fulminant pulmonary embolism and died during attempted resuscitation. An autopsy revealed a massive IVC thrombosis without deep vein thrombosis.
The last patient with postoperative IVC thrombosis (Tables 2 and 3, #3), aged 51 years, received LT with simultaneous creation of an 8-mm portocaval shunt for post-alcoholic cirrhosis with extensive portal hypertension and grade IV varices. The shunt was implanted to reduce the immediate pressure on the transplant. Due to primary non-function of the transplanted organ, re-transplantation was done 4 days later with simultaneous shunt explantation. After 4 months, CT scanning (Figure 1E) was performed due to acute dyspnea, revealing IVC stenosis with a floating thrombus. Anticoagulation with heparin was induced immediately. Due to the size of the thrombus, the multidisciplinary team decided to use a trans-atrial approach under cardiac arrest in an on-pump setting via cannulation of the superior vena cava and arteria femoralis. The thrombus was removed trans-diaphragmally by additional phrenotomy, and an augmentation of the suprahepatic IVC anastomosis was performed. Clinical follow-up 1 year later showed unrestricted general health.
Furthermore, we investigated whether we had a systematic problem with IVC stenosis after transplantation. During thorough clinical and imaging examinations at follow-up, an asymptomatic stenoses was found in a 68-year-old patient (Tables 2 and 3, #4) who received an LT for chronic hepatitis B cirrhosis and simultaneous kidney transplantation for chronic terminal renal failure. After a conspicuous finding in ultrasound, venography revealed a stenosis of the suprahepatic IVC anastomosis due to kinking, with a pressure gradient of 5 mmHg (IVC pressure before stenosis 13 mmHg; right atrium (RA) pressure after stenosis 8 mmHg). Due to the clinical condition, no intervention was performed. The patient died due multiorgan failure 6 months after transplantation.
However, symptoms of lower venous congestion such as ascites or edemas are more common reasons for initiation of imaging diagnostics. A 38-year-old patient (Tables 2 and 3, #5) received LT for post-alcoholic cirrhosis and 3 days later by primary non-function re-transplantation. An early postoperative abdominal CT revealed an IVC stenosis within the anastomosis 11 days after transplantation. Following progressive hemodynamic relevance, PTA was performed 9 months after transplantation with excellent results – the pressure gradient decreased from 10 mmHg before dilatation (IVC 14 mmHg; RA 4 mmHg) to 3 mmHg (IVC 10 mmHg; RA 13 mmHg). PTA was repeated 1 year after transplantation and the pressure gradient slightly decreased from 9 mmHg to 7 mmHg, presumably because of a rigid stenosis (before PTA: IVC 10 mmHg; RA 1 mmHg, after PTA: IVC 6 mmHg; RA 13 mmHg). Nevertheless, the hemodynamic status remained unaltered without need for stent implantation or surgical intervention.
Another case with symptomatic IVC stenosis was found in a 70-year-old patient (Tables 2 and 3, #6) who received LT due to hepatocellular carcinoma in hepatitis C cirrhosis after listing 1 month ahead. Ascites remained high 9 months after transplantation. CT scanning showed a narrow stenosis of the suprahepatic IVC and subsequent cavography revealed an IVC pressure gradient of 11 mmHg (IVC 23 mmHg; RA 12 mmHg). One year after transplantation, the pressure gradient had nearly vanished (IVC 10 mmHg, RA 8 mmHg), although the clinical condition was significantly restricted. The patient died due to multiorgan failure 13 months after transplantation.
Discussion
IVC obstruction is less common than radiologically indicated, but remains a severe complication with possible fatal outcome [14,20,24]. The clinical significance of radiological findings is only determined by correlation of the imaging with clinical symptoms. In contrast, subtle symptoms in the complex presentation of a patient after LT should be carefully balanced and early diagnostic imaging should be performed.
Anastomosis technique has less influence on IVC stenosis than expected – comparison of end-to-end [25–27] with piggyback [28–31] technique revealed no difference in thrombotic occurrence. Interestingly, the piggyback method [4, 32], which was introduced to reduce venal and cardiac compromise during LT, does not seem to prevent anastomotic stenosis of the venous outflow tract or the IVC [5,33–37]. In particular, torsion is a more frequent finding in this patient cohort; however, therapy was in general satisfactory with PTA and stenting [5,35–37]. Reports on IVC stenosis following LDLT remain rare [38]. In addition, patients after LDLT suffer venous outflow stenosis, although it seemed to be less common than in deceased-donor liver transplantation (DDLT) [10,38–41]. The Korean standardization of right-lobe LDLT suggests preservation of retro-hepatic IVC and unification venoplasty techniques for reconstruction of the inferior right hepatic vein [42], which was likewise performed in our center. Venous augmentation is frequently performed in highly experienced LDLT centers [41,43,44], and PTFE grafts [41,43] or homologous vascular grafts [43,45] can be used. Although remaining a rare complication after LDLT, IVC stenosis can be successfully treated by PTA [44]. In our cohort, 8 patients received an LDLT and 8 received a right-lobe split LT, and we did not experience any stenoses in these groups.
In general, treatment options range from conservative monitoring to re-transplantation. A watch-and-wait approach is reserved for selected cases with special regard to the patient’s overall condition. Conservative treatment using anticoagulation should be reserved for partial thrombosis or prophylaxis in patients with subclinical stenosis. Regional thrombolysis is the most common form of therapy for thrombosis [25–27], although local thrombolysis using a catheter technique is also described [31,33]. More recently, interventional thrombectomy via Fogarty catheter and subsequent stenting has been successfully performed [26,27]. PTA and stent placement commonly produce better results than surgical treatment [14,17,46]. Nevertheless, relapse of stenosis after PTA is common, and repeated angioplasties may be necessary [9]. Systemic reviews of interventional
We hereby present the possibility of simultaneous trans-diaphragmal anastomosis augmentation after trans-atrial thrombectomy, showing that this approach is suitable for both interventions while avoiding abdominal complications when interventional treatment does not promise acceptable results (case #3). In addition, trans-thoracic thrombectomy was performed in case #1 with secondary PTA after convalescence 2 weeks later without recurring thrombosis. This surgical approach was chosen due to the size of the thrombosis. The time sequence after LT was considered convenient for anastomosis healing and the subsequent stenosis dilatation showed total pressure gradient reduction. In general, operative treatment for thrombectomy was individually chosen depending on the thrombus localization and dimension as well as the time of occurrence after LT.
We decided on a postponed PTA in case #5 due to initially moderate clinical restriction and presumed transitory stenosis caused by edema. Based on appearance of symptoms, PTA was performed 9 months later with satisfying results, although repeated PTA failed, most probably due to fibrotic stenosis. The untreated patients (cases #4 and #6) remained without intervention due to deteriorated general health unlikely to be improved by therapy. IVC obstruction is reported to be more common in cases of re-transplantation [46], resembling our results with 2 out of 8 patients suffering IVC stenosis after re-transplantation within the same hospital stay. We could not determine the cause in our case with fatal IVC thrombosis (#2), which led to the unfortunate outcome of in-hospital death after LT, but kinking or edema seems likely. In general, we found less mortality in the observational period after 2015 compared to transplantation before 2016 despite routinely performed ultrasound imaging due to implementation of early interventional treatment.
Conclusions
In conclusion, IVC constriction and subsequent thrombosis is a relatively rare complication as indicated in the literature. Among 117 transplantations, we found 3 cases with thrombosis and 3 cases with IVC stenosis. Since patients often present with subclinical symptoms, vascular diagnosis should be performed early to detect caval anastomosis pathologies. Diagnostic tools include easily available Doppler ultrasound routinely performed in the postoperative course as well as CT imaging or angiography. However, pathologies of caval anastomosis are typically diagnosed in a later stage by CT or angiography. IVC constriction after LT requires immediate surgical or interventional treatment in specialized centers. Treatment options in the early course can be conservative (watch-and-wait as well as anticoagulation) or angiographic procedures with PTA and stent placement. Furthermore, surgical intervention seems feasible in the early period after LT, but is more complicated in the later period due to collaterals and adhesions prompting alternative surgical approaches such as a potential trans-atrial access. In that respect, we demonstrated that retrograde IVC thrombectomy in special cases via cardiac arrest is a feasible treatment option. Caval constrictions remain a severe complication, but accurate and diagnosis and early individually adapted treatment can prevent graft failure and the need for re-transplantation.
References
1. Piardi T, Lhuaire M, Bruno O, Vascular complications following liver transplantation: A literature review of advances in 2015: World J Hepatol, 2016; 8(1); 36-57
2. Wozney P, Zajko AB, Bron KM, Vascular complications after liver transplantation: A 5-year experience: Am J Roentgenol, 1986; 147(4); 657-63
3. Duffy JP, Hong JC, Farmer DG, Vascular complications of orthotopic liver transplantation: Experience in more than 4,200 patients: J Am Coll Surg, 2009; 208(5); 896-903 discussion 905
4. Calne RY, Williams R, Liver transplantation in man. I. Observations on technique and organization in five cases: Br Med J, 1968; 4(5630); 535-40
5. Bilbao JI, Herrero JI, Martinez-Cuesta A, Ascites due to anastomotic stenosis after liver transplantation using the piggyback technique: Treatment with endovascular prosthesis: Cardiovasc Intervent Radiol, 2000; 23(2); 149-51
6. Khorsandi SE, Athale A, Vilca-Melendez H, Presentation, diagnosis, and management of early hepatic venous outflow complications in whole cadaveric liver transplant: Liver Transpl, 2015; 21(7); 914-21
7. Brouwers MA, de Jong KP, Peeters PM, Inferior vena cava obstruction after orthotopic liver transplantation: Clin Transplant, 1994; 8(1); 19-22
8. Settmacher U, Nussler NC, Glanemann M, Venous complications after orthotopic liver transplantation: Clin Transplant, 2000; 14(3); 235-41
9. Darcy MD, Management of venous outflow complications after liver transplantation: Tech Vasc Interv Radiol, 2007; 10(3); 240-45
10. Takeda K, Tanaka K, Kumamoto T, Severe outflow block syndrome caused by compression by the swollen caudate lobe after living donor liver transplantation: Report of a case: Surg Today, 2012; 42(2); 177-80
11. Tokai H, Eguchi S, Soyama A, Compressive stenosis of the inferior vena cava due to localized ascites after living-donor liver transplantation: J Hepatobiliary Pancreat Surg, 2008; 15(5); 528-30
12. Cardella JF, Castaneda-Zuniga WR, Hunter D, Angiographic and interventional radiologic considerations in liver transplantation: Am J Roentgenol, 1986; 146(1); 143-53
13. Stiglbauer R, Barton P, Jantsch HAngiography following liver transplantation: Rofo, 1990; 153(4); 357-61
14. Raby N, Karani J, Thomas S, Stenoses of vascular anastomoses after hepatic transplantation: Treatment with balloon angioplasty: Am J Roentgenol, 1991; 157(1); 167-71
15. Kok T, Slooff MJ, Thijn CJ, Routine Doppler ultrasound for the detection of clinically unsuspected vascular complications in the early postoperative phase after orthotopic liver transplantation: Transpl Int, 1998; 11(4); 272-76
16. Buell JF, Funaki B, Cronin DC, Long-term venous complications after full-size and segmental pediatric liver transplantation: Ann Surg, 2002; 236(5); 658-66
17. Jiang L, Yang J, Chen W, Zhuang W, Vascular and biliary complications after liver transplantation: Interventional treatment: Chin Med J (Engl), 2002; 115(11); 1679-82
18. Jia YP, Lu Q, Gong S, Postoperative complications in patients with portal vein thrombosis after liver transplantation: Evaluation with Doppler ultrasonography: World J Gastroenterol, 2007; 13(34); 4636-40
19. Yilmaz A, Arikan C, Tumgor G, Vascular complications in living-related and deceased donation pediatric liver transplantation: Single center’s experience from Turkey: Pediatr Transplant, 2007; 11(2); 160-64
20. Ma Y, He XS, Zhu XFThe cause and management of postoperative venous outflow obstruction after orthotopic liver transplantation: Zhonghua Wai Ke Za Zhi, 2008; 46(15); 1133-35 [in Chinese]
21. Boraschi P, Donati F, Rossi M, Role of MDCT in the detection of early abdominal complications after orthotopic liver transplantation: Clin Imaging, 2016; 40(6); 1200-6
22. Galloux A, Pace E, Franchi-Abella S, Diagnosis, treatment and outcome of hepatic venous outflow obstruction in paediatric liver transplantation: 24-year experience at a single centre: Pediatr Radiol, 2018; 48(5); 667-79
23. : Qualitätsreport (quality report) 2019 [press release], 2019, Gemeinsamer Bundesausschuss, Berlin, IQTIG – Institut für Qualitätssicherung und Transparenz im Gesundheitswesen, Berlin
24. Fujimori M, Yamakado K, Takaki H, Long-term results of stent placement in patients with outflow block after living-donor-liver transplantation: Cardiovasc Intervent Radiol, 2016; 39(4); 566-74
25. Kraus TW, Rohren T, Manner M, Successful treatment of complete inferior vena cava thrombosis after liver transplantation by thrombolytic therapy: Br J Surg, 1992; 79(6); 568-69
26. Baccin CE, Haskal ZJ, Power-pulse thrombolysis and stent recanalization for acute post-liver transplant iliocaval venous thrombosis: Cardiovasc Intervent Radiol, 2008; 31(Suppl 2); S166-70
27. Mindikoglu AL, Miller JS, Borge MA, Van Thiel DH, Post-transplant IVC occlusion and thrombosis treated with tPA, heparin, and sharp recanalization: J Gastroenterol, 2005; 40(3); 302-5
28. Mazzaferro V, Regalia E, Pulvirenti A, Renal-splenic shunt for infrahepatic caval occlusion after piggy-back liver transplantation: Transpl Int, 1997; 10(5); 392-94
29. Bertani H, Pinna AD, Di Benedetto F, Hepatic allograft salvage with early doppler ultrasound diagnosis of acute vena cava thrombosis: Abdom Imaging, 2004; 29(5); 606-8
30. Williams AM, Hundley JC, Daily MF, Thrombectomy and cavocavostomy for inferior vena cava thrombosis and torsion after piggyback liver transplantation: Liver Transpl, 2012; 18(8); 993-94
31. Latchana N, Dowell JD, Al Taani J, Ultrasound-accelerated, catheter-directed thrombolysis for inferior vena cava thrombosis after an orthotopic liver transplant: Exp Clin Transplant, 2015; 13(1); 96-99
32. Tzakis A, Todo S, Starzl TE, Orthotopic liver transplantation with preservation of the inferior vena cava: Ann Surg, 1989; 210(5); 649-52
33. Orons PD, Hari AK, Zajko AB, Marsh JW, Thrombolysis and endovascular stent placement for inferior vena caval thrombosis in a liver transplant recipient: Transplantation, 1997; 64(9); 1357-61
34. Iberer F, Grasser B, Schaffellner S, Extracorporeal circulation for repair of suprahepatic vena cava stenosis after liver transplantation: Transpl Int, 2002; 15(11); 589-90
35. Brostoff JM, Bhati CS, Syn WK, Late venous outflow obstruction after liver transplant: The ‘piggy-back’ syndrome: Eur J Intern Med, 2008; 19(5); 374-76
36. Kim IG, Kim BS, Jeon JY, Cavo-caval intervention stent insertion after deceased-donor liver transplantation using side-to-side piggyback technique: Report of a case: Korean J Hepatobiliary Pancreat Surg, 2011; 15(3); 184-88
37. Ferro C, Andorno E, Guastavino A, Endovascular treatment with primary stenting of inferior cava vein torsion following orthotopic liver transplantation with modified piggyback technique: Radiol Med, 2014; 119(3); 183-88
38. Yamagiwa K, Yokoi H, Isaji S, Intrahepatic hepatic vein stenosis after living-related liver transplantation treated by insertion of an expandable metallic stent: Am J Transplant, 2004; 4(6); 1006-9
39. Mizuno S, Yokoi H, Yamagiwa K, Outflow block secondary to stenosis of the inferior vena cava following living-donor liver transplantation?: Clin Transplant, 2005; 19(2); 215-19
40. Liu XL, Li FQ, Li X, Treatment of hepatic venous outflow stenosis after living donor liver transplantation by insertion of an expandable metallic stent: Hepatobiliary Pancreat Dis Int, 2009; 8(4); 424-27
41. Thorat A, Hsu SC, Yang HR, Reconstruction of isolated inferior right hepatic vein(s) in right lobe living donor liver transplantation using polytetrafluoroethylene grafts: A new feasible concept, technique of ‘bridging conduit venoplasty’ and outcomes: Ann Transplant, 2016; 21; 735-44
42. Hwang S, Ha TY, Ahn CS, Reconstruction of inferior right hepatic veins in living donor liver transplantation using right liver grafts: Liver Transpl, 2012; 18(2); 238-47
43. Gonultas F, Akbulut S, Barut B, Usability of inferior vena cava interposition graft during living donor liver transplantation: Is this approach always necessary?: J Gastrointest Surg, 2019 [Online ahead of print]
44. Barut B, Akbulut S, Kutluturk K, Eligibility of circumferential fence with the autologous peritoneal patch for venous reconstruction in right lobe living-donor liver transplant: A case control study: Exp Clin Transplant; 2019 [Online ahead of print]
45. Ara C, Akbulut S, Ince V, Living donor liver transplantation for Budd-Chiari syndrome: Overcoming a troublesome situation: Medicine (Baltimore), 2016; 95(43); e5136
46. Pfammatter T, Williams DM, Lane KL, Suprahepatic caval anastomotic stenosis complicating orthotopic liver transplantation: Treatment with percutaneous transluminal angioplasty, Wallstent placement, or both: Am J Roentgenol, 1997; 168(2); 477-80
47. Goelitz BW, Darcy M, Longitudinal stent fracture and migration of a stent fragment complicating treatment of hepatic vein stenosis after orthotopic liver transplantation: Semin Intervent Radiol, 2007; 24(3); 333-36
48. Eid A, Rahamimov R, Ilan Y, Cavoatrial shunt: A graft salvage procedure for suprahepatic caval anastomosis obstruction after liver transplantation: Liver Transpl Surg, 1998; 4(3); 239-40
49. Molmenti EP, Grover DS, Thuluvath PJ, Cavoatrial shunt in the treatment of suprahepatic vena cava stricture after liver transplantation: Liver Transpl, 2004; 10(9); 1216-17
50. Delay D, Lardi C, Jaussi A, von Segesser LK, Hepato-atrial anastomosis, the “other Senning operation” for treatment of Budd-Chiari syndrome: Swiss Med Wkly, 2005; 135(15–16); 235-37
51. Saeb-Parsy K, Jah A, Butler AJ, Use of a donor aortic interposition allograft to treat stenosis of the suprahepatic inferior vena cava after liver transplantation: Liver Transpl, 2009; 15(6); 662-65
52. Gunasekaran G, Bencsath K, Hupertz V, Deep hypothermia with circulatory arrest to aid in the management of suprahepatic vena cava stenosis after liver transplantation: Liver Transpl, 2010; 16(12); 1434-36
53. Salizzoni S, Romagnoli R, Rispoli P, Solution to recurrent suprahepatic caval stenosis after liver transplantation: cardiac surgery after repeated dilatations and stenting: Liver Transpl, 2014; 20(5); 624-26
54. Weeks SM, Gerber DA, Jaques PF, Primary Gianturco stent placement for inferior vena cava abnormalities following liver transplantation: J Vasc Interv Radiol, 2000; 11(2 Pt 1); 177-87
55. Glanemann M, Settmacher U, Stange B, Caval complications after orthotopic liver transplantation: Transplant Proc, 2000; 32(3); 539-40
Tables
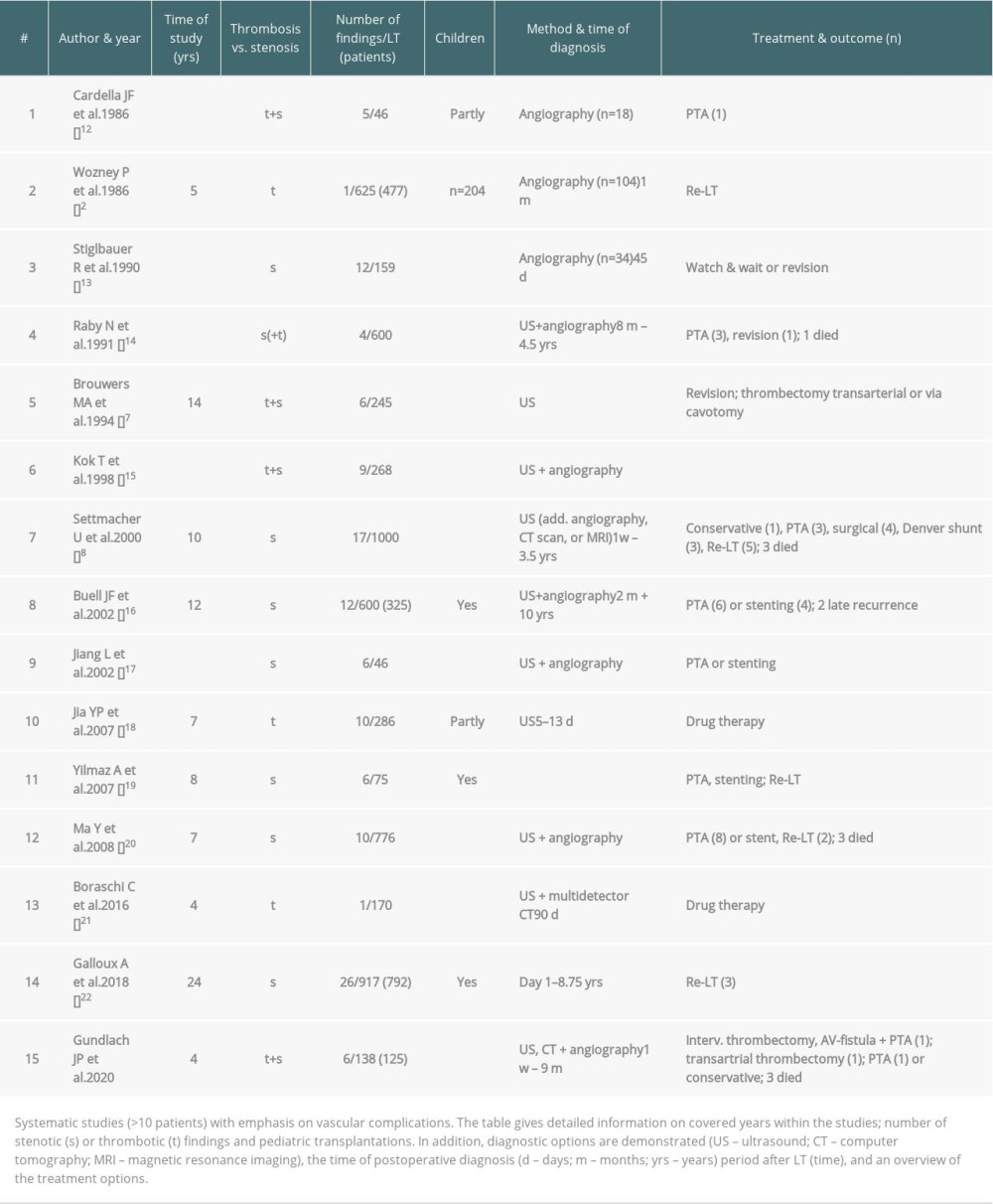 Table 1. Literature overview of systematic studies comprising thrombosis and stenosis with respective diagnostic tools and treatment options.
Table 1. Literature overview of systematic studies comprising thrombosis and stenosis with respective diagnostic tools and treatment options.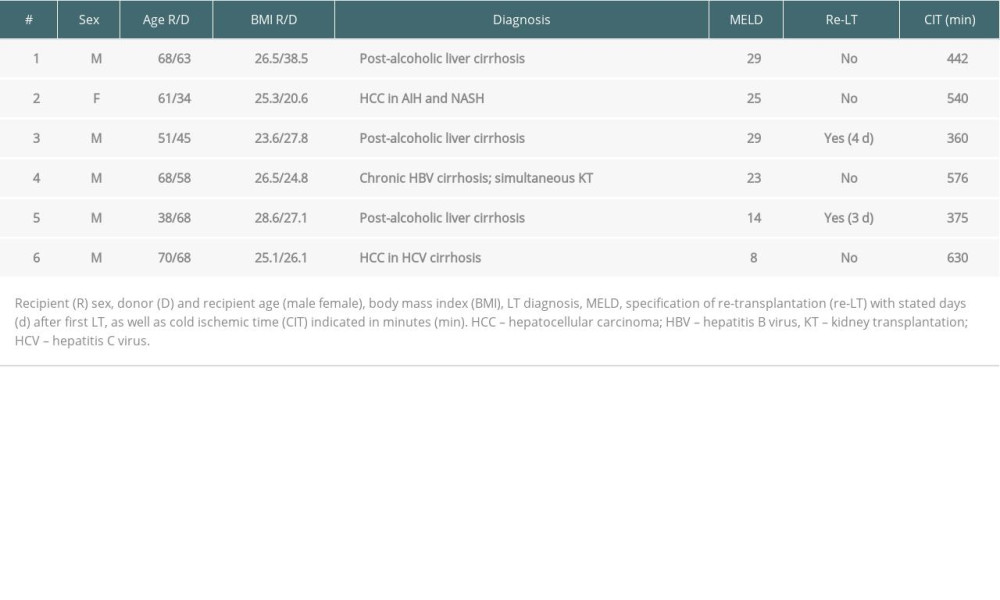 Table 2. Transplantation characteristics.
Table 2. Transplantation characteristics.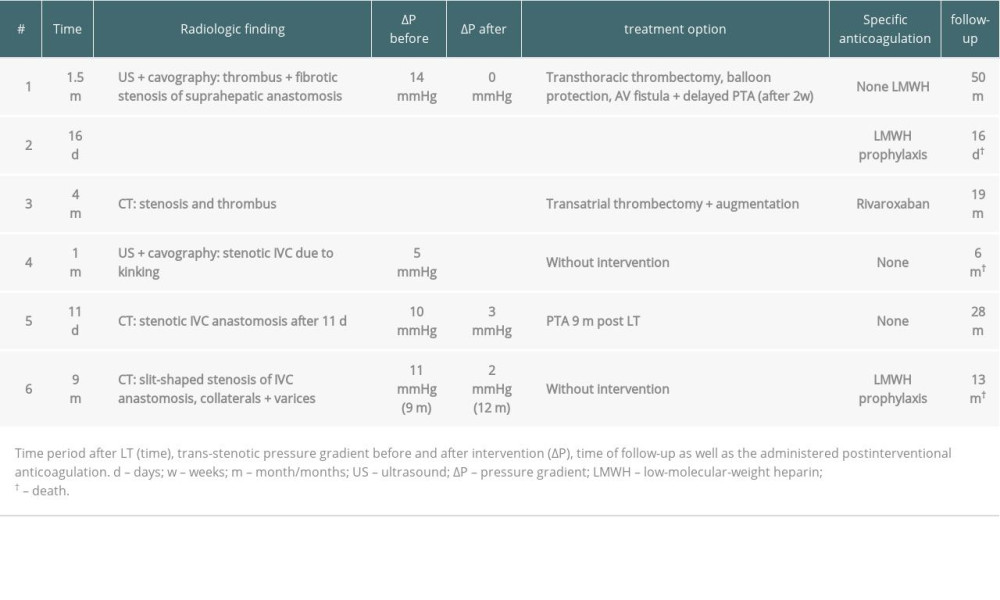 Table 3. Diagnostic and therapeutic procedures in case of IVC occlusion.
Table 3. Diagnostic and therapeutic procedures in case of IVC occlusion. Table 1. Literature overview of systematic studies comprising thrombosis and stenosis with respective diagnostic tools and treatment options.
Table 1. Literature overview of systematic studies comprising thrombosis and stenosis with respective diagnostic tools and treatment options. Table 2. Transplantation characteristics.
Table 2. Transplantation characteristics. Table 3. Diagnostic and therapeutic procedures in case of IVC occlusion.
Table 3. Diagnostic and therapeutic procedures in case of IVC occlusion. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860