27 August 2021: Original Paper
Can Microscopic Biliary Reconstruction Reduce Biliary Complication Rate in ABO-Incompatible Adult Living Donor Liver Transplantation?
Yu-Cheng Lin1BCDEF, Tsan-Shiun Lin1ABCDF, Chih-Che Lin1ABCDEF, Yueh-Wei Liu1B, Shih-Ho Wang1B, Yi-Ju Wu1B, Wei-Feng Li1B, Yu-Hung Lin1BCDEF, Ting-Lung Lin1B, Yi-Chia Chan1B, Cheng-Hsi Yeh1B, Chih-Chi Wang1B, Chao-Long Chen1ABDEF, Chee-Chien Yong1ABCDEF*DOI: 10.12659/AOT.931963
Ann Transplant 2021; 26:e931963
Abstract
BACKGROUND: With the introduction of rituximab, ABO-incompatible (ABOi) living donor liver transplantation (LDLT) has been considered a feasible and safe procedure to overcome the shortage of organ donors. However, higher biliary complication rates remain an unresolved problem in the ABOi group. In our center, biliary anastomosis has been done with microscopic biliary reconstruction (MBR), which effectively reduced the biliary complication rate. The aim of the current study was to investigate whether the microscopic approach reduced anastomotic biliary complications in ABOi LDLT.
MATERIAL AND METHODS: From March 2006 to December 2018, 30 adult ABOi and 60 ABO-compatible (ABOc) LDLT patients were selected from over 1300 recipients through 1: 2 propensity score-matched cohorts. All patients received MBR during the transplantation. Biliary complications included bile leakage and biliary stricture. Patients with diffuse intrahepatic biliary stricture were excluded from analysis.
RESULTS: Patient characteristics were similar in the 2 groups. There was no in-hospital mortality in the ABOi LDLT. The long-term survival rates of the ABOi patients were comparable to those of the patients that underwent ABOc LDLT (87.1% vs 87.4%, P=0.964). Those in the ABOi group with anastomotic biliary complications were about 40%, which was higher than in the ABOc patients (40% vs 15%, P=0.01).
CONCLUSIONS: Microscopic biliary reconstruction does not help to reduce the high biliary complication rate in ABOi LDLT. Further investigation and identification regarding other risk factors and precautionary measures involving immunologic and adaptation mechanisms are needed.
Keywords: ABO Blood-Group System, Liver Transplantation, Living Donors, Anastomosis, Surgical, Biliary Tract, Blood Group Incompatibility, Carcinoma, Hepatocellular, End stage liver disease, Female, Graft Rejection, Humans, Liver Neoplasms, Severity of Illness Index
Background
Living donor liver transplantation (LDLT) flourishes in Asia as a response to a severe organ shortage in a population with high demand for liver transplantation due to the endemicity of diseases related to hepatitis B virus and hepatitis C virus infection and the high incidence of hepatocellular carcinoma [1]. ABO incompatibility (ABOi) was previously considered a contraindication for adult LDLT due to the possibilities of antibody-mediated rejection, vascular thrombosis, and biliary complications [2,3]. Since the introduction of the anti-CD20 monoclonal antibody rituximab and the development of various strategies to improve the outcome of ABOi LDLT, including plasma exchange (PE), splenectomy, aggressive immunosuppressive protocols, and intrahepatic portal and arterial infusion, promising results has been seen in recent studies [4]. Nevertheless, biliary complications remain the most difficult challenge in the rituximab era [5]. The mechanism is not well established because reports of biliary complications are based on heterogenous patient sources, various desensitization protocols, and different surgical techniques. The biliary complications include diffuse intrahepatic biliary stricture (DIHBS) from the immune response, as evidenced by the presentation of multiple segmental intrahepatic biliary strictures, and those resulting from surgical techniques.
A systematic review and meta-analysis in 2019 highlighted that a higher biliary complication rate (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.14 to 1.96,
Importantly, the 2 generally accepted methods of biliary reconstruction among most centers are conventional Roux-en-Y hepaticojejunostomy and duct-to-duct anastomosis, with or without the modification of using stents [7]. In our institute, biliary anastomosis has been routinely performed through microscopic biliary reconstruction (MBR) by a single surgeon since March 2006. Previous reports showed the routine use of MBR overcomes the difficulties due to anatomical variations and size discrepancies between the graft and recipient hepatic ducts with an effective reduction in biliary complications of 10.2% [8–11].
Most studies have focused on higher biliary complications in ABOi LDLT and its immunologic mechanisms, while few studies have discussed the impact of biliary reconstruction method in regard to biliary complications of ABOi. We have obtained great success in reducing biliary complications in ABOc LDLT, but the role of MBR in ABOi LDLT has never been investigated.
Here, we aimed to determine whether MBR could reduce anastomotic biliary complications from a surgical approach in the ABOi LDLT.
Material and Methods
PREOPERATIVE EVALUATION AND SELECTION:
Both donors and recipients underwent our standard evaluation protocol. The evaluation protocols were as described in our earlier publications [12,13]. For ABOi adult recipients, the CD19+ B lymphocyte count and anti-A and/or anti-B isoagglutinin (IA) titers were measured during the evaluation phase. The exclusion criteria for ABOi recipients included highly sensitized patients with anti-A and/or anti-B IA over 1: 2046, acute hepatic failure, MELD score over 25 points, and multiple severe comorbidities. The ABOi donors were selected either because they were the only one available or because other possible ABOc donors were refused owing to clinical or anatomical reasons.
ABOI DESENSITIZATION PROTOCOL:
A pretransplant desensitization regimen with a single dose of anti-CD20 monoclonal antibody, rituximab (MabThera®; Roche, Mannheim, Germany), 375 mg/m2, was given 2–3 weeks prior to the transplantation. The CD19+ count was checked before rituximab injection and before transplantation. The anti-A and -B IA titers were measured before and after rituximab injection and every PE. The frequency and timing of PE before transplantation depended on the hemagglutinin titer. The target titers of anti-A and -B IA before LT were ≤1: 32. If the IA titers were high, PE was performed and repeated as needed to achieve anti-A or anti-B antibody titers within ≤1: 32 at the time of LDLT. No local graft infusion therapy or routine splenectomy was performed during the operation (Figure 1).
SURGICAL TECHNIQUES:
Donor and recipient operations were performed in the usual manner. Surgical details and techniques were identical in both the ABOi and the ABOc groups. With regard to biliary reconstruction in our institution, MBR to overcome the complexities brought by anatomic variations has been routinely conducted by a single microsurgeon (TS Lin) since 2006 [8,11]. The classification of biliary reconstruction was based on the number of ducts in the graft, the method in which these ducts were reconstructed (with or without ductoplasty), and the conduit used (recipient duct or jejunum) to reconstruct the biliary tree. All biliary reconstructions were performed under an operating microscope (Carl Zeiss, Jena, Germany) with a magnification of ×5 to ×15. Duct-to-duct anastomosis was carried out in the majority of the cases (82.67%). However, Roux-en-Y jejunal reconstruction was chosen in patients with diseased or absent extrahepatic bile ducts (eg, patients with biliary atresia or primary sclerosing cholangitis) and when the recipient duct was unfit for reconstruction (ie, devascularized and short) [8,10,11].
IMMUNOSUPPRESSIVE PROTOCOL:
The posttransplant immunosuppressants protocol is presented in Figure 1. Basiliximab (Simulect; Novartis Pharma AG, Basel, Switzerland) was intravenously administered (20 mg) twice, 6 h after portal vein reperfusion and on post-LDLT day 4. Steroid therapy consisted of intraoperative intravenous methylprednisolone (500 mg) followed by 20 mg/d (switched to oral prednisolone 20 mg/d once the patient could tolerate oral medication), which was tapered down and withdrawn after 3 months if no acute cellular rejection occurred. Patients with stable vital signs and renal function were given tacrolimus (Prograf; Fujisawa, Kerry, Ireland) at a dose to maintain trough levels at 8–10 ng/mL during the first week after LDLT. Mycophenolate mofetil (CellCept; Roche, Humacoa, Puerto Rico) was continuously administered at 1 g/d. Patients with a diagnosis of unfavorable tumor histology or confirmed recurrences were given sirolimus (Rapamune; Pfizer, NY, USA) at a dose to maintain trough levels at 3–8 ng/mL (Figure 1) [14].
POST-LT MONITORING FOR ANTIBODY-MEDIATED REJECTION IN ABOI LDLT:
During the first week after transplantation, the anti-A and -B IA titers were checked daily. Thereafter, both parameters were measured every other day during the first month after LDLT, and followed at every outpatient clinic visit after discharge or suspicion of rejection. For any clinical suspicion of antibody-mediated rejection, including increased IA titers, hemolysis, and nonsurgical vascular complications for an abnormal liver function test, additional PE was arranged, the dosage of immunosuppressants was increased, and liver biopsy was performed. If biopsy revealed histological evidence of rejection, steroid pulse therapy (10–20 mg/kg per dose) was administered with repeated PE and/or high-dose intravenous immunoglobulin (0.8 g/kg/d).
POST-LT FOLLOW-UP AND DIAGNOSIS OF BILIARY COMPLICATIONS:
Laboratory tests including liver function tests were performed daily during the Intensive Care Unit stay after transplantation, 3 times a week during ordinary ward stay, and at every outpatient clinic visit after discharge. Ultrasonography was routinely used to monitor biliary and vascular complications. Patients with ultrasound findings suspicious for vascular complications or biliary complications were further evaluated with computed tomography angiography or magnetic resonance cholangiography [8].
Biliary complications included bile leakage and biliary stricture. Bile leakage was defined as the presence of bile content in the drainage tubes that persisted beyond 1 week after transplantation or as the presence of a biloma. An anastomotic biliary stricture was defined as intrahepatic duct dilatation >3 mm in the presence of a notable extrahepatic biliary narrowing and symptomatic or with abnormal liver function tests [8,11]. Patients with DIHBS were not included in this study.
STATISTICAL ANALYSIS:
Data are expressed as percentages and continuous values as means±standard deviation, or as medians and interquartile range if data were not normally distributed. Fisher’s exact or chi-square test was used to compare categorical variables. Continuous variables between groups were compared using the
Results
PROPENSITY SCORE-MATCHING REPORT:
We chose the greedy data-matching method with a 0.25×(standard deviation of the propensity score) caliper half-width, using NCSS 11. The standardized mean difference after matching was less than 10%.
PATIENT CHARACTERISTICS:
Thirty adult patients, including 25 men and 5 women, underwent ABOi LDLT between 2013 and 2018. The mean age was 53.9±7.49 years. The MELD scores ranged from 6 to 21, with a median of 11. The underlying liver etiologies were hepatitis B-related liver failure (n=15, 50.0%) followed by alcoholic cirrhosis (n=7, 23.3%). Seventeen of the 30 patients (56.7%) had combined hepatocellular carcinoma. The mean graft-to-recipient weight ratio was 0.87±0.2%. The clinical characteristics of patients receiving ABOi LDLT did not differ from those of patients who underwent ABOc LDLT, except for body mass index (P=0.02) (Table 1).
With regard to surgical factors, most ABOi grafts were right lobe grafts (n=19, 63%). In terms of biliary reconstruction, most of the ABOi biliary anastomoses were duct-to-duct reconstruction (n=28, 93.3%). The number of bile duct orifices with a 1-to-1 anastomosis was 23 (76.7%) followed by those with a 2-to-2 anastomosis (n=3, 10%). There were no significant differences compared with the ABOc group, including cold/warm ischemic time, operation time, and blood loss (Table 2).
OUTCOMES:
There was no in-hospital mortality associated with ABOi LDLT, but 2 patients in the ABOc-matched group died during the same admission after LT owing to bleeding and sepsis. The long-term survival rates of the ABOi patients were comparable to those of the patients who underwent ABOc LDLT. None of the ABOi or ABOc LDLT recipients underwent re-transplantation. Therefore, graft survival and patient survival were the same in this study. The 5-year ABOi graft and patient survival rate was 87.1%, which did not differ from that of the ABOc recipients (87.4%; P=0.964; Figures 2, 3).
Two ABOi recipients had hepatic artery complications. One patient was found to have hepatic artery kinking and underwent repositioning of the hepatic artery on postoperative day 2. Another patient was found to have no hepatic artery inflow 1 month after LT. CT angiography showed hepatic artery thrombosis and the patient was treated successfully with urokinase infusion. In addition, 1 ABOi recipient encountered portal vein stenosis 10 months after the transplantation and percutaneous transhepatic angioplasty was successfully performed. There was no significant difference regarding acute cellular rejection within 1 year in either group (23.3% vs 21.7%, P=0.86) (Table 3).
BILIARY COMPLICATIONS:
Twelve (40%) recipients of ABOi LDLT had biliary complications, which were less common in ABOc LDLT recipients (15%, P=0.01, Table 4). Among the ABOi LDLT patients with biliary complications, 1 experienced bile leak and the other 11 had anastomotic strictures. These complications were successfully treated with surgical revision (n=1), endoscopic retrograde biliary stenting (n=10), and combined biliary stents with percutaneous transhepatic cholangial drainage (n=1). Most of the complications were completely resolved.
No histologically proven antibody-mediated rejection was documented in either group. Four ABOi recipients experienced a rebound of IA titers after LT. Among them, 3 were treated successfully by additional plasma therapy, while the fourth received observation only due to having no abnormal liver functions. In addition, there was no correlation between the incidence of biliary complications between rebound of IA and the incidence of acute cellular rejection (Table 5).
In a univariate analysis of risk factors of biliary complication in 90 adult LDLT patients, ABO incompatibility was the only significant risk factor. Other variables, including age, sex, disease, comorbidity with hepatocellular carcinoma, and graft type did not differ. Multivariate analysis was performed using binary logistic regression, including the variables (ABO compatibility, graft type, single/multiple biliary anastomosis) with a P value of <0.1 in the univariate analysis (Table 6). Table 7 shows the variables that were entered into the multivariate analysis for the development of biliary complications. ABO incompatibility, multiple biliary anastomosis, and left lobe graft harvest were significantly related to an increased risk of biliary complications.
Discussion
With the introduction of rituximab and robust development of a desensitization protocol, we undertook our first ABOi adult LDLT in March 2013 and achieved a 5-year graft and patient survival rate of 87.1%. We observed similar survival outcomes in the propensity score-matched cohort in our study, which was comparable to other centers. However, biliary complications are still a major concern in ABOi LDLT. Among patients undergoing this procedure, 8.57% developed DIHBS. In terms of anastomotic biliary complications, the incidence was significantly higher (40% vs 15%,
Our literature review revealed that biliary complications in LDLT range from 10% to 50%. In most of these cases, the complications involve DIHBS or anastomotic stricture, with DIHBS occurring in about 7% to 20% of cases, and anastomotic stricture rate being about 10% to 50% [2,3,6,15–17]. Song et al [2] reported an overall incidence of ABOi biliary complications, including DIHBS, of around 19.6%, which was significantly higher than the incidence of biliary stricture in the ABOc group (12.0%,
Previously, anastomotic biliary strictures were thought to result from technical surgical problems or local ischemia. In terms of surgical techniques, our center has developed MBR, which effectively addresses the difficulties in biliary reconstructions due to anatomical variations and size discrepancies between graft and recipient ducts. The routine use of MBR can decrease the number of anastomotic biliary complications in LDLT. Based on previous experiences, a classification system for biliary reconstruction was also used, which reduced the biliary complication rates from 40.0% to 10.2% [8–11].
It is believed that the microsurgical biliary reconstruction could reduce the incidence of biliary complications in ABOi, as shown by our experience in ABOc LDLT. However, our data revealed that MBR did not further reduce the biliary complication rate in ABOi, and the incidence was actually higher than reported in some previous studies. The current study identified additional phenomena indicating that the anastomotic strictures not only derive from surgical techniques but also from other risk factors. ABOi, multiple biliary anastomosis, and left lobe graft harvest were the 3 risk factors for biliary complications identified in multivariate analysis. While our previously published results showed no significant differences in the biliary complication rate between single duct opening and multiple duct openings (6.14% vs 8.89%,
The role of the ABO antibody titer remains poorly defined and controversial. Based on the published theory, ABO antigens in the donor graft activate the preformed IA and proliferation of B cells, causing damage to liver grafts. The main targets are the endothelial cells on the graft hepatic artery and portal vein and the epithelial cells on the bile duct. The activation of the immune response contributes to biliary strictures and vascular thrombosis, leading to graft ischemia, severe cholestasis, and finally, graft loss [10].
Currently, the anti-CD20 monoclonal antibody rituximab plays a fundamental role in desensitization by depleting B cells through its complement-dependent cellular cytotoxicity. Some centers have even proposed the use of rituximab alone without plasmapheresis to achieve sufficient desensitization [18,19]. However, the effectiveness of preventing posttransplant antibody rebound is still debated. Anti-CD20 monoclonal antibody eliminates CD20+ B cells for up to 6 months, but it does not directly suppress antibody-producing plasma cells [20]. Plasma B cells are activated after encountering the allograft and produce antibodies thereafter. Since rituximab cannot eliminate plasma cells that are present on the epithelium of the bile ducts, higher biliary stricture may occur despite desensitization [6]. Furthermore, antibody production at low levels is still possible. Therefore, Rummler et al [20] proposed a plasma treatment procedure combined with quadruple immunosuppression (steroids, calcineurin inhibitors, antimetabolites, and monoclonal antibodies) to address antibody rebound. Other effective measures to conquer post-LT B-cell responses have also been published, including monoclonal antibodies that target plasma cells and memory B cells or the complement system. Previous research has indicated the potential utility of including bortezomib and eculizumab, recently introduced proteasome inhibitors, as plasma cell-depleting agents [10,15]. However, future study is needed to prove the efficacy and safety.
However, some studies have shown no correlation between a high IA titer and biliary complications, including a single-center study by Song et al [2] and a large multicenter by Egawa et al [21]. Our data are also compatible with such findings. Although some hypotheses and theories on intra-graft expression of ABO antigen and adaptation process have been introduced [5], strong evidence or definite proof to support this phenomenon is lacking. Further investigations need to be conducted in this field. Thus, lowering IA titers should always be the aim according to current knowledge.
Since MBR did not reduce the biliary complications from the surgical technique aspect, the remaining unresolved problem is to understand the detailed immunologic mechanism in ABOi LDLT and subsequently create a sufficient desensitization protocol. Based on results from the present study, we are now developing a modified desensitization protocol, including the use of mycophenolate mofetil 1 week before LT, with an emphasis on lowering the pretransplant IA titer to <1: 16 and using PE and intravenous immunoglobulin to overcome the antibody rebound (Figure 4). Continued patient enrollment and longer follow-up are the next steps if we are to overcome the biliary complications in ABOi LDLT.
This study has some limitations. First, our sample size was small. Second, this was a single-center, retrospective study. There was undoubtedly some patient selection bias. Finally, we were not able to evaluate any data concerning biochemical or immunological responses to identify definite risk factors and precautionary measures in the present study.
Conclusions
Although the current desensitization protocol gives comparable survival outcomes in adult ABOi LDLT, which successfully reduces the burden of organ shortage, the higher incidence of biliary complications still remains the Achilles heel of LT and is beyond the issue of surgical techniques. Further research in transplant immunology is mandatory to solve the obstacle.
Figures
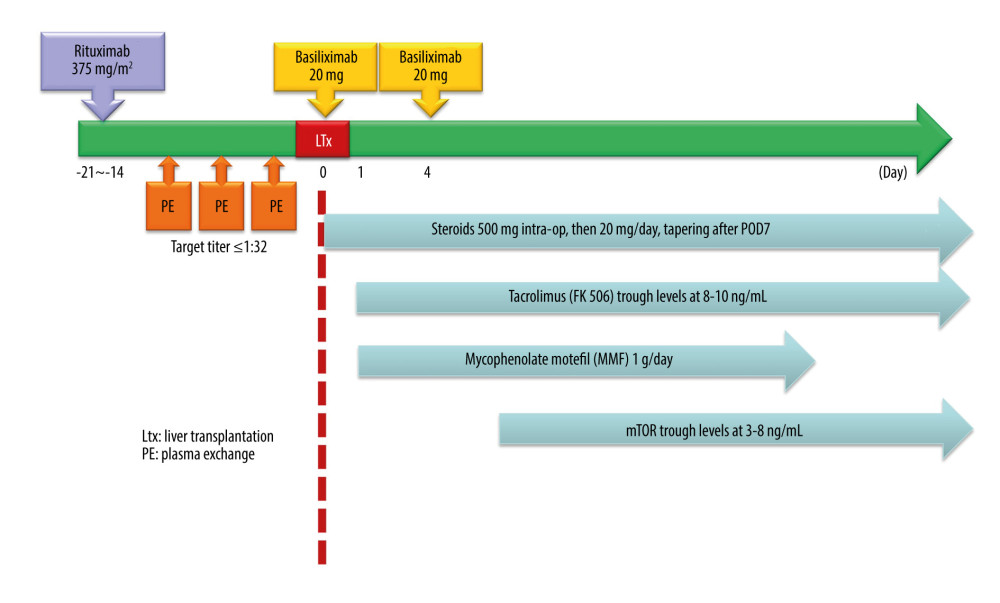 Figure 1. Desensitization protocol for ABO-incompatible living donor liver transplantation.
Figure 1. Desensitization protocol for ABO-incompatible living donor liver transplantation. 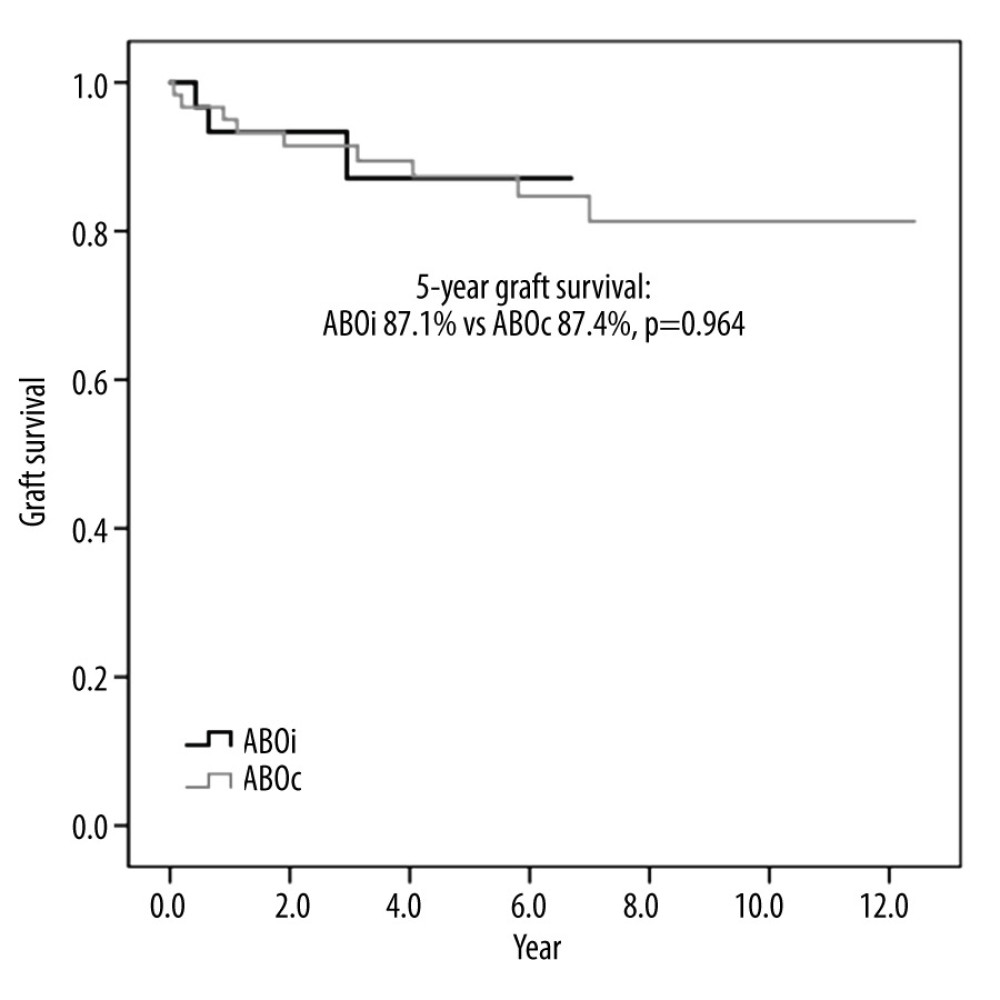 Figure 2. Graft survival of ABO-incompatible and ABO-compatible living donor liver transplantation
Figure 2. Graft survival of ABO-incompatible and ABO-compatible living donor liver transplantation 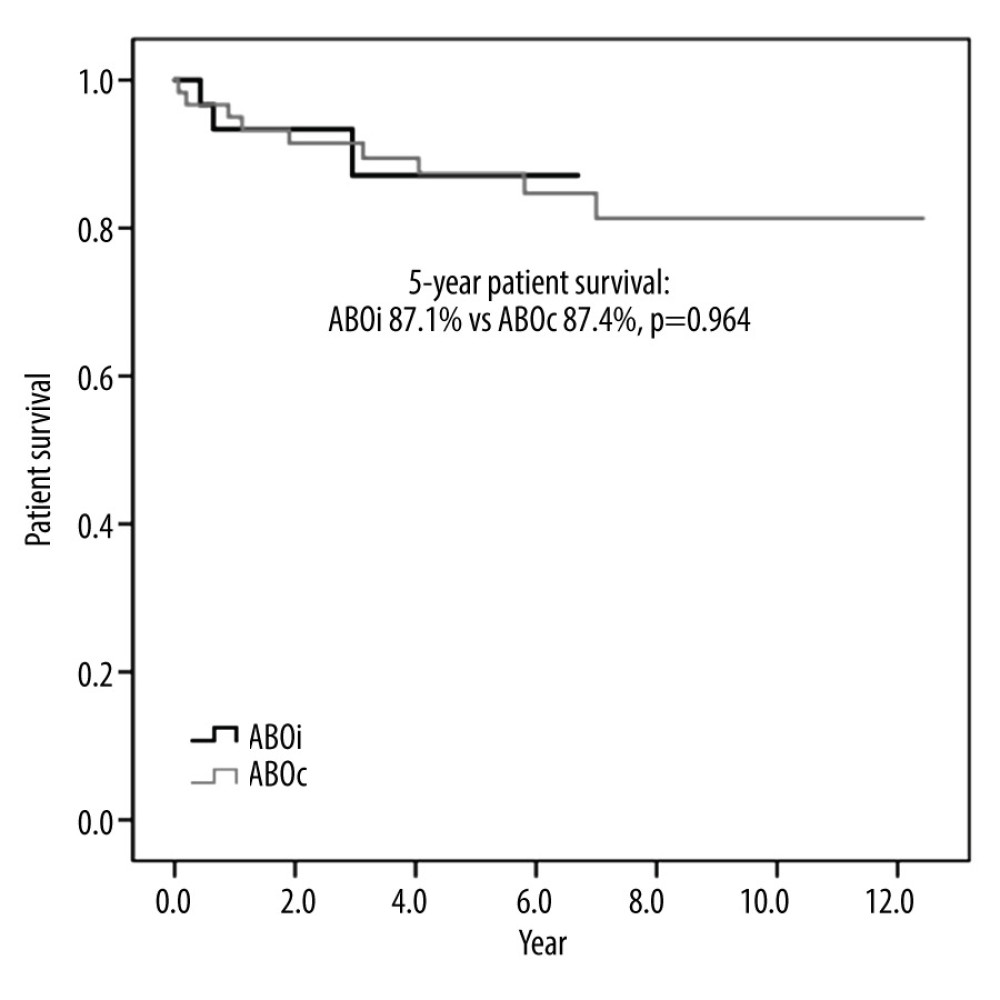 Figure 3. Patient survival of ABO-incompatible and ABO-compatible living donor liver transplantation.
Figure 3. Patient survival of ABO-incompatible and ABO-compatible living donor liver transplantation. 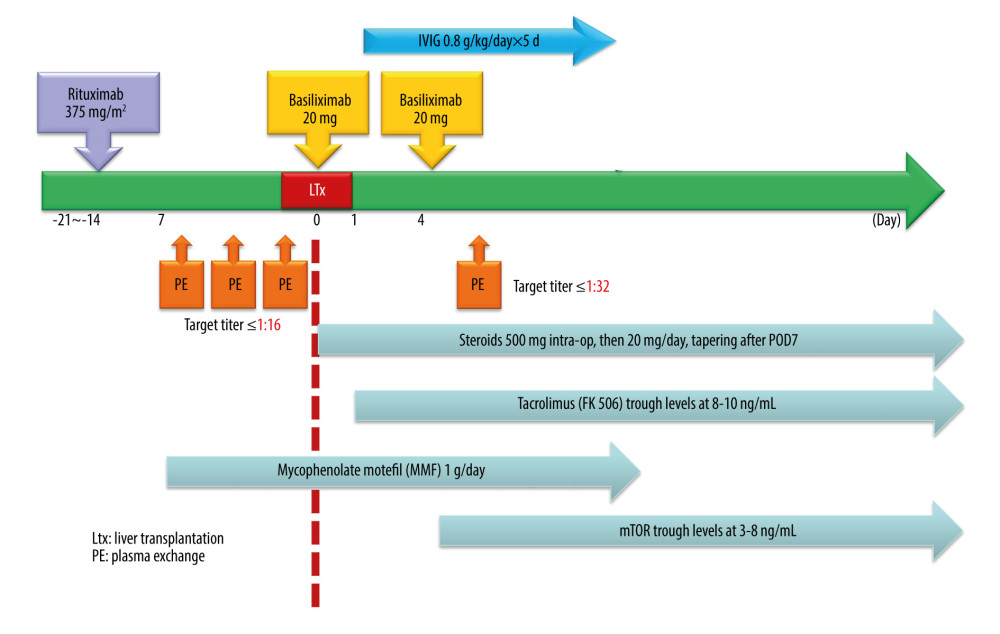 Figure 4. Proposed modification of desensitization protocol of ABO-incompatible living donor liver transplantation.
Figure 4. Proposed modification of desensitization protocol of ABO-incompatible living donor liver transplantation. Tables
Table 1. Clinical characteristics of ABO-incompatible and ABO-compatible patients.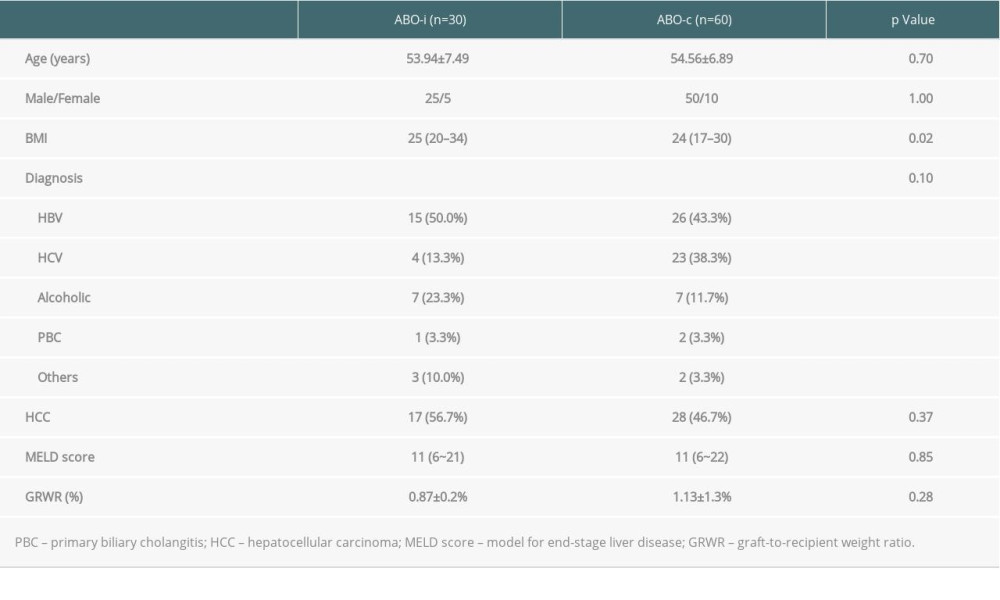 Table 2. Surgical data of ABO-incompatible and ABO-compatible patients.
Table 2. Surgical data of ABO-incompatible and ABO-compatible patients.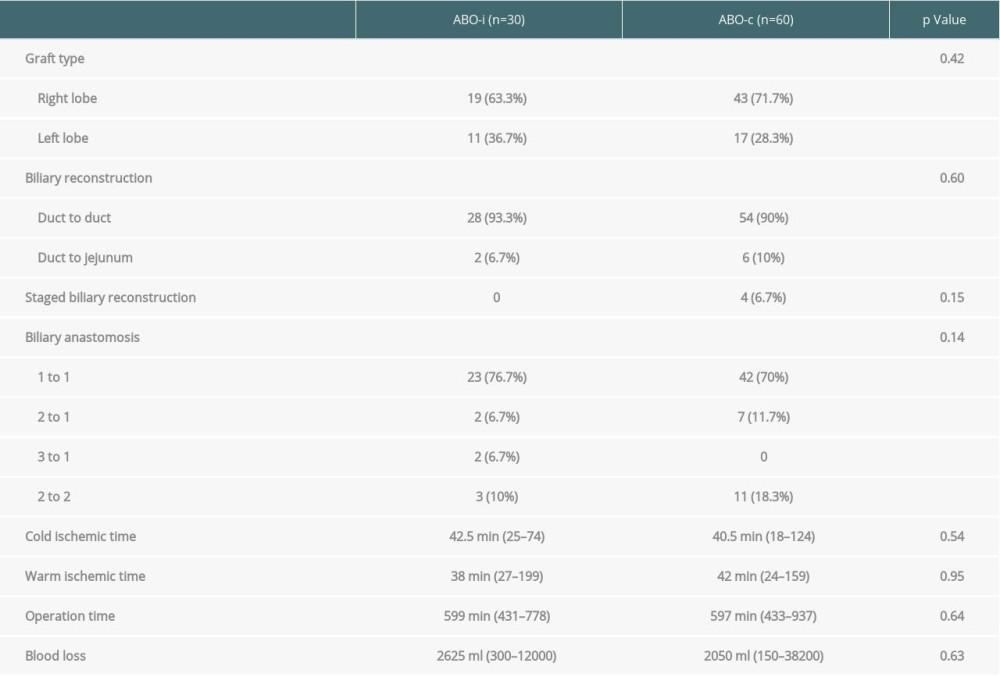 Table 3. Medical and surgical complications of ABO-incompatible and ABO-compatible patients.
Table 3. Medical and surgical complications of ABO-incompatible and ABO-compatible patients.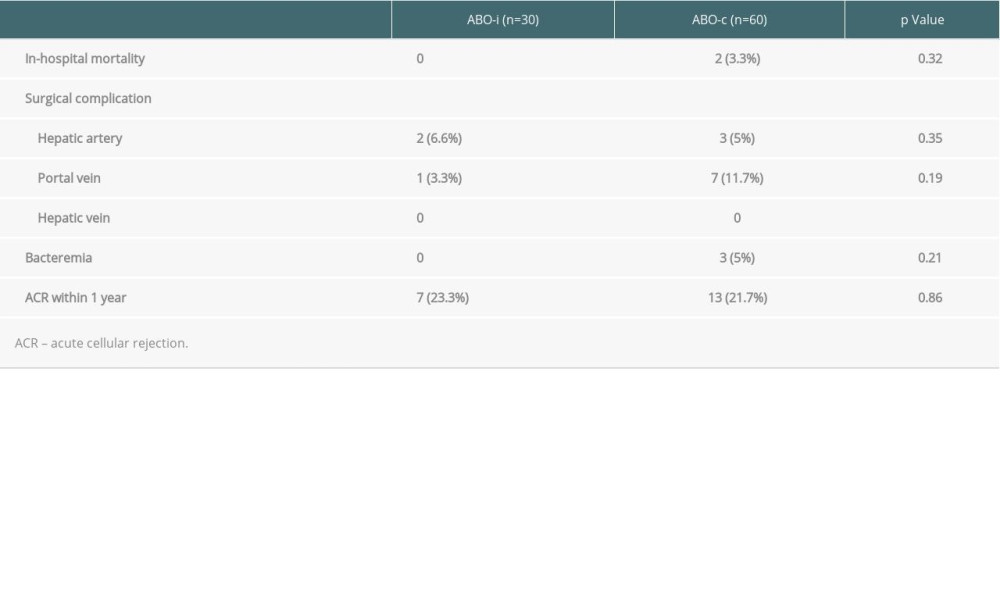 Table 4. Biliary complications of ABO-incompatible and ABO-compatible patients.
Table 4. Biliary complications of ABO-incompatible and ABO-compatible patients.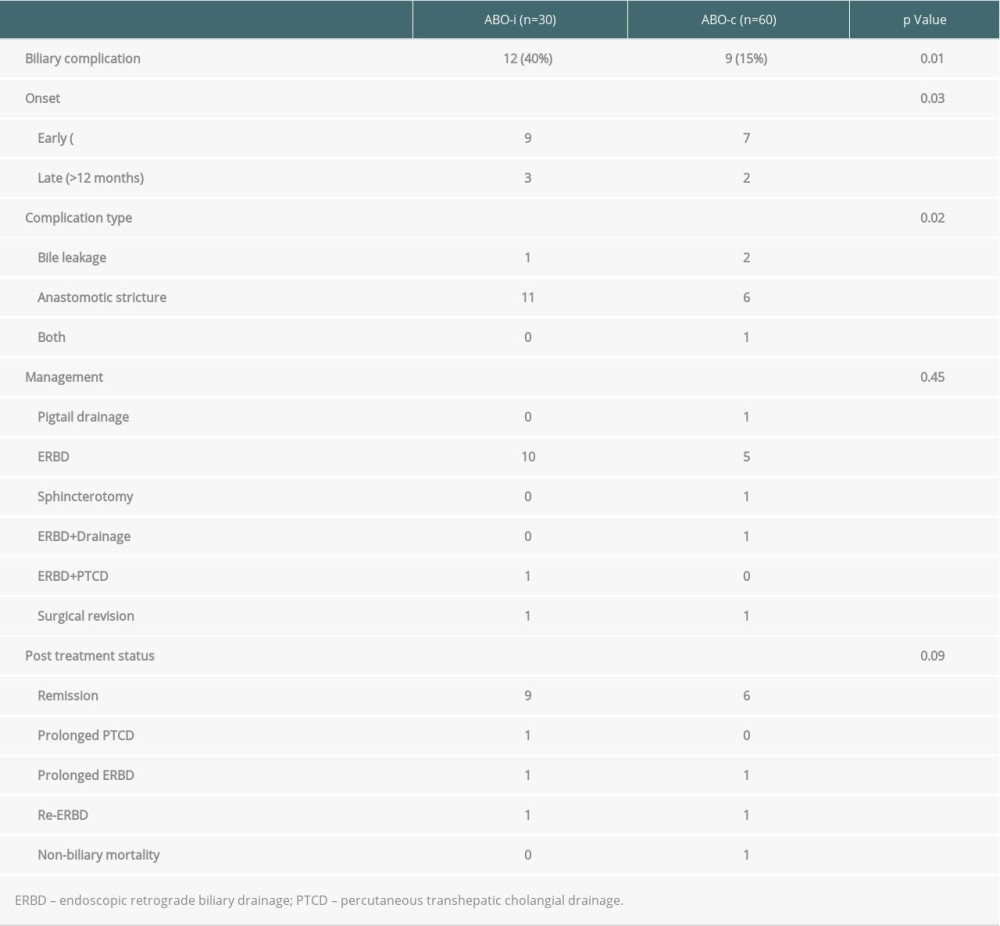 Table 5. Univariate analysis of the risk factors for biliary complication in 30 ABO-incompatible patients.
Table 5. Univariate analysis of the risk factors for biliary complication in 30 ABO-incompatible patients.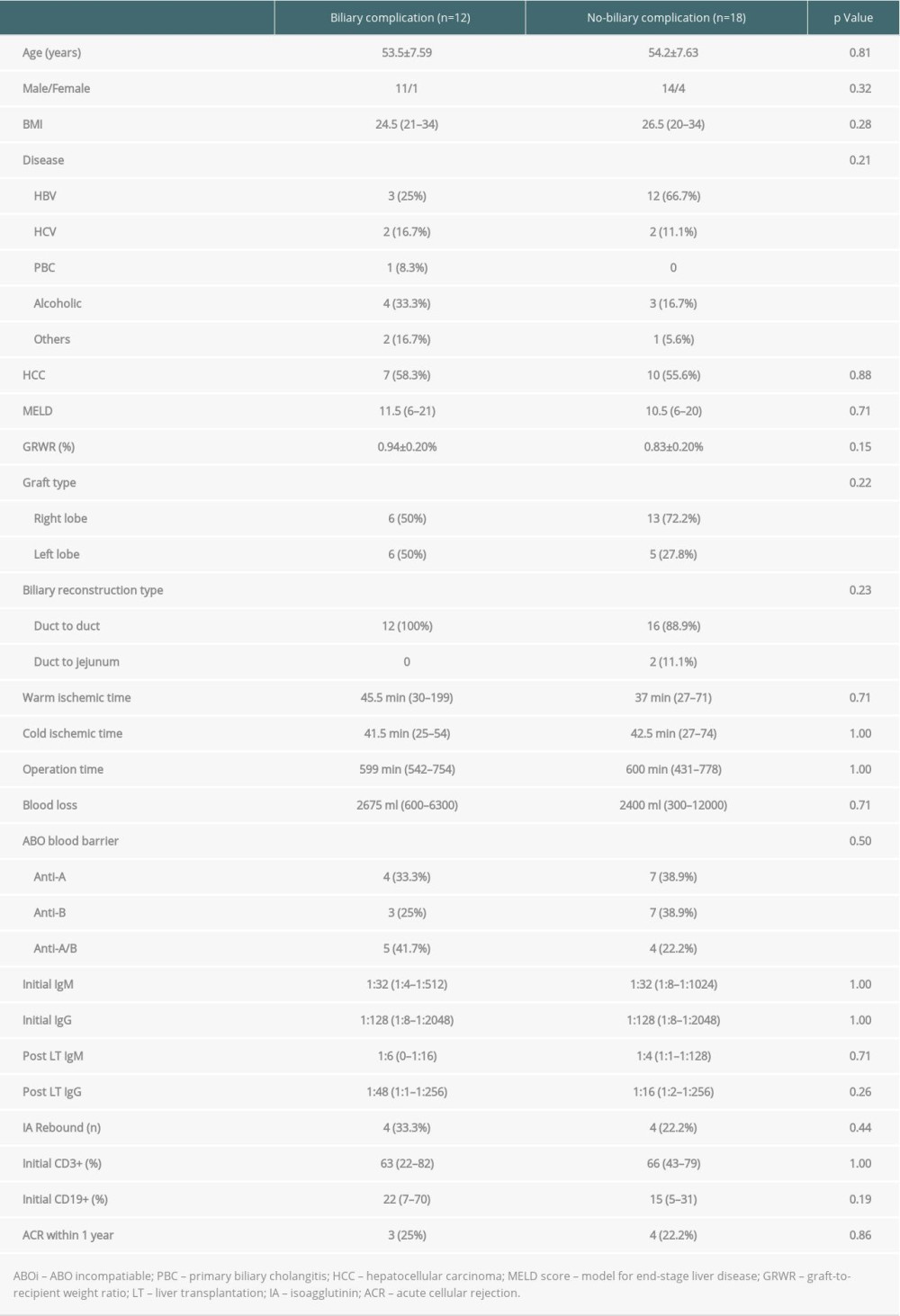 Table 6. Univariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.
Table 6. Univariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.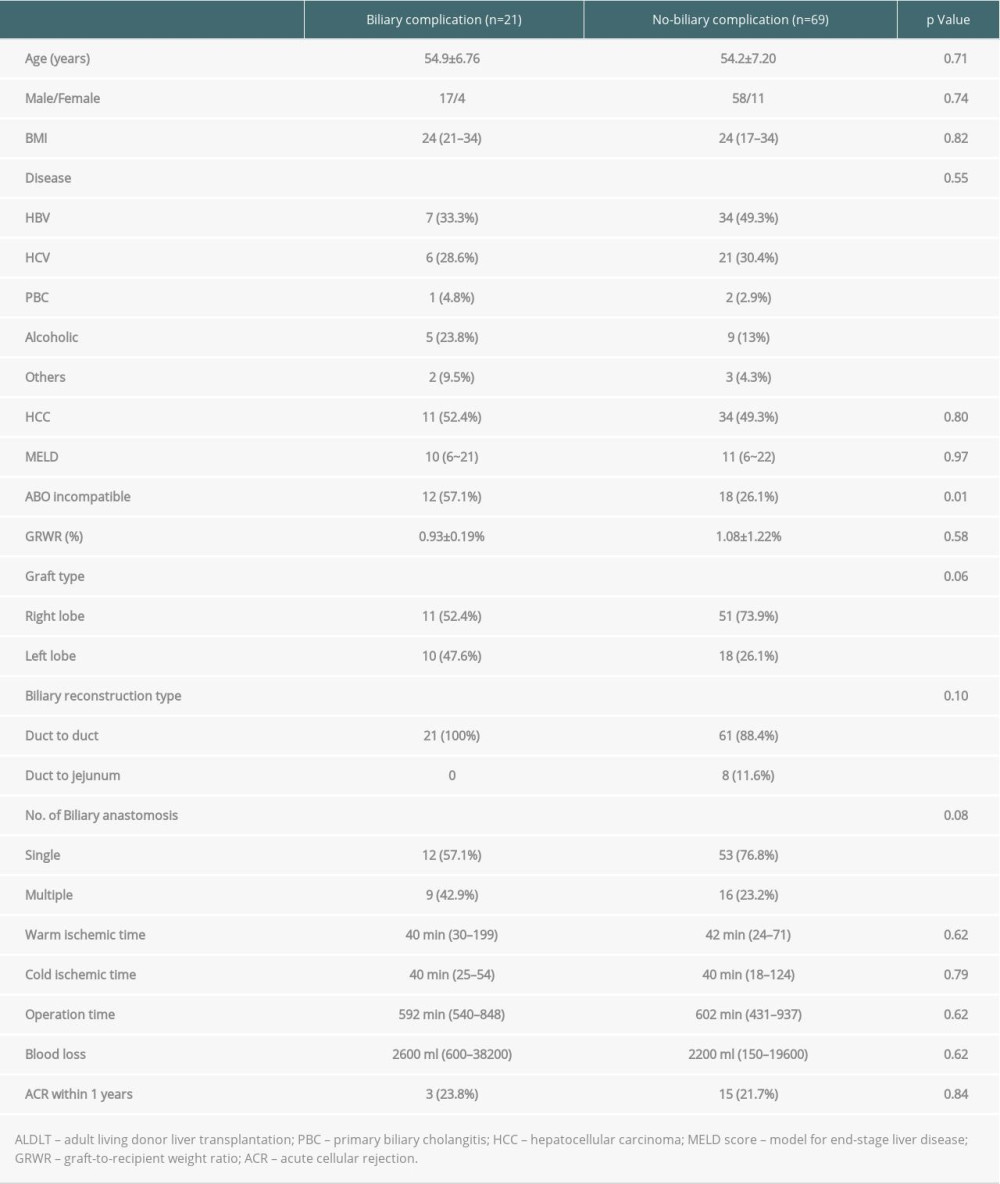 Table 7. Multivariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.
Table 7. Multivariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.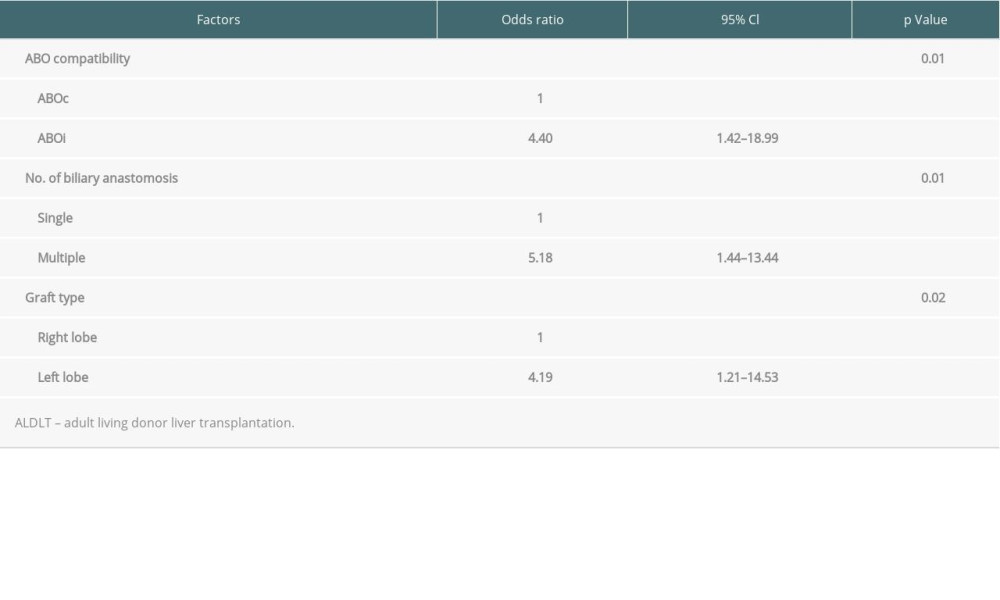
References
1. Chen CL, Kabiling CS, Concejero AM, Why does living donor liver transplantation flourish in Asia?: Nat Rev Gastroenterol Hepatol, 2013; 10(12); 746-51
2. Song GW, Lee SG, Hwang S, ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab: Am J Transplant, 2016; 16(1); 157-70
3. Lee CF, Cheng CH, Wang YC, Adult living donor liver transplantation across ABO-incompatibility: Medicine (Baltimore), 2015; 94(42); e1796
4. Tanabe M, Kawachi S, Obara H, Current progress in ABO-incompatible liver transplantation: Eur J Clin Invest, 2010; 40(10); 943-49
5. Song GW, Lee SG, Hwang S, Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era: J Hepatol, 2014; 61(3); 575-82
6. Yadav DK, Hua YF, Bai X, ABO-incompatible adult living donor liver transplantation in the era of rituximab: A systematic review and meta-analysis: Gastroenterol Res Pract, 2019; 2019; 8589402
7. Jung DH, Ikegami T, Balci D, Bhangui P, Biliary reconstruction and complications in living donor liver transplantation: Int J Surg, 2020; 82S; 138-44
8. Lin TS, Chen CL, Concejero AM, Early and long-term results of routine microsurgical biliary reconstruction in living donor liver transplantation: Liver Transpl, 2013; 19(2); 207-14
9. Lin TS, Co JS, Chen CL, Ong AD, Optimizing biliary outcomes in living donor liver transplantation: Evolution towards standardization in a high-volume center: Hepatobiliary Pancreat Dis Int, 2020; 19(4); 324-27
10. Chen CL, Cheng YF, Yu CY, Living donor liver transplantation: The Asian perspective: Transplantation, 2014; 97(Suppl 8); S3
11. Lin TS, Concejero AM, Chen CL, Routine microsurgical biliary reconstruction decreases early anastomotic complications in living donor liver transplantation: Liver Transpl, 2009; 15(12); 1766-75
12. Chen YS, Cheng YF, De Villa VH, Evaluation of living liver donors: Transplantation, 2003; 75(3 Suppl); S16-19
13. Chang CC, Chen YJ, Huang TH, Living donor liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma: experience of a single center: Ann Transplant, 2017; 22; 115-20
14. Ling LL, Hsu CC, Yong CC, FDG-PET predicted unfavorable tumor histology in living donor liver transplant recipients: A retrospective cohort study: Int J Surg, 2019; 69; 124-31
15. Hsu SC, Thorat A, Jeng LB, ABO-incompatible living donor liver transplantation with reduced rituximab dose: A retrospective analysis of 65 patients – can we fast-track liver transplant surgery and improve long-term survival?: Ann Transplant, 2020; 25; e923502
16. Testa G, Vidanovic V, Chejfec G, Adult living-donor liver transplantation with ABO-incompatible grafts: Transplantation, 2008; 85(5); 681-86
17. Ikegami T, Yoshizumi T, Soejima Y, Feasible usage of ABO incompatible grafts in living donor liver transplantation: Hepatobiliary Surg Nutr, 2016; 5(2); 91-97
18. Lee EC, Kim SH, Shim JR, Park SJ, A comparison of desensitization methods: Rituximab with/without plasmapheresis in ABO-incompatible living donor liver transplantation: Hepatobiliary Pancreat Dis Int, 2018; 17(2); 119-25
19. Yamamoto H, Uchida K, Kawabata S, Feasibility of monotherapy by rituximab without additional desensitization in ABO-incompatible living-donor liver transplantation: Transplantation, 2018; 102(1); 97-104
20. Rummler S, Bauschke A, Baerthel E, ABO-incompatible living donor liver transplantation in focus of antibody rebound: Transfus Med Hemother, 2017; 44(1); 46-51
21. Egawa H, Teramukai S, Haga H, Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: A Japanese multicenter study: Am J Transplant, 2014; 14(1); 102-14
Figures
 Figure 1. Desensitization protocol for ABO-incompatible living donor liver transplantation.
Figure 1. Desensitization protocol for ABO-incompatible living donor liver transplantation. Figure 2. Graft survival of ABO-incompatible and ABO-compatible living donor liver transplantation
Figure 2. Graft survival of ABO-incompatible and ABO-compatible living donor liver transplantation Figure 3. Patient survival of ABO-incompatible and ABO-compatible living donor liver transplantation.
Figure 3. Patient survival of ABO-incompatible and ABO-compatible living donor liver transplantation. Figure 4. Proposed modification of desensitization protocol of ABO-incompatible living donor liver transplantation.
Figure 4. Proposed modification of desensitization protocol of ABO-incompatible living donor liver transplantation. Tables
 Table 1. Clinical characteristics of ABO-incompatible and ABO-compatible patients.
Table 1. Clinical characteristics of ABO-incompatible and ABO-compatible patients. Table 2. Surgical data of ABO-incompatible and ABO-compatible patients.
Table 2. Surgical data of ABO-incompatible and ABO-compatible patients. Table 3. Medical and surgical complications of ABO-incompatible and ABO-compatible patients.
Table 3. Medical and surgical complications of ABO-incompatible and ABO-compatible patients. Table 4. Biliary complications of ABO-incompatible and ABO-compatible patients.
Table 4. Biliary complications of ABO-incompatible and ABO-compatible patients. Table 5. Univariate analysis of the risk factors for biliary complication in 30 ABO-incompatible patients.
Table 5. Univariate analysis of the risk factors for biliary complication in 30 ABO-incompatible patients. Table 6. Univariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.
Table 6. Univariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients. Table 7. Multivariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.
Table 7. Multivariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients. Table 1. Clinical characteristics of ABO-incompatible and ABO-compatible patients.
Table 1. Clinical characteristics of ABO-incompatible and ABO-compatible patients. Table 2. Surgical data of ABO-incompatible and ABO-compatible patients.
Table 2. Surgical data of ABO-incompatible and ABO-compatible patients. Table 3. Medical and surgical complications of ABO-incompatible and ABO-compatible patients.
Table 3. Medical and surgical complications of ABO-incompatible and ABO-compatible patients. Table 4. Biliary complications of ABO-incompatible and ABO-compatible patients.
Table 4. Biliary complications of ABO-incompatible and ABO-compatible patients. Table 5. Univariate analysis of the risk factors for biliary complication in 30 ABO-incompatible patients.
Table 5. Univariate analysis of the risk factors for biliary complication in 30 ABO-incompatible patients. Table 6. Univariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.
Table 6. Univariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients. Table 7. Multivariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients.
Table 7. Multivariate analysis of the risk factors for biliary complications in 90 living donor liver transplantation patients. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








