06 August 2021: Original Paper
Invasive Fungal Infections After Liver Transplantation: A Retrospective Matched Controlled Risk Analysis
Halil-Ibrahim Karadag1ACEF, Oya Andacoglu2CDEF, Marios Papadakis3BCEF, Andreas Paul1ACD, Arzu Oezcelik1ACDE*, Eugen Malamutmann1ADDOI: 10.12659/AOT.930117
Ann Transplant 2021; 26:e930117
Abstract
BACKGROUND: Invasive fungal infections (IFI) are major risks for mortality after liver transplantation (LT). The aim of this study was to evaluate possible risk factors for the development of IFI after LT.
MATERIAL AND METHODS: All adult patients with IFI after LT between January 2012 and December 2016 at Essen University were identified. Pre-, intra-, and postoperative data were reviewed. These were compared to a 1-to-3 matched control group. Multinominal univariate and multivariate regression analyses were performed.
RESULTS: Out of the 579 adults who underwent LT, 33 (5.6%) developed postoperative IFI. Fourteen had invasive aspergillosis with 7 (50%) mortality, and 19 had Candida sepsis with 7 (37%) mortality. The overall mortality due to invasive fungal infections was 42%. Perfusion fluid contamination with yeast was detected in 5 patients (15%). Multivariate regression analyses showed that preoperative dialysis (OR=1.163; CI: 1.038-1.302), Eurotransplant donor risk index (OR=0.04; CI=0.003-0.519), length of hospital stay (OR=25.074; CI: 23.99-26.208), and yeast contamination of the preservation fluid (OR=47.8; CI: 4.77-478, 96) were associated with IFI in the Candida group, whereas duration of surgery (OR=1.013; CI: 1.005-1.022), ventilation hours (OR=0.993; CI=0.986-0.999), and days of postoperative dialysis (OR=1.195; CI: 1.048-1,362) were associated with IFI in the aspergillosis group.
CONCLUSIONS: Post-LT IFI had 42% mortality in our cohort. Prophylactic antifungal therapy should be expanded to broader risk groups as defined above.
Keywords: Antifungal Agents, Aspergillosis, Liver Transplantation, End stage liver disease, invasive fungal infections, Risk Assessment, Severity of Illness Index
Background
Liver transplantation (LT) is the ultimate treatment for end-stage liver disease [1–3]. Despite improvements in liver transplantation, infections in the early postoperative phase play a significant role in postoperative morbidity and mortality [3–5]. Among those that lead to sepsis, invasive fungal infections (IFI) are detrimental for patient outcomes [5]. Fungal infections are the cause of 12% of all sepsis-related deaths and 2% of all deaths after LT [3]. Candida and Aspergillus species are the most common pathogens of an IFI after liver transplantation [5,6].
The pathogenesis is different for these 2 types of fungi. Invasive fungal infections caused by Candida spp (species pluralis) are primarily endogenous infections, which start from a colonization in the body or from an already existing microbial biofilm [7]. If the milieu conditions are fulfilled, these fungal species can spread superficially or invasively [8]. The most common pathogen of this genus is
Antimycotic prophylaxis during LT is variable among institutes [10–13]. Various risk factors for IFI after LT are described in the literature [5,13,16–26]. To date, there has been no multinominal multivariate analysis of these known risk factors. Our primary aim was to investigate the incidence and mortality of IFI after LT at our center. Our secondary aim was identification of possible risk factors for the development of IFI, which could potentially help us to select patients with high risk for IFI who would benefit from proper prophylaxis or early treatment.
Material and Methods
STATISTICAL ANALYSIS:
According to distribution, values are reported either as median and interquartile range (IQR) or as mean and standard deviation. Continuous variables were compared using the Mann-Whitney test. Comparisons of proportions were performed using Fisher’s exact test or chi-square test. The survival rates were presented by Kaplan-Meier analysis and compared by log-rank test. At first, a univariate multinomial logistic regression analysis, ie, a logistic regression with more than 2 characteristics (Aspergillus, Candida, no IFI), was performed. In order to minimize presumed relevance, the significant variables were then included in a conditional logistic multinomial multivariate analysis with a stepwise forward selection. The level of significance was defined with a p-value <0.05. All statistical analyses were performed with IBM SPSS Statistics for Windows, Version 25.0. (IBM Corp. Released 2017. Armonk, NY: IBM Corp).
Results
Out of the 579 adults who underwent LT in 2012–2016, 33 patients (5.6%) developed postoperative IFI. Of these, 14 patients (42.4%) had invasive aspergillosis (AG) and 19 patients (57.6%) had Candida sepsis (CG). There were 19 male (58%) and 14 female (42%) patients. The mean age was 48 years (SD11.89) and the mean lab MELD score was 22 (SD 9.4). The etiology of the liver cirrhosis was primary sclerosing cholangitis in 9 patients (27%), hepatitis B or C virus infection in 7 patients (21%), alcoholic liver disease in 8 patients (24%), hepatocellular tumor in 2 patients (6%), 1 acute liver failure (3%), and other reasons in 6 patients (18%). Twenty-two (66.7%) patients required hemodialysis preoperatively. The median ET-DRI of the IFI study group was 1.74. There was no statistically significant difference between IFI and matched control group in recipient characteristics, such as age of the recipient, gender distribution, diagnoses leading to liver transplantation, BMI, pre-transplant abdominal surgery, portal vein status, and lab MELD score (Table 1).
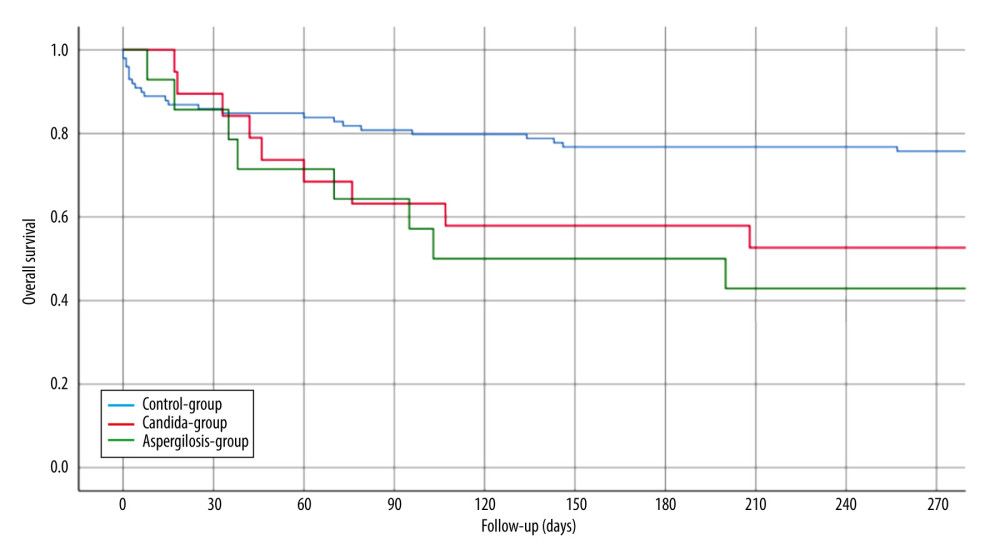
Perfusion fluid contamination with yeast was detected in 5 patients (15%): 1 in the AG group and 4 in the CG group. None of the patients received prophylactic antifungal therapy based on the fluid culture result but rather based on active infection signs or symptoms after LT. The mean duration of surgery was 327 minutes (SD 140). Intraoperative blood transfusion occurred in 22 patients (66.7%). There were no intraoperative complications. The mean LOS-ICU was 29.9 days (SD 23.2). The mean hospital LOS was 54.6 days (SD 43.8). Major postoperative complications were observed in 13 patients (39.4%). Perioperative death within 90 days after LT occurred in 4 patients (12.1%). Of the patients with AG, 7 (50.0%) died within 102 days (mean) after LT and of the patients with CG, 7 (37%) died within 98 days (mean) after LT. The 1- and 3-year overall survival rates of IFI group were 48.5% and 24%, whereas the control group had 75.5% 1-year survival (p<0.003) (Figure 1). In the univariate analysis, the duration of surgery (DOS), LOS-ICU, postoperative ventilation time, the need for preoperative dialysis, as well as the duration of dialysis, transaminase peak, re-operation rate and the total LOS, were significantly higher in patients who developed invasive aspergillosis or Candida sepsis (Tables 2, 3). In the Candida group, regarding donor-related factors, only the incidence of perfusion fluid contamination was significantly higher in the study group compared to the control group (p<0.001; OR=47.8; CI: 4.77–478) and remained significant for Candida in multivariate multinominal analysis but not for aspergillosis. Multivariate regression analyses showed that preoperative dialysis (OR=1.163; CI: 1.038–1.302), Eurotransplant donor risk index (OR=0.04; CI=0.003–0.519), length of hospital stay (OR=25.074; CI: 23.99–26.208), and yeast contamination of the preservation fluid (OR=47.8; CI: 4.77–478, 96) were associated with IFI in the Candida group, whereas duration of surgery (OR=1.013; CI: 1.005–1.022), ventilation hours (OR=0.993; CI=0.986–0.999), and days of postoperative dialysis (OR=1.195; CI: 1.048–1,362) were associated with IFI in the aspergillosis group (Tables 4,5).
Discussion
Systemic fungal infection after LT is one of the important causes of perioperative death within the first month after LT [17]. Consequently, the identification of significant risk factors and evaluation of the role of prophylactic antifungal treatment is essential. The incidence of IFI after organ transplantation is reported to be 5–20% in the literature [18,19]. Our IFI incidence was 5.6% among 579 adult patients following LT. The ratio of aspergillosis to Candida septicemia was rather atypical (42.4% to 57.5%). In international comparison, the rate of Candida species as the cause of IFI is described in the literature is up to 90% [20]. One-year mortality after an IFI is up to 62.5% [21,22]. One-year survival of patients with IFI in our series was 48.5%, which is significantly lower than that of the control group. Levesque et al described a significant correlation between the contamination of the preservative fluid and the development of invasive mycosis [21]. Of all the possible risk factors analyzed, the presence of fungi in the preservation fluid was the greatest risk factor for a fungal infection by Candida spp after liver transplantation in our analysis but not for Aspergillus infection. Existing donor invasive fungal infections can contaminate the donor organ and can be detected as a “contamination” of the preservative liquid. Yeast contamination of the preservation fluid was identified as an independent risk factors to develop IFI after LT in our study. In our practice, donor cultures are reviewed regularly at the time of accepting the donor and we do not accept donors with active fungal infection; however, the donor culture data and fungal infection data of the donors were not available at the time of this study. Preservation fluid culture is also a routine practice in our center following findings in this report. After the findings in our study, we changed our practice so that any patient with preservation fluid contamination with yeast is now receiving empiric antifungal treatment independent from clinical signs and symptoms.
The DRI is used as a predictive value to determine the probable survival of the transplant recipient after LT; however, the influence of DRI on the incidence of postoperative infections is still unclear. Rosenberger et al analyzed the relation between DRI and post-transplant infections, concluding that infections depend more heavily on recipient factors [23]. There were 673 infectious complications in 378 patients, including 53 cases with
An association between a prolonged duration of surgery and increased rate of infection after LT is described in many studies [24–26]. The duration of surgery is also one of the significant predictive factors for invasive fungal infection and is associated with postoperative complications as shown previously [27]. We also report that duration of surgery was significantly longer in the study group compared to the control group and was identified as a significant predictive factor for development of IFI by Aspergillus species; therefore, transplant teams may consider this risk factor for antifungal prophylaxis.
The total LOS among patients in the mycosis groups was significantly longer than that of the patients in the control group) (p<0.0005) These results are concordant with the study by Levesque et al in which a prolonged LOS was also demonstrated for postoperative invasive mycoses (p=0.002) [17]. Biological plausibility has a key role in assessing the association between variables considered as risk factors and outcomes. In that sense, the length of hospitalization, as well as the duration of ventilation, might be the consequence of the fungal infection rather than the cause.
The Infectious Diseases Society of America (IDSA) Treatment of Candidiasis guidelines stated patients who undergo liver transplantation with at least 2 key risk factors, including re-transplantation, creatinine level 12.0 mg/dL, choledochojejunostomy, intraoperative use of >40U of blood products, prolonged intraoperative time (defined as >11 h), and fungal colonization detected at least 2 days before and 3 days after transplantation, are at higher risk of invasive candidiasis and are recommended to receive prophylaxis treatment [28]. In our study, median duration of surgery was 5.2 h in AG, 4.45 h in CG, and 4.08 h in the control group (p<0.05). So, we can argue that DOS could still be considered prolonged when approaching 6 h, which is a much shorter cut off as defined by IDSA. Considering it is common that LT takes longer than 6 h, surgeons could consider antifungal prophylaxis more often if there are other risk factors as well.
According to the Guidelines of the American Society of Transplantation, Infectious Diseases Community of Practice, Singh et al reported Candida has been isolated in about 4% of the preservation fluids from liver transplant recipients [29]. They also stated cultures of blood, urine, and drainage fluids should be performed prior to initiating empiric therapy when Candida is identified in preservation fluid cultures or following organ procurement complicated by intestinal contamination [29]. The authors concluded liver transplant recipients in whom Candida species are identified in the preservation fluid cultures or in patients with surgeries complicated by intestinal contamination during organ recovery should receive empiric antifungal therapy for 2 weeks [29]. As stated above, we now imply empiric antifungal treatment independent from clinical signs and symptoms when contamination is detected.
Renal replacement or chronic kidney disease have been reported as risk factor for post-liver transplant yeast infection [30,31]. Eschenauer et al also commented that dialysis and re-transplantation patients should be considered for fungal prophylaxis [32]. The IFI group in our study had higher incidence of hemodialysis and the duration of dialysis was significantly longer compared to the control group. Although we had a small number of patients and we performed a retrospective review, we agree that liver transplant patients who have been on hemodialysis prior to transplant should be considered for antifungal prophylaxis.
The limitations of our study include its retrospective nature and small sample size. Given that only patients with systemic fungal infections were included in the analysis, a selection bias is inevitable. Furthermore, due to the multimodal analysis, an over-interpretation of the collected data is possible as well. Lastly, the control group was not fully randomized with the IFI group, which is suboptimal, thus we cannot make firm conclusions.
Conclusions
Based on the results of the study, duration of surgery, hours of mechanical ventilation, and preoperative dialysis days were significant factors for the development of postoperative invasive aspergillosis. Yeast contamination of the preservation fluid, ET-DRI, dialyses days, and hospital stay were identified as significant factors for the development postoperative Candida sepsis. Despite the small cohort and retrospective nature of the study, we believe patients with these risk factors may benefit from prophylactic antifungal treatment. Larger cohorts and prospective analyses are necessary to improve the power of these findings.
Tables
Table 1. Comparison of the preoperative recipient data.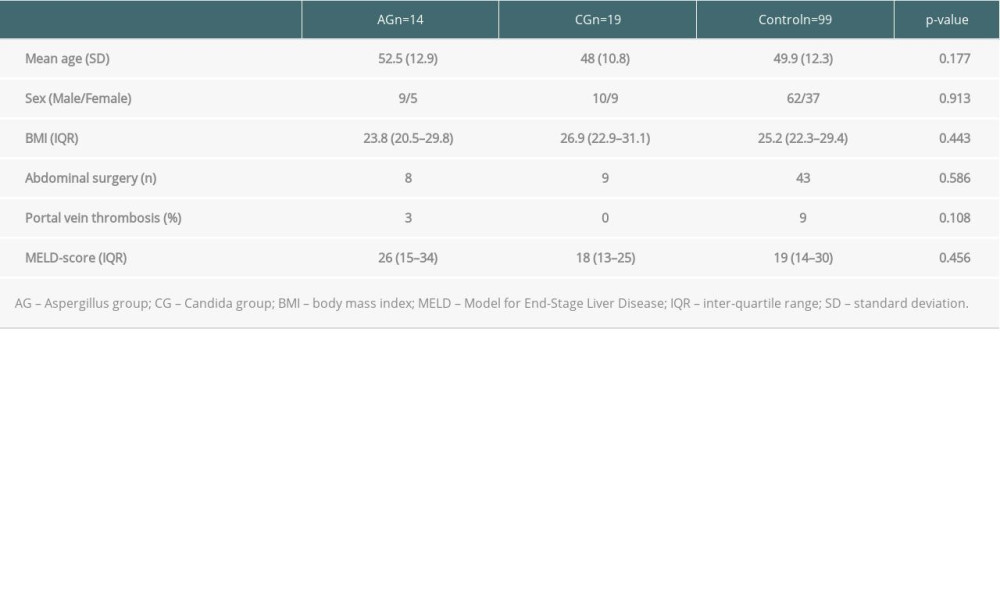 Table 2. Comparison of the peri and postoperative recipient data.
Table 2. Comparison of the peri and postoperative recipient data.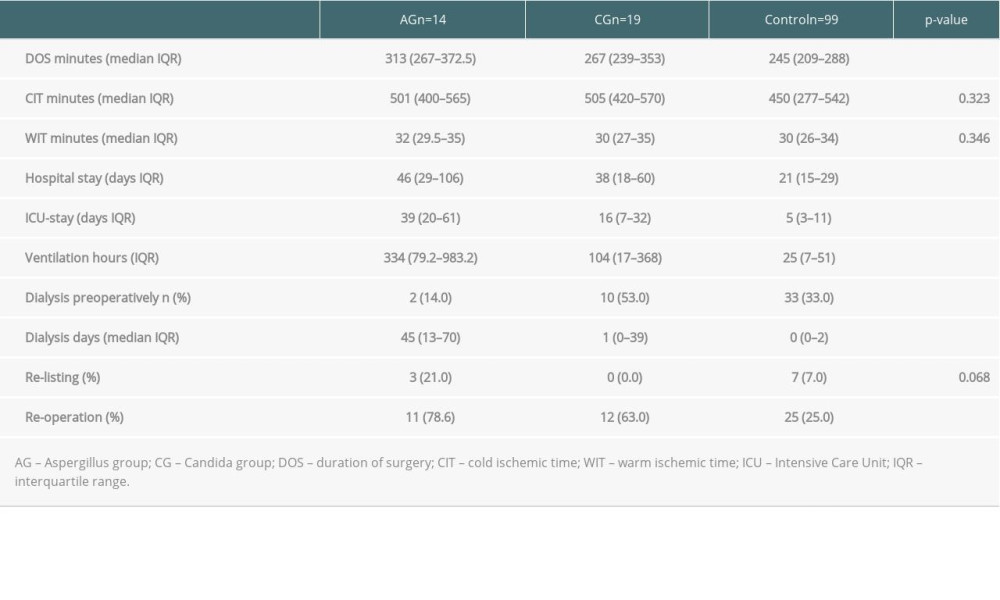 Table 3. Comparison of the donor data.
Table 3. Comparison of the donor data.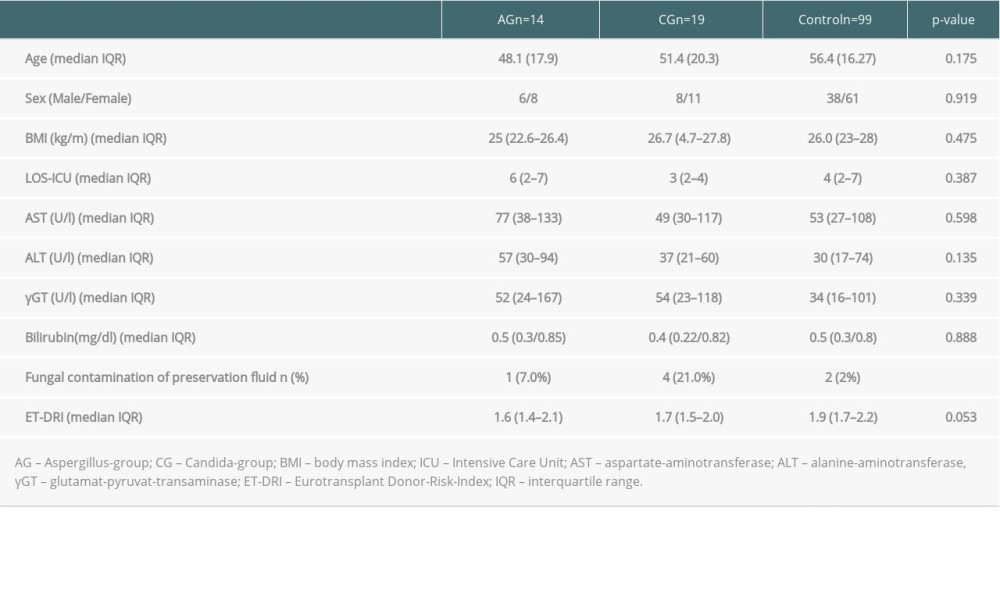 Table 4. Multivariate multinominale analysis of the Aspergillus group.
Table 4. Multivariate multinominale analysis of the Aspergillus group.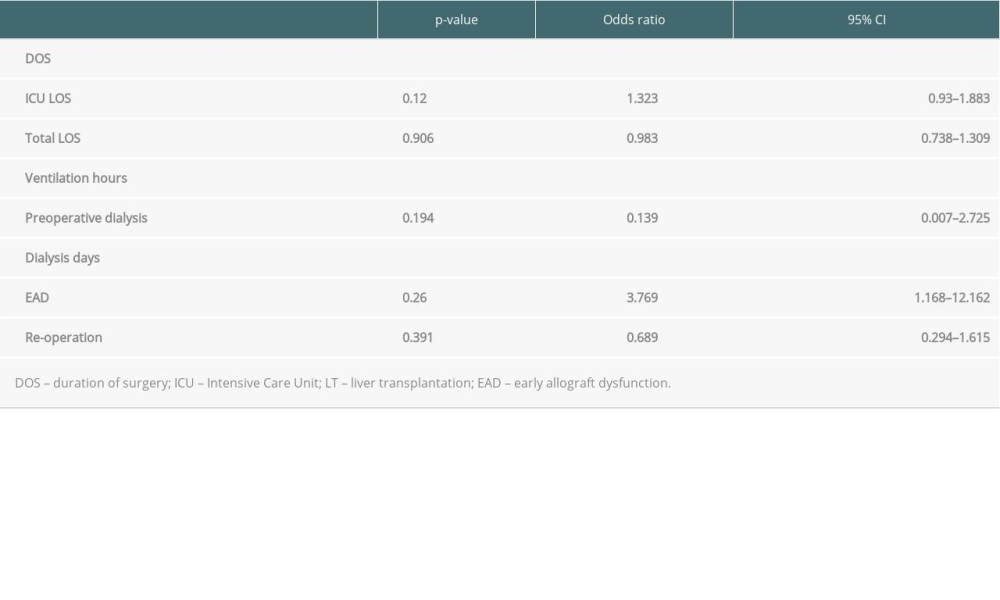 Table 5. Multivariate multinominal analysis of the Candida group.
Table 5. Multivariate multinominal analysis of the Candida group.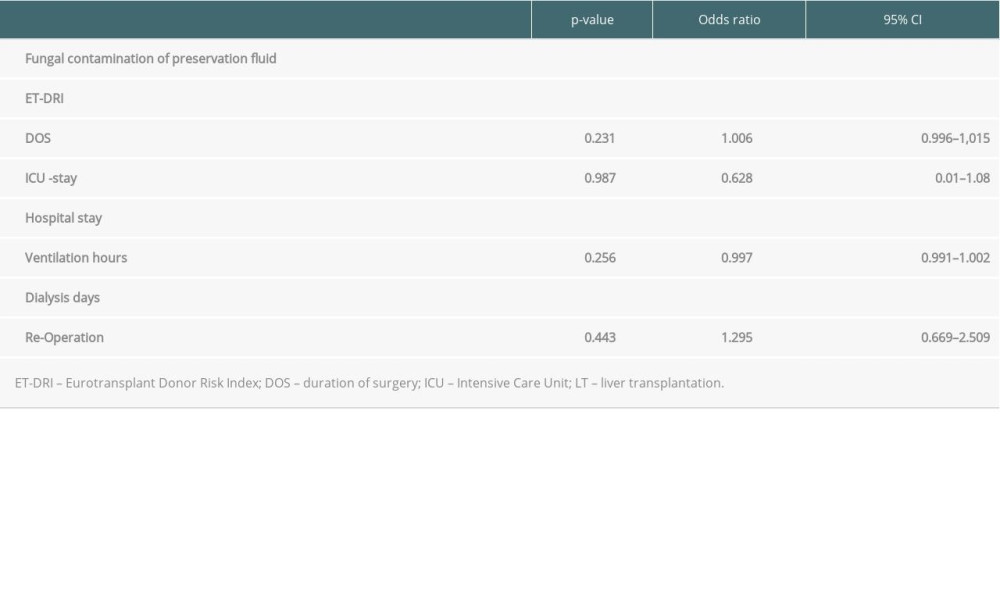
References
1. Carithers RL, Liver transplantation. American Association for the Study of Liver Diseases: Liver Transpl, 2000; 6(1); 122-35
2. Rai R, Liver transplantation – an overview: Indian J Surg, 2013; 75(3); 185-91
3. Adam R, Karam V, Delvart V, Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR): J Hepatol, 2012; 57(3); 675-88
4. Martin M, Kusne S, Alessiani M, Infections after liver transplantation: Risk factors and prevention: Transplant Proc, 1991; 23(3); 1929-30
5. Liu X, Ling Z, Li L, Ruan B, Invasive fungal infections in liver transplantation: Int J Infect Dis, 2011; 15(5); e298-304
6. Zhang W, Wang W, Kang M, Bacterial and fungal infections after liver transplantation: Microbial epidemiology, risk factors for infection and death with infection: Ann Transplant, 2020; 25; e921591
7. von Lilienfeld-Toal M, Wagener J, Einsele H, Invasive fungal infection: Dtsch Arztebl Int, 2019; 116; 271-78
8. Hof H, Dörries R, Medizinische Mikrobiologie, 5: Auflage (Thieme), 2014; 466-90 [in German]
9. Sotiropoulos GC, Steinmann J, Stern S, Donor leucocytosis predicts bacterial and fungal contamination of the preservation solution in visceral organ transplantation: Prog Transplant, 2018; 28(1); 24-28
10. Cruciani M, Mengoli C, Malena M, Antifungal prophylaxis in liver transplant patients: A systematic review and meta-analysis: Liver Transpl, 2006; 12(5); 850-58
11. Evans JD, Morris PJ, Knight SR, Antifungal prophylaxis in liver transplantation: A systematic review and network meta-analysis: Am J Transplant, 2014; 14(12); 2765-76
12. Raghuram A, Restrepo A, Safadjou S: Liver Transpl, 2012; 18(9); 1100-9
13. Koval C, Echinocandins for antifungal prophylaxis in liver transplant recipients: Advance in the field or variation on a theme?: Liver Transpl, 2016; 22(4); 396-98
14. De Pauw B, Walsh TJ, Donnelly JP, Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group: Clin Infect Dis, 2008; 46(12); 1813-21
15. Dindo D, Demartines N, Clavien PA, Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey: Ann Surg, 2004; 240(2); 205-13
16. Olthoff KM, Kulik L, Samstein B, Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors: Liver Transpl, 2010; 16(8); 943-49
17. Singh N, Fungal infections in the recipients of solid organ transplantation: Infect Dis Clin North Am, 2003; 17(1); 113-34
18. Chen YC, Huang TS, Wang YC, Effect of prophylactic antifungal protocols on the prognosis of liver transplantation: A propensity score matching and multistate model approach: Biomed Res Int, 2016; 2016; 6212503
19. Pacholczyk M, Lagiewska B, Lisik W, Invasive fungal infections following liver transplantation – risk factors, incidence and outcome: Ann Transplant, 2011; 16(3); 14-16
20. Pappas PG, Kauffman CA, Andes D, Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America: Clin Infect Dis, 2009; 48(5); 503-35
21. Levesque E, Paugam-Burtz C, Saliba F: Transpl Int, 2015; 28(11); 1308-16
22. Singh N, Avery RK, Munoz P, Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients: Clin Infect Dis, 2003; 36(1); 46-52
23. Rosenberger LH, Gillen JR, Hranjec T, Donor risk index predicts graft failure reliably but not post-transplant infections: Surg Infect (Larchmt), 2014; 15(2); 94-98
24. Cheng H, Chen BP, Soleas IM, Prolonged operative duration increases risk of surgical site infections: A systematic review: Surg Infect (Larchmt), 2017; 18(6); 722-35
25. George DL, Arnow PM, Fox AS, Bacterial infection as a complication of liver transplantation: Epidemiology and risk factors: Rev Infect Dis, 1991; 13(3); 387-96
26. Kusne S, Dummer JS, Singh N, Infections after liver transplantation. An analysis of 101 consecutive cases: Medicine (Baltimore), 1988; 67(2); 132-43
27. Bennett-Guerrero E, Feierman DE, Barclay GR, Preoperative and intraoperative predictors of postoperative morbidity, poor graft function, and early rejection in 190 patients undergoing liver transplantation: Arch Surg, 2001; 136(10); 1177-83
28. Pappas PG, Kauffman CA, Andes DInfectious Diseases Society of America, Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America: Clin Infect Dis, 2009; 48(5); 503-35
29. Singh N, Huprikar S, Burdette SDAmerican Society of Transplantation, Infectious Diseases Community of Practice, Donor-Derived Fungal Infection Working Group, Donor-derived fungal infections in organ transplant recipients: guidelines of the American Society of Transplantation, infectious diseases community of practice: Am J Transplant, 2012; 12(9); 2414-28
30. Shi SH, Lu AW, Shen Y: Chin Med J (Engl), 2008; 121(7); 625-30
31. Lavezzo B, Patrono D, Tandoi F, A simplified regimen of targeted antifungal prophylaxis in liver transplant recipients: A single-center experience: Transpl Infect Dis, 2018; 20(2); e12859
32. Eschenauer GA, Lam SW, Carver PL, Antifungal prophylaxis in liver transplant recipients: Liver Transpl, 2009; 15(8); 842-58 [Erratum in: Liver Transpl. 2010:16(6):797–805]
Tables
 Table 1. Comparison of the preoperative recipient data.
Table 1. Comparison of the preoperative recipient data. Table 2. Comparison of the peri and postoperative recipient data.
Table 2. Comparison of the peri and postoperative recipient data. Table 3. Comparison of the donor data.
Table 3. Comparison of the donor data. Table 4. Multivariate multinominale analysis of the Aspergillus group.
Table 4. Multivariate multinominale analysis of the Aspergillus group. Table 5. Multivariate multinominal analysis of the Candida group.
Table 5. Multivariate multinominal analysis of the Candida group. Table 1. Comparison of the preoperative recipient data.
Table 1. Comparison of the preoperative recipient data. Table 2. Comparison of the peri and postoperative recipient data.
Table 2. Comparison of the peri and postoperative recipient data. Table 3. Comparison of the donor data.
Table 3. Comparison of the donor data. Table 4. Multivariate multinominale analysis of the Aspergillus group.
Table 4. Multivariate multinominale analysis of the Aspergillus group. Table 5. Multivariate multinominal analysis of the Candida group.
Table 5. Multivariate multinominal analysis of the Candida group. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860