26 January 2022: Review Paper
Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models
Wojciech Andrzej Straś1AEF, Dariusz Wasiak1F, Beata Łągiewska2F, Olga Tronina3EF*, Marta Hreńczuk1F, Joanna Gotlib4F, Wojciech Lisik2AF, Piotr Małkowski1AEFGDOI: 10.12659/AOT.934924
Ann Transplant 2022; 27:e934924
Abstract
ABSTRACT: Liver transplantation (LTx) is the best treatment for patients with early-stage hepatocellular carcinoma (HCC). The Milan criteria positively influenced results of liver transplantation and were adopted by the majority of cancer centers, becoming the criterion standard treatment for early-stage HCC. Despite the use of restrictive criteria, recurrence is still high, affecting between 8% and 20% of cases, and is a significant predictor of survival after LTx. The diagnosis of both micro-and macro-invasion of vessels, which are significant factors in determining the frequency of recurrence and overall survival, significantly decreases the success of transplantation, causing an increase in mortality of 50% in comparison to recipients with no vascular invasion. The risk of recurrence depends on several factors, which are discussed in this review. The authors also discuss the clinical presentation and treatment methods of recurrence and its prognosis. In addition, the role of different models developed to identify groups of patients with high versus low risk of recurrence is discussed, enabling the planning of recommendations and screening protocols after transplantation to help early diagnosis and guide effective treatment. In the era of an increasing numbers of liver transplants due to HCC, the need to create robust screening tools is urgent.
Keywords: Carcinoma, Hepatocellular, Liver Transplantation, Risk Factors, Humans, Liver Neoplasms, Neoplasm Recurrence, Local
Background
Liver transplantation is included in the therapeutic methods of treatment for hepatocellular carcinoma (HCC). The simultaneous removal of the tumor and the underlying liver disease, usually cirrhosis, which is responsible for oncogenesis, seemed a perfect and reasonable procedure [1]. Nevertheless, the beginning was not easy. In the 1980s, a high percentage of recurrence, reaching as high as 50%, caused high mortality of recipients, with 5-year-long survival not exceeding 20% to 40% [1–3]. Such dismal outcomes influenced the decision of the US Department of Health and Human Services to list HCC as a contradiction for LTx [1,4].
Criteria introduced by Mazzafero (the Milan criteria, MC) for determining the eligibility of early-stage HCC patients (1 tumor up to 5 cm, or 3 tumors none of which exceeds 3 cm, lack of vascular invasion, and absence of extrahepatic spread) for LTx based on morphologic criteria of the tumor (ascertained by pretransplant imaging) changed the situation [1,5]. The Milan criteria improved the results of liver transplantation [5,6] and were adopted by the majority of cancer centers, becoming the criterion standard treatment for early-stage HCC [7,8]. With a growing proportion of LTx being performed for HCC, other criteria appeared (eg, University of California San Francisco, UCSF) that extended the indications through less restrictive requirements for the size and number of tumors. This expanded liver transplant treatment to patients with more advanced HCC [9–13]. However, the results of these transplantations tended to be poor. In the 5-year period after transplantation, rates of recurrence increased and 5-year survival decreased compared with patients meeting the Milan criteria [11,14]. The majority of investigators estimate that tumor recurrence (TR) occurs in 8% to 20% of cases [15–21], and increasing the rates of survival is still a challenge to transplant surgeons. It is believed that recurrence is contributed to by insufficient preoperative radiological assessment of the tumor’s parameters, which influences qualification of patients for transplantation. The assessment is unable to determine the aggressiveness of the tumor based on vascular invasion and histopathologic degree of differentiation [7,14,20–22]. The tumor’s biology, including its potential for recurrence, can only be evaluated through analysis of the explant, which is unavailable in the preoperative period. Numerous studies on genetic tumor biomarkers associated with recurrence involved postoperative assessment of extracted tumors. Due to this, the biomarkers could not be readily used in the qualification of patients for transplantation [23–26]. Research into some of the more widely used HCC biomarkers in clinical practice, which correlate with tumor progression, risk of recurrence, and general prognosis, has been ongoing for over a decade. They include alpha-fetoprotein (AFP) level and an indicator based on the neutrophil-to-lymphocyte ratio (NLR). Their preoperative assessment may be useful in qualification for transplantation and determining the degree of recurrence risk [19,20,27].
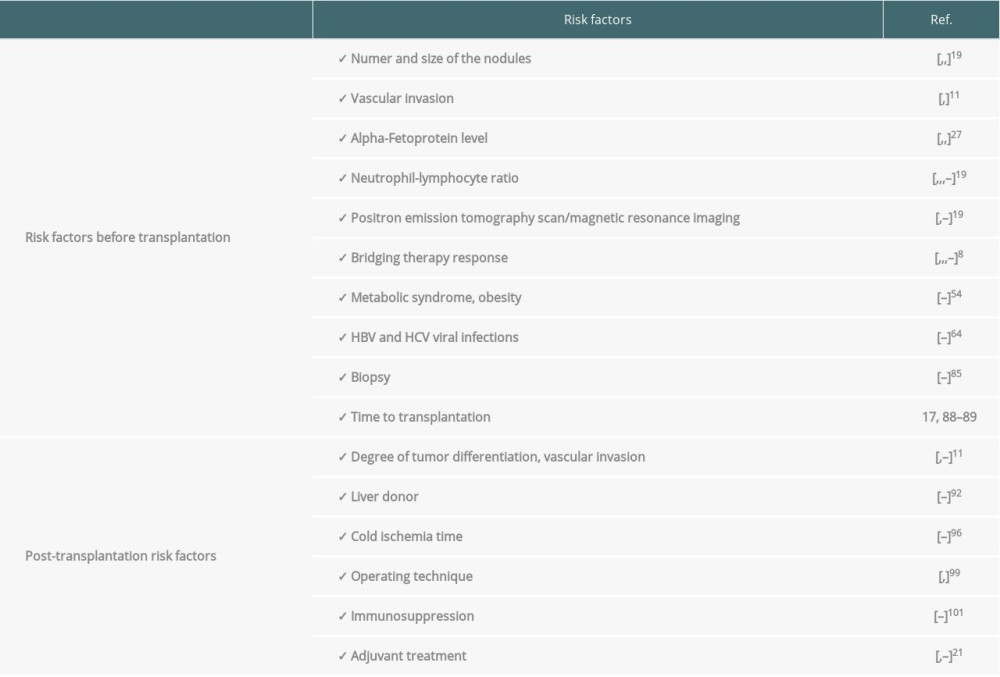
In the present study, we discuss risk factors influencing the frequency of recurrence, including both those that may be assessed before and after transplantation. These include factors to help determine transplantation eligibility among patients that have the lowest recurrence risk, as well as classification and duration of follow-up of liver recipients to aid in the early diagnosis of possible recurrence, which may allow for more prompt and effective treatment (Table 1) [7,19,20,27–30].
Risk Factors Before Procedure
NUMBER AND SIZE OF NODULES:
Use of the Milano criteria shows that restrictions on the size and number of tumors improves LTx results. Liver transplantation for patients whose tumors are above 5 cm increases the risk of recurrence [19]. However, it is different when there are more small tumors. The risk of recurrence does not increase with an increased number of tumors. In the case of small tumors, recurrence is likely caused by a small percentage of vascular invasion [31]. This was reported by Welling et al, who showed that an increase in the diameter of the largest tumor by 1 cm is associated with an increase of recurrence risk by 36%, with no connection to the other tumors [32].
VASCULAR INVASION:
The diagnosis of both micro-and macro-invasion of vessels, which are significant factors in determining the frequency of recurrence and overall survival (OS), significantly decrease the success of transplantation, causing an increase in mortality of 50% in comparison to recipients with no vascular invasion [11,19]. Any vascular macro-invasion, portal vein branch or hepatic vein, may sometimes be diagnosed in preoperative imaging and may be a basis to eliminate a patient from a transplant waiting list. However, accurate evaluation of this phenomenon is only possible after the procedure. The assessment of postoperative factors derived from detailed analysis of the tumor and vascular invasion from the extracted liver will be discussed in detail later.
AFP LEVEL:
Increased AFP level is found in around 60% of HCC cases and is an auxiliary marker in the diagnosis of HCC. High AFP levels may also be an indicator of prognosis and offer insight into the biological aggressiveness of the tumor, likely during the course of vascular microinvasion. Berry et al showed an inversely proportional relationship between AFP levels and patient survival after LTx [33]. Giard et al reported that an AFP increase of more than 7.5 ng/mL per month, independent of applied locoregional bridging therapy (radiofrequency ablation, RFA; transarterial chemoembolization, TACE), is associated with HCC recurrence after LTx and may be a component of vascular microinvasion [34]. Mahmud et al suggested that a high preoperative AFP level is associated with increased mortality caused by tumor recurrence [27].
Researchers have suggested different AFP cut-off values above which patients should not be qualified for LTx due to significant recurrence risk. Toso et al and Hameed recommended values of 400 ng/mL and 1000 ng/mL, respectively [35–37]. Recently, there are reports concerning homocysteine levels, which if >12.75 μmol/L, may be a marker of tumor aggressiveness in patients without increased AFP concentrations (AFP ≤20 ng/mL) [38]. Among patients with normal AFP levels, increased concentration of homocysteine (>12.75 μmol/L) was associated with a 53% decrease in the 3-year recurrence-free survival (RFS) rate in comparison to patients with low homocysteine levels, for whom the RFS was 92% [38].
NEUTROPHIL-TO-LYMPHOCYTE RATIO (NLR):
NLR is evaluated in peripheral blood and may indicate an inflammatory response to the tumor, increasing the risk of metastasis or recurrence, and, hence, a worse prognosis [19,39]. Halazun et al were the first to show that an NLR greater than 5 determined in the indirect preoperative period is associated with increased recurrence risk as well as shorter survival among patients operated on due to metastasis of colon cancer to the liver [40]. In their subsequent paper [41], they reported that an increase of the NLR by 5 is an independent factor for worse prognosis, increased risk of HCC recurrence, and shorter recurrence-free survival among patients who underwent LTx due to HCC, and this was confirmed by other authors [20,42,43].
A number of retrospective studies have conducted morphological and biological risk assessments aimed to create objective models of factors responsible for HCC recurrence in patients deemed eligible for LTx [22,29,44,45]. Since they were mostly based on factors determined after transplantation (size and volume measurements, number of tumors, vascular invasion, the degree of tumor aggressiveness), they will be discussed in detail in the latter parts of this paper.
POSITRON EMISSION TOMOGRAPHY (PET) SCAN; MAGNETIC RESONANCE IMAGING, MRI:
The effectiveness of PET in HCC diagnosis does not exceed 50% due to a high degree of heterogeneity among HCC tumors, in which some tumors metabolize glucose similarly to normal hepatocytes [19]. However, the use of the glucose analog (18)F-fluorodeoxyglucose ((18)F-FDG) as an isotopic marker may be useful in diagnosing more aggressive HCC [46]. PET visualization of HCC in the liver showing vascular invasion has been shown to be associated with recurrence and decreased 5-year survival after LTx [47,48]. Similarly, the presence of microsatellite instability (MSI) in tumors, determined by radiomic features assessed by MRI through the use of gadolinium derivatives, can affect vascular microinvasion and increase the risk of HCC recurrence after LTx [49].
BRIDGING THERAPY, BT, LRT RESPONSE:
Bridging therapy in the form of radiofrequency ablation and chemoembolization of the tumor is recommended in patients that meet the Milan criteria and are eligible for LTx when the waiting period for transplant is longer than 6 months [8], which could avoid having to remove patients from waiting lists due to tumor development. The benefits of bridging therapies, especially when they can achieve complete necrosis of the tumor, are currently questioned by some authors, who suggest there is a higher risk of recurrence in patients in whom locoregional therapy causes only partial necrosis of the tumor [50,51].
Apart from bridging therapy, locoregional therapy for tumors in HCC patients is used to downstage the tumor and allow a patient to meet the eligibility criteria for LTx. It has been shown that results of the therapy may be used to select LTx candidates more effectively. A positive response to treatment enables eligibility and decreases recurrence risk [16,30,51–53].
METABOLIC SYNDROME, OBESITY:
The percentage of liver transplantations performed to treat HCC caused by non-alcoholic steatohepatitis (NASH), often related to metabolic syndrome, has been increasing [54–56]. The risk of occurrence of HCC in patients with obesity and diabetes is 5 times higher than in patients without these disorders [57]. The patho-mechanism of oncogenesis is multifactorial, with hepatitis, cirrhosis, diabetes, insulin resistance, lipid metabolism disorders, intestinal dysbiosis, and genetic defects deemed to play a significant role in HCC occurrence [58–60]. According to some reports, obesity doubles mortality after LTx because of HCC. The risk of vascular invasion with a tendency for more frequent recurrence and shorter recurrence-free survival also decreases [61,62]. Some suggest that obesity stimulates oncogenesis by disrupting the balance between adiponectin and leptin levels, which causes an increase in the activity of vascular endothelial growth factor (VEGF), which stimulates angiogenesis and tumor proliferation [61,62]. In contrast to the above, in a study that compared transplantation outcomes in HCC patients with NASH etiology and cause of cancer other than NASH, no differences were observed in the frequency of recurrence, which was to 13.3% and 14%, respectively. Moreover, longer general survival and recurrence-free survival was noticed in the NASH patients [63]. A meta-analysis performed on 40 495 cases of LTx due to HCC published in 2021 showed an 8% lower rate of recurrence in NASH patients in comparison to those with a viral or alcohol etiology [64].
VIRAL INFECTIONS:
Infection with the hepatitis B and C virus (HBV and HCV, respectively) also increases recurrence risk. In the above-mentioned meta-analysis, Tan et al reported that among patients with HCC recurrence after LTx, 18% had an HBV etiology and 11% had an HCV etiology [64]. HBV infections are dominant in Asia; therefore, the majority of studies come from this region. Based on their meta-analyses, Li et al and Yuan et al reported that HBV therapy with nucleotide and nucleoside analogs decreases recurrence risk, extends recurrence-free survival, and increases 5-year overall survival in comparison to untreated patients [65,66]. The risk of HCC recurrence after LTx increases together with high viremia and may be connected to specific, carcinogenic mutation of the virus [67,68]. Teng et al suggested the possible significance of the oncogenic protein “Pre-S mutant”, which is a biomarker of increased risk of HCC and its recurrence in patients with chronic HBV infection [69].
In the case of HCV infection, the situation is unclear, especially in the context of treatment with non-interferon direct-acting antivirals (DAA). The first reports warned against increased HCC incidence (both de novo and recurrence after treatment) in patients with chronic HCV infection who underwent DAA therapy [70,71]. The majority of authors of subsequent meta-analyses and papers based on other research did not confirm the initial reports. Initially, the lack of proof for DAA therapy causing HCC development was pointed out. Currently, arguments for the decrease in other cancers and recurrence in patients with chronic HCV infection treated with non-interferon drugs have been presented [72–77]. Kohli et al and a group of French authors published papers showing a lower risk of HCC recurrence in patients after transplantation treated with both interferon and DAA [72,78]. In guidelines from the International Liver Transplantation Society (ILTS) published in 2017, DAA treatment for patients with HCC and cirrhosis during the course of HCV infection is recommended when the expected time of waiting for transplantation exceeds 3–6 months [79]. An international, multi-center study on the effect of antiviral therapy on transplantation outcomes in HCC cases with HCV infection showed that the 5-year recurrence-free survival in patients preoperatively treated with DAA, with interferon, and untreated was 93%, 84.8%, and 73.9%, respectively. DAA therapy while awaiting transplantation as well as postoperatively increased recurrence risk [80]. Studies by Jain et al and Lim et al among small numbers of HCC and HCV patients showed a higher recurrence risk in patients treated with DAA before liver transplantation compared with untreated patients. In the first study, 3 out of 5 patients did not obtain a sustained virologic response (SVR) [81,82]. A study conducted at a center in Padua, Italy did not show any differences in the percentage of dropout patients and in recurrence after LTx in HCC-HCV patients treated with DAA and those that were untreated [83]. Similarly, a multi-center retrospective study conducted in South America did not report tumor progression in patients treated with DAA awaiting LTx or higher recurrence rates (independent of DAA treatment pre- or postoperatively) in comparison to HCC patients treated for HCV [84].
BIOPSY:
Although the diagnosis of the majority of HCC tumors is based on imaging (CT and NMR with contrast [85]), in some diagnostically complex cases, tumor biopsy may be performed. Some authors suggest biopsy increases recurrence risk after transplantation, including extrahepatic metastasis [86,87].
TIME TO TRANSPLANTATION:
The waiting time for LTx for HCC patients has to be optimal. Too short a time does not allow the elimination of patients with aggressive cancer and poor prognosis, which is associated with high recurrence risk and short overall survival after the procedure [17,88]. A long wait period may cause the tumor to progress, which would exceed the Milan criteria and may cause the patient to drop out. Mehta et al showed that dropout was 3.2% and 12.4% when the time from HCC diagnosis to transplantation was 6 and 18 months, respectively. Moreover, a higher 5-year recurrence risk in patients who underwent transplantation earlier than 6 months or after 18 months since diagnosis was reported [89].
Post-Transplantation Risk Factors
DEGREE OF TUMOR DIFFERENTIATION, VASCULAR INVASION:
Tumor differentiation and vascular invasion can be precisely determined in a morphological examination of the liver explant. Both low-differentiated tumors and those with vascular invasion have a worse prognosis. Compared with more differentiated tumors, low-differentiated tumors increase recurrence risk (39.9% versus 13%) and reduce 5-year recurrence-free survival (39.9% versus 57.7%) [11,90]. Similarly, in 16.6% of patients meeting the Milan criteria, vascular invasion is a significant factor determining recurrence and that doubles mortality [11]. The diagnosis of micro- or macro-invasion increases recurrence risk by 2 and 8 times, respectively, while the 5-year recurrence-free survival is 44% and 13%, respectively, in comparison to 64% of patients without vascular invasion [91].
LIVER DONOR:
According to Sharma et al and Vagefi et al, donor age above 60 years is a factor for HCC recurrence risk after liver transplantation [92,93]. In a randomized study involving 9724 liver recipients, Orci et al showed that donor age above 60 years, diabetes, BMI ≥35, hepatic steatosis ≥60%, and an organ obtained after circulatory arrest with prolonged warm ischemia time increase the risk of HCC recurrence [94]. Based on findings from Kim et al, an increase in the weight of the right liver graft due to perfusion obtained from a living donor increases recurrence risk and reduces recurrence-free survival and overall survival among groups of increased versus decreased graft weight that have comparable tumor parameters [95].
ISCHEMIA TIME:
Although it is known that an extended cold ischemia time increases the risk of losing the graft [96], there have been few studies on the influence of ischemia time on HCC recurrence risk. Nagai et al studied 60 patients with HCC recurrence and found that prolonged cold ischemia time (CIT) ≥10 hours and ≥50 minutes of warm ischemia are independent risk factors of early recurrence (within one year of transplantation) [97]. Kornberg et al confirmed these reports, finding prolonged times of cold, warm and total ischemia in 24 patients with HCC recurrence in comparison to patients without recurrence [98].
OPERATING TECHNIQUE:
The potentially higher risk of not maintaining the proper margin of the removed tumor during transplantation by means of the “piggy-back” method (preserving the flow through the inferior vena cava) in comparison to the classic technique has not been shown [99,100]. Mangus et al did not observe differences in the recurrence frequency or recurrence-free survival time in HCC patients operated on with both techniques [99]. Grąt et al showed a higher risk of recurrence in the classic liver transplantation method [100].
IMMUNOSUPPRESSION:
Studies on immunosuppression after LTx in HCC patients have reported the effects of a high concentration of calcineurin inhibitors, prolonged steroid therapy, and the use of polyclonal antibodies on increased recurrence risk [101–104]. Simultaneously, studies have reported encouraging results for anti-cancer treatment with mTOR inhibitors. According to several studies, their use in immunosuppression regimens decreased the risk of recurrence and prolonged survival [105–109]. Publication of the results of a randomized, prospective, multi-center clinical study called the SILVER trial provided a more reliable assessment of the suitability of use of rapamycin derivatives in patients who underwent LTx due to HCC [110]. The study did not show a long-term recurrence-free survival of more than 5 years, but in the period between 3 and 5 years after transplantation, both recurrence-free and overall survival were clearly prolonged in the group of patients that met the Milan criteria. In a complementary exploratory analysis of a group of patients from the SILVER study treated with sirolimus, Schnitzbauer et al reported that use of the drug for longer than 3 months after transplantation reduced the risk of death. In patients with AFP ≥10 ng/mL, the benefits are even more evident with respect to increased overall survival and recurrence-free survival and reduced recurrence risk [111].
Two recently published papers assessed the association between acute liver rejection and risk of HCC recurrence. Gul-Klein et al found that recurrence was over twice as frequent in patients with a history of acute rejection incidents in comparison to patients without such incidents. Lai et al reported that the recurrence risk is 18 times higher in patients treated with steroid bolus in comparison to patients without rejection or without clinical manifestation of rejection who were not treated with steroids [112,113].
To complete the literature review on immunosuppression in HCC patients who underwent LTx, the ILTS guidelines published in 2020 should be cited. In the context of the lack of apparent proof for the effectiveness of mTOR inhibitors, the guidelines do not recommend a specific therapy and only recommend use of low doses of calcineurin inhibitors [21].
ADJUVANT TREATMENT:
Attempts to use tyrosine kinase inhibitors (such as sorafenib) after liver transplantations to reduce recurrence frequency, especially in patients not meeting the Milan criteria, was found to be fruitless due to the multiple adverse effects of sorafenib and the lack of effectiveness of such therapies [114–117]. According to oncology and ILTS experts, adjuvant treatment in HCC recurrence prevention is not recommended [21].
Recurrence Risk Stratification Models
Most of the above-mentioned factors for recurrence risk may eventually only be diagnosed postoperatively through the assessment of parameters determined in pathomorphological examination. Therefore, their usefulness in determining eligibility of patients for transplant is limited. There have been attempts at creating models and scales of risk of LTx failure based on pathomorphological, biochemical, and clinical parameters. Some of them reflect the biology of the neoplasm, indicating its aggressiveness [22,29,44,45,91,118]. Duvoux et al created a simple system for evaluation of recurrence risk based on the measure and number of tumors, as well as AFP. A maximum of 3 tumors, no bigger than 3 cm and AFP ≤100 ng/mL, were evaluated as 0 points, indicating no risk. Patients with ≤2 points belonged to the low-risk group and those with >2 points were in the high-risk group, with the recurrence percentages of 8.8% and 50.6%, respectively [45]. Lai et al proposed criteria based on AFP concentration and total diameter of the tumors. When AFP exceeds 400 ng/mL and the tumor diameter is 8 cm, LTx results are similar to the results of patients that met the Milan criteria [44].
The MORAL and RETREAT scales are the most commonly used [22,29]. The MORAL scale is divided into the following: preoperative criteria (Pre MORAL) in which 3 factors are evaluated (NLR >5, AFP >200 ng/mL, tumor >3 cm), postoperative criteria (Post MORAL) which assesses 4 factors (fourth degree of histopathological development of the tumor, vascular invasion, tumor >3 cm, number of alterations >3), and total (combo-MORAL). A numeric evaluation was introduced. Based on this evaluation, 4 risk groups (low, average, high, very high) were created. Five-year recurrence-free survival in the very high-risk group was 17.9% and 98.6% in the low-risk group. The Milan criteria are certainly less precise with respect to risk recurrence in comparison to MORAL [22]. In a study by Mehta et al, 721 HCC patients meeting the Milan criteria who underwent LTx between 2002 and 2012 were verified. The following criteria were evaluated: vascular invasion, AFP, the diameter of the largest tumor, and the number of tumors, allocating points to specific factors. The scale of degrees of risk ranged between 0 and 5 or more points, with 3% 5-year recurrence-free survival for 0 points and over 75% recurrence chance in the case of 5 or more points [29].
On the basis of factors like the size, number of tumors, AFP level, presence of vascular invasion, and cancer etiology (HCV or another), a new calculator [14] was designed in 2018. It is a modified version, expanded with postoperative factors of the Metroticket model [11], which allows determining the 5-year survival prognosis of a patient after LTx with a certain probability.
Another new model is the verified, modified, and expanded HALTHCC model, which was developed using data from 4089 patients [118,119]. Researchers from 16 centers in 8 European, North American, and Asian countries created a personalized, numeric model based on AFP level, MELD classification extended by the sodium level (Model for End-Stage Liver Disease-sodium-MELD-Na), tumor parameters, cirrhosis etiology, NLR, locoregional therapy results, and Milan criteria, as well as the significant postoperative factors of vascular invasion and histopathological differentiation of the tumor. Patients with low risk (0–5 points) have a very low percentage of cases of vascular invasion and low-differentiated tumors (7.7% and 4.6%) in comparison to high-risk patients (>35 points) in whom the frequency of occurrence was to 70.6% and 47.1%, respectively. According to the authors, this model is the most precise out of the existing models in the evaluation of recurrence risk and overall survival of patients who underwent LTx due to HCC [118].
The meta-analysis based on 40 495 cases of LTx due to HCC that was mentioned previously is very interesting. The authors do not suggest a model of recurrence risk, but they present its frequency in connection to AFP level with a cut-off of 50 ng/mL, type of transplantation (from a deceased or a living donor), meeting the Milan criteria, and cirrhosis etiology, as well as income and patient ethnicity. The overall average frequency of recurrence was 13%, while it was 11% in patients with AFP <50 ng/mL, 15% at AFP ≥50 ng/mL, 17% after LTx from a living donor, 14% after LTx from a deceased donor, 8% in patients meeting the Milan criteria, 28% in those outside these criteria, 18% in those with HBV, 11% in those with HCV, 8% in those with NASH, 10% in those with ALD, 13% in high-income patients, 15% in average-income patients, 19% in Asians, 16% in patients from Arab countries, 11% in patients from South America, and 12% in patients from the West [64].
Surveillance, Treatment of Recurrence
The majority of recurrence occurs within 2 years of transplantation. The average time to recurrence ranges between 15.8 and 17.8 months [7,91,120,121]. Late recurrences happen at between 2 and 5 years. A recurrence after 5 years is rare [18]. They concern the liver (from 27% to 45%), but more frequently they appear in other locations (around 60%), mainly in lungs and bones, and less often in the adrenal glands, peritoneum, and lymph nodes [7,18,91,121–123].
The most effective radical treatment for recurrence after LTx is resection. Repeated liver transplantation is not recommended. Recommendations for other methods of treatment of recurrence are not different from those used in cancer therapy before transplantation [7–8,21,28,120]. Screening after transplantation allows for early detection of recurrence and can identify patients who qualify for radical treatment, increasing the chances of survival [18,21,123].
Eligibility criteria for transplantation due to HCC [5,9–11], as well as numerous models of recurrence risk (which include RETREAT, MORAL and HALTHCC among others) [22,29,118–119], are useful in the creation of protocols for screening, especially for patients with increased recurrence risk. Each HCC patient eligible for LTx should have a planned scheme of postoperative screening [124]. However, there are no universal, uniform protocols of such screening, and transplantation centers follow their own regulations in the choice of tools for prognosis and screening [7,18,21]. According to work by Aggrawal et al, 80% of American liver transplantation centers conduct screening, but only 11% of centers use one of the above-mentioned models to forecast recurrence risk [18]. The majority of experts and investigators recommend performing CT/NMR with contrast of the abdominal cavity and chest as well as AFP every 3–6 months for 2–3 years. After this time, the interval between the tests is extended to 6–12 months. When recurrence is diagnosed, bone scintigraphy is usually performed [7,18,28,120,124–126].
Studies have shown that the average survival time until recurrence is diagnosed ranges between 10.1 and 12.4 months [7,28,121–122] and depends on various factors. Based on these factors, several scales of risk of death for patients diagnosed with recurrence have been created. Goldaracena et al [7] confirmed previous reports by Sapisochin et al [28] indicating 3 significant factors for poor prognosis: lack of possibility to treat radically (resection or thermoablation), AFP level at time of diagnosis ≥100 ng/mL, and early recurrence (<12 months after LTx). Patients who do not meet any of the above-mentioned criteria (possible resection of the tumor, low AFP level, late recurrence) are classified as a low-risk group. Patients meeting 1–2 criteria belong to an average-risk group, and those meeting all 3 criteria belong to a high-risk group. One-year, 3-year, and 5-year survival, depending on the risk group, were 73% versus 55% versus 17%, 41% versus 19% versus 0%, and 34% versus 6% versus 0%, respectively [7].
Researchers from a center in San Francisco analyzed numerous factors such as recipient’s MELD score before transplantation >23, time of recurrence, less than 3 tumors, the maximum size of the tumor, recurrence in bones, AFP level at recurrence diagnosis, donor’s sodium level, and preoperative neutrophil-to-lymphocyte ratio, divided patients into 3 groups: low-risk, average-risk, and high-risk. The average survival in the low-risk, average-risk, and high-risk groups were 70.6, 12.2, and 3.4 months, respectively [120]. Other authors also reported poor prognosis for patients with early recurrence [122] and not qualifying for radical treatment, while 1-year and 2-year survival after radical recurrence resection was 93.8% and 52.6%, respectively [127]. Some authors indicate the benefits of immunosuppression conversion to sirolimus in patients who are not treated surgically [121,127,128]. In one report, survival time of patients treated with sirolimus from the moment of recurrence diagnosis was 12 months in comparison to 8 months in the group treated with the unmodified immunosuppression protocol [128]. Overall survival of patients who tolerated sorafenib well and were treated with lenvatinib was 19.5 months and was 7 months longer than in patients who discontinued sorafenib treatment or received regorafenib as the second-line drug [127]. According to De Simone et al, the use of sorafenib in recurrence treatment is debatable due to inconclusive results and frequent complications forcing discontinuation of therapy [129]. Patients in the highest-risk group, treated symptomatically, did not survive 1 year [120,127].
Conclusions
Liver transplantation is the best therapeutic option for patients with early-stage HCC, especially those meeting the Milan criteria. Numerous studies on risk factors of poor prognosis after transplantation have allowed some centers to expand the criteria to include patients with more advanced cancer to be eligible for the procedure. While it allowed broader access to definitive treatment, on the other hand, it increased rates of recurrence, which due to an unfavorable prognosis, became a challenge for patients and clinicians. Identification of more recurrence risk factors by postoperative pathological evaluation of the extracted liver has allowed the generation of various risk models of transplantation failure, which enable the classification of patients as low-risk or high-risk patients. There are no prospective studies allowing the creation of individual screening protocols based on the risk group, which would enable early diagnosis of recurrence and effective treatment. There are also no recommendations concerning optimal treatment methods for recurrence. Due to the increasing number of liver transplantations performed due to HCC, the need for such evidence-based recommendations is urgent.
References
1. Ishizaki Y, Kawasaki S, The evolution of liver transplantation for hepatocellular carcinoma (past, present, and future): J Gastroenterol, 2008; 43(1); 18-26
2. Ringe B, Pichlmayr R, Wittekind C, Tusch G, Surgical treatment of hepatocellular carcinoma: Experience with liver resection and transplantation in 198 patients: World J Surg, 1991; 15; 270-85
3. Bismuth H, Chiche L, Adam R, Liver resection versus transplantation for hepatocellular carcinoma in cirrhosis: Ann Surg, 1993; 218; 145-51
4. Stone MJ, Fulmer JM, Klintmalm GB, Transplantation for primary hepatic malignancy: Transplantation of the liver, 2005; 211-32, Philadelphia, Saunders
5. Mazzaferro V, Regalia E, Doci R, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis: N Engl J Med, 1996; 334; 693-99
6. Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ, The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time: J Clin Oncol, 2003; 21; 4329-35
7. Goldaracena N, Mehta N, Scalera , Multi-center validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation: HPB, 2019; 21(6); 731-38
8. Clavien P-A, Lesurtel M, Bossuyt PHCC Consensus Group, Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report: Lancet Oncol, 2012; 13; e11-22
9. DuBay D, Sandroussi C, Sandhu L, Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion: Ann Surg, 2011; 253; 166-72
10. Yao FY, Ferrell L, Bass NM, Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival: Hepatology, 2001; 33(6); 1394-403
11. Mazzaferro V, Llovet JM, Miceli RMetroticket Investigator Study Group, Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis: Lancet Oncol, 2009; 10; 35-43
12. Onaca N, Davis GL, Goldstein RM, Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: A report from the International Registry of Hepatic Tumors in Liver Transplantation: Liver Transpl, 2007; 13(3); 391-99
13. Ito T, Takada Y, Ueda M, Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation: Liver Transpl, 2007; 13; 1637-44
14. Mazzaferro V, Sposito C, Zhou J, Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma: Gastroenterology, 2018; 154(1); 128-39
15. Pomfret EA, Washburn K, Wald C, Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States: Liver Transpl, 2010; 16(3); 262-78
16. Yao FY, Mehta N, Flemming J, Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria: Hepatology Jun, 2015; 61(6); 1968-77
17. Schlansky B, Chen Y, Scott DL, Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: A cohort study using the United Network for Organ Sharing registry: Liver Transpl, 2014; 20; 1045-56
18. Aggarwal A, Te HS, Verna EC, Desai AP, A national survey of hepatocellular carcinoma surveillance practices following liver transplantation: Transplant Direct, 2020; 7(1); e638
19. Filgueira N, A Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation: World J Hepatol, 2019; 11(3); 261-72
20. Najjar M, Agrawal S, Emond JC, Halazun KJ, Pretreatment neutrophil-lymphocyte ratio: Useful prognostic biomarker in hepatocellular carcinoma: J Hepatocell Carcinoma, 2018; 5; 17-28
21. Berenguer M, Burra P, Ghobrial M, Posttransplant management of recipients undergoing liver transplantation for hepatocellular carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference: Transplantation, 2020; 104(6); 1143-49
22. Halazun KJ, Najjar M, Abdelmessih RM, Recurrence after liver transplantation for hepatocellular carcinoma: A mew MORAL to the story: Ann Surg, 2017; 265(3); 557-64
23. Dvorchik I, Schwartz M, Fiel MI, Fractional allelic imbalance could allow for the development of an equitable transplant selection policy for patients with hepatocellular carcinoma: Liver Transpl, 2008; 14; 443-50
24. Nault JC, De Reyniès A, Villanueva A, A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection: Gastroenterology, 2013; 145(1); 176-87
25. Shim JH, Lee HC, Han S, Hepatocyte nuclear factor 1β is a novel prognostic marker independent of the Milan criteria in transplantable hepatocellular carcinoma: A retrospective analysis based on tissue microarrays: Liver Transpl, 2013; 19(3); 336-45
26. Shim JH, Kang HJ, Han S, Prognostic value of hepatocyte nuclear factors 4α and 1α identified by tissue microarray in resectable hepatocellular carcinoma: J Gastroenterol Hepatol, 2014; 29(3); 524-32
27. Mahmud N, John B, Taddei TH, Pre-transplant alpha-fetoprotein is associated with post-transplant hepatocellular carcinoma recurrence mortality: Clinical Transplantation, 2019; 33(7/e); 13634
28. Sapisochin G, Goldaracena N, Astete S, Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American series: Ann Surg Oncol, 2015; 22(7); 2286-94
29. Mehta N, Heimbach J, Harnois DM, Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant: JAMA Oncol, 2017; 3(4); 493-500
30. Bürger Ch, Maschmeier M, Hüsing-Kabar A, Achieving complete remission of hepatocellular carcinoma: A significant predictor for recurrence-free survival after liver transplantation: Can J Gastroenterol Hepatol, 2019; 2019; 5796074
31. Germani G, Gurusamy K, Garcovich M, Which matters most: number of tumors, size of the largest tumor, or total tumor volume?: Liver Transpl, 2011; 17(Suppl 2); S58-66
32. Welling TH, Eddinger K, Carrier K, Multicenter study of staging and therapeutic predictors of hepatocellular carcinoma recurrence following transplantation: Liver Transpl, 2018; 24; 1233-42
33. Berry K, Ioannou GN, Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma: Liver Transpl, 2013; 19; 634-45
34. Giard J-M, Mehta N, Dodge JL, Alpha-fetoprotein slope >7.5 ng/mL per month predicts microvascular invasion and tumor recurrence after liver transplantation for hepatocellular carcinoma: Transplantation, 2018; 102(5); 816-22
35. Toso C, Asthana S, Bigam DL, Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database: Hepatology, 2009; 49; 832-38
36. Toso C, Meeberg G, Hernandez-Alejandro R, Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation: Hepatology, 2015; 62; 158-65
37. Hameed B, Mehta N, Sapisochin G, Alpha-fetoprotein level >1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria: F Liver Transplantation, 2014; 20(8); 945-51
38. Yang M, Tan W, Yang X, Homocysteine: A novel prognostic biomarker in liver transplantation for alpha-fetoprotein-negative hepatocellular carcinoma: Cancer Biomarkers, 2020; 29(2); 197-206
39. Templeton AJ, McNamara MG, Šeruga B, Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and metaanalysis: J Natl Cancer Inst, 2014; 106; dju124
40. Halazun KJ, Aldoori A, Malik HZ, Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases: Eur J Surg Oncol, 2008; 34(1); 55-60
41. Halazun KJ, Hardy MA, Rana AA, Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma: Ann Surg, 2009; 250; 141-51
42. Brodsky SV, Mendelev N, Melamed M, Ramaswamy G, Vascular density and VEGF expression in hepatic lesions: J Gastrointestin Liver Dis, 2007; 16(4); 373-77
43. Motomura T, Shirabe K, Mano Y, Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment: J Hepatol, 2013; 58(1); 58-64
44. Lai Q, Avolio AW, Manzia TM, Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation: Clin Transplant, 2012; 26(2); E125-31
45. Duvoux C, Roudot-Thoraval F, Decaens TLiver Transplantation French Study Group, Liver transplantation for hepatocellular carcinoma: A model including α-fetoprotein improves the performance of Milan criteria: Gastroenterology, 2012; 143; 986-94e3 quiz e14–15
46. Kornberg A, Küpper B, Tannapfel A, Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation: Liver Transpl, 2012; 18; 53-61
47. Takada Y, Kaido T, Shirabe KLTx-PET study group of the Japanese Society of Hepato-Biliary-Pancreatic Surgery and the Japanese Liver Transplantation Society, Significance of preoperative fluorodeoxyglucose-positron emission tomography in prediction of tumor recurrence after liver transplantation for hepatocellular carcinoma patients: A Japanese multi-center study: J Hepatobiliary Pancreat Sci, 2017; 24; 49-57
48. Kornberg A, Freesmeyer M, Bärthel E, 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients: Am J Transplant, 2009; 9(3); 592-600
49. Kim AY, Sinn DH, Jeong WK, Hepatobiliary MRI as novel selection criteria in liver transplantation for hepatocellular carcinoma: J Hepatol, 2018; 68; 1144-52
50. Agopian VG, Harlander-Locke MP, Ruiz RM, Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: Analysis of 3601 patients from the US multicenter HCC transplant consortium: Ann Surg, 2017; 266; 525-35
51. Xu M, Doyle MM, Banan B, Neoadjuvant locoregional therapy and recurrent hepatocellular carcinoma after liver transplantation: J Am Coll Surg, 2017; 225; 28-40
52. Otto G, Schuchmann M, Hoppe-Lotichlus M, How to decide about liver transplantation in patients with hepatocellular carcinoma: Size and number of lesions or response to TACE?: J Hepatol, 2013; 59; 279-84
53. Kulik L, Heimbach JK, Zaiem F, Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and metaanalysis: Hepatology, 2018; 67; 381-400
54. Straś W, Małkowski P, Tronina O, Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis – epidemiology, risk factors, clinical implications and treatment: Clin Exp Hepatol, 2020; 6(3); 170-75
55. Mikolasevic I, Filipec-Kanizaj T, Milic S, Non-alcoholic fatty liver disease and liver transplantation – where do we stand?: World J Gastroenterol, 2018; 24; 1491-506
56. Cholankerii G, Patel R, Khurana S, Satapathy SK, Hepatocellular carcinoma in non-alcoholic steatohepatitis: Current knowledge and implications for management: World J Hepatol, 2017; 9; 533-43
57. Berkan-Kawinska A, Piekarska A, Hepatocellular carcinoma in non-alcohol fatty liver disease – changing trends and specific challenges: Curr Med Res Opin, 2020; 36; 235-43
58. Zoller H, Tilg H, Nonalcoholic fatty liver disease and hepatocellular carcinoma: Metabolism, 2016; 65; 1151-60
59. Takakura K, Oikawa T, Nakano M, Recent insights into the multiple pathways driving non-alcoholic steatohepatitis derive hepatocellular carcinoma: Front Oncol, 2019; 9; 762
60. Desterke C, Chiappini F, Lipid related genes altered in NASH connect inflammation in liver pathogenesis progression to HCC: A canonical pathway: Int J Mol Sci, 2019; 20; 5594
61. Siegel AB, Lim EA, Wang S, Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation: Transplantation, 2012; 94; 539-43
62. Mathur A, Franco ES, Leone JP, Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma: HPB (Oxford), 2013; 15; 504-10
63. Sadler EM, Mehta N, Mamatha Bhat M, Liver transplantation for NASH-related hepatocellular carcinoma versus non-NASH etiologies of hepatocellular carcinoma: Transplantation, 2018; 102(4); 640-47
64. Tan DJH, Wong C, Han Ng CH, A metaanalysis on the rate of hepatocellular carcinoma recurrence after liver transplant and associations to etiology, alpha-fetoprotein, income and ethnicity: J Clin Med, 2021; 10(2); 238
65. Li MS, Hou ZH, Yao GZ, Tan DM, The strategy and efficacy of prophylaxis against hepatitis B virus recurrence after liver transplantation for HBV-related diseases in the era of potent nucleos(t)ide analogues: A metaanalysis: J Dig Dis, 2021; 22(2); 91-101
66. Yuan P, Chen P, Qian Y, Evaluation of antiviral therapy performed after curative therapy in patients with HBV-related hepatocellular carcinoma: An updated meta-analysis: Can J Gastroenterol Hepatol, 2016; 2016; 5234969
67. Wu TJ, Chan KM, Chou HS, Liver transplantation in patients with hepatitis B virus-related hepatocellular carcinoma: The influence of viral characteristics on clinical outcome: Ann Surg Oncol, 2013; 20; 3582-90
68. Li MR, Chen GH, Cai CJ, High hepatitis B virus DNA level in serum before liver transplantation increases the risk of hepatocellular carcinoma recurrence: Digestion, 2011; 84; 134-41
69. Teng C-F, Wu H-C, Su I-J, Jeng L-B, Hepatitis B virus pre-S mutants as biomarkers and targets for the development and recurrence of hepatocellular carcinoma: Viruses, 2020; 12(9); 945
70. Reig M, Mariño Z, Perelló C, Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy: J Hepatol, 2016; 65(4); 719-26
71. Conti F, Buonfiglioli F, Scuteri A, Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals: J Hepatol, 2016; 65(4); 727-33
72. ANRS collaborative study group on hepatocellular carcinoma, Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts: J Hepatol, 2016; 65(4); 734-40
73. Nault J-C, Colombo M, Hepatocellular carcinoma and direct acting antiviral treatments: Controversy after the revolution: J Hepatol, 2016; 65(4); 663-65
74. Guarino M, Sessa A, Cossiga V, Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver: World J Gastroenterol, 2018; 24(24); 2582-95
75. Singal AG, Rich NE, Mehta N, Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multi-center North American cohort study: Gastroenterology, 2019; 156(6); 1683-92
76. Singal AG, Lim JK, Kanwal F, AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: Expert review: Gastroenterology, 2019; 156(8); 2149-57
77. Adhoute X, Penaranda G, Raoul JL, Hepatocellular carcinoma recurrence in hepatitis C virus-related cirrhosis treated with direct-acting antivirals: A case-control study: Eur J Gastroenterol Hepatol, 2018; 30(4); 368-75
78. Kohli V, Singhal A, Elliott L, Jalil S, Antiviral therapy for recurrent hepatitis C reduces recurrence of hepatocellular carcinoma following liver transplantation: Transpl Int, 2012; 25; 192-200
79. Terrault NA, Berenguer M, Strasser SI, International Liver Transplantation Society consensus statement on hepatitis C management in liver transplant recipients: Transplantation, 2017; 101(5); 956-67
80. Gorgen A, Galvin Z, Huang AC, The impact of direct-acting antivirals on overall mortality and tumoral recurrence in patients with hepatocellular carcinoma listed for liver transplantation: An international multicenter study: Transplantation, 2020; 104(10); 2087-96
81. Jain A, Miller D, Schreibman I, Is there increased risk of hepatocellular carcinoma recurrence in liver transplant patients with direct-acting antiviral therapy?: Hepatol Int, 2019; 13(2); 190-98
82. Lim N, Singh D, Jackson S, Lake JR, Recurrence of hepatocellular carcinoma in hepatitis C virus (HCV) liver transplant recipients treated with pretransplant direct-acting antiviral (DAA) therapy: Gastrointest Tumors, 2020; 7(4); 134-43
83. Zanetto A, Shalaby S, Vitale A, Dropout rate from the liver transplant waiting list because of hepatocellular carcinoma progression in hepatitis C virus-infected patients treated with direct-acting antivirals: Liver Transpl, 2017; 23(9); 1103-12
84. Piñero F, Boin I, Chagas A, Direct-acting antivirals and hepatocellular carcinoma: No evidence of higher wait-list progression or posttransplant recurrence: Liver Transpl, 2020; 26(5); 640-50
85. European Association for the Study of the Liver, EASL clinical practice guidelines: Management of hepatocellular carcinoma: J Hepatol, 2018; 69; 182-236
86. Saborido BP, Díaz JC, de Los Galanes SJ, Does preoperative fine needle aspiration-biopsy produce tumor recurrence in patients following liver transplantation for hepatocellular carcinoma?: Transplant Proc, 2005; 37; 3874-77
87. Lopez KT, Kuwada SK, Wong LL, Consequences of needle tract seeding of hepatocellular cancer after liver transplant: Clin Transplant, 2013; 27; E400-6
88. Samoylova ML, Dodge JL, Yao FY, Roberts JP, Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation: Liver Transpl, 2014; 20; 937-44
89. Mehta N, Heimbach J, Lee D, Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: Proposing a wait time “sweet spot”: Transplantation, 2017; 101; 2071-78
90. Donat M, Alonso S, Pereira F, Impact of histological factors of hepatocellular carcinoma on the outcome of liver transplantation: Transplant Proc, 2016; 48; 1968-77
91. Agopian VG, Harlander-Locke M, Zarrinpar A, A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: Analysis of 865 consecutive liver transplant recipients: J Am Coll Surg, 2015; 220; 416-27
92. Sharma P, Welch K, Hussain H, Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era: Dig Dis Sci, 2012; 57; 806-12
93. Vagefi PA, Dodge JL, Yao FY, Roberts JP, Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation: Liver Transpl, 2015; 21; 187-94
94. Orci LA, Berney T, Majno PE, Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation: Br J Surg, 2015; 102(10); 1250-57
95. Kim JM, Chung YJ, Kim S, Impact of graft weight change during perfusion on hepatocellular carcinoma recurrence after living donor liver transplantation: Front Oncol, 2021; 10; 609844
96. Lozanovski VJ, Döhler B, Weiss KH, The differential influence of cold ischemia time on outcome after liver transplantation for different indications – who is at risk? A collaborative transplant study report: Front Immunol, 2020; 11; 892
97. Nagai S, Yoshida A, Facciuto M, Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation: Hepatology, 2015; 61(3); 895-904
98. Kornberg A, Witt U, Kornberg J, Extended ischemia times promote risk of HCC recurrence in liver transplant patients: Dig Dis Sci, 2015; 60(9); 2832-39
99. Mangus RS, Fridell JA, Vianna RM, Use of the piggyback hepatectomy technique in liver transplant recipients with hepatocellular carcinoma: Transplantation, 2008; 85; 1496-99
100. Grąt M, Kornasiewicz O, Lewandowski Z, The impact of surgical technique on the results of liver transplantation in patients with hepatocellular carcinoma: Ann Transplant, 2013; 18; 448-59
101. Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma: J Hepatol, 2013; 59; 1193-99
102. Schwartz M, Konstadoulakis M, Roayaie S, Recurrence of hepatocellular carcinoma after liver transplantation: Is immunosuppression a factor?: Liver Transpl, 2005; 11(5); 494-96
103. Chen ZS, He F, Zeng FJ, Early steroid withdrawal after liver transplantation for hepatocellular carcinoma: World J Gastroenterol, 2007; 13(39); 5273-76
104. Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: A multi-center study of 412 patients: World J Gastroenterol, 2006; 12(45); 7319-25
105. Menon KV, Hakeem AR, Heaton ND, Metaanalysis: Recurrence and survival following the use of Sirolimus in liver transplantation for hepatocellular carcinoma: Aliment Pharmacol Ther, 2013; 37; 411-19
106. Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P, Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: A systematic review: Transpl Int, 2014; 27; 1039-49
107. Toso C, Meeberg GA, Bigam DL, De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: Long-term outcomes and side effects: Transplantation, 2007; 83(9); 1162-68
108. Castroagudín JF, Molina-Pérez E, Ferreiro-Iglesias R, Varo-Pérez E, Strategies of immunosuppression for liver transplant recipients with hepatocellular carcinoma: Transplant Proc, 2011; 43(3); 711-13
109. Tarantino G, Magistri P, Ballarin R, Oncological impact of M-Tor inhibitor immunosuppressive therapy after liver transplantation for hepatocellular carcinoma: Review of the literature: Front Pharmacol, 2016; 7; 387
110. Geissler EK, Schnitzbauer AA, Zülke C, Sirolimus use in liver transplant recipients with hepatocellular carcinoma: A randomized, multicenter, open-label phase 3 trial: Transplantation, 2016; 100(1); 116-25
111. Schnitzbauer AA, Filmann N, Adam R, mTOR inhibition is most beneficial after liver transplantation for hepatocellular carcinoma in patients with active tumors: Ann Surg, 2020; 272(5); 855-62
112. Gül-Klein S, Kästner A, Haber PK, Recurrence of hepatocellular carcinoma after liver transplantation is associated with episodes of acute rejections: J Hepatocell Carcinoma, 2021; 8; 133-43
113. Lai Q, Iesari S, Finkenstedt A, Hoppe-Lotichius M, Hepatocellular carcinoma recurrence after acute liver allograft rejection treatment: A multi-center European experience: Hepatobiliary Pancreat Dis Int, 2019; 18(6); 517-24
114. Saab S, McTigue M, Finn RS, Busuttil RW, Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: Feasibility and efficacy: Exp Clin Transplant, 2010; 8; 307-13
115. Teng CL, Hwang WL, Chen YJ, Sorafenib for hepatocellular carcinoma patients beyond Milan criteria after orthotopic liver transplantation: A case control study: World J Surg Oncol, 2012; 10; 41
116. Satapathy SK, Das K, Kocak M, No apparent benefit of preemptive sorafenib therapy in liver transplant recipients with advanced hepatocellular carcinoma on explant: Clin Transplant, 2018; 32; e13246
117. Dikilitas M, Why adjuvant and neoadjuvant therapy failed in HCC. Can the new immunotherapy be expected to be better?: J Gastrointest Cancer, 2020; 51(4); 1193-96
118. Firl DJ, Sasaki K, Agopian VG, Charting the path forward for risk prediction in liver transplant for hepatocellular carcinoma: International validation of HALTHCC among 4,089 patients: Hepatology, 2020; 71(2); 569-82
119. Sasaki K, Firl DJ, Hashimoto K, Development and validation of the HALT-HCC score to predict mortality in liver transplant recipients with hepatocellular carcinoma: A retrospective cohort analysis: Lancet Gastroenterol Hepatol, 2017; 2(8); 595-603
120. Bodzin AS, Lunsford KE, Markovic D, Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: Impact of treatment modality and recurrence characteristics: Ann Surg, 2017; 266; 118-25
121. Ekpanyapong S, Philips N, Loza Bao-Li, Predictors, presentation, and treatment outcomes of recurrent hepatocellular carcinoma after liver transplantation: A large single center experience: J Clin Exp Hepatol, 2020; 10(4); 304-15
122. Ho CM, Lee CH, Lee MC, Survival after treatable hepatocellular carcinoma recurrence in liver recipients: A nationwide cohort analysis: Front Oncol, 2021; 10; 616094
123. Lee DD, Sapisochin G, Mehta N, Surveillance for HCC after liver transplantation: increased monitoring may yield aggressive treatment options and improved postrecurrence survival: Transplantation, 2020; 104; 2105-12
124. Verna EC, Patel YA, Aggarwal A, Liver transplantation for hepatocellular carcinoma: Management after the transplant: Am J Transplant, 2020; 20; 333-47
125. Roayaie S, Schwartz JD, Sung MW, Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis: Liver Transpl, 2004; 10; 534-40
126. Toso C, Cader S, Mentha-Dugerdil A, Factors predicting survival after post-transplant hepatocellular carcinoma recurrence: J Hepatobiliary Pancreat Sci, 2013; 20; 342-47
127. Yang Z, Wang S, Tian XY, Impact of treatment modalities on patients with recurrent hepatocellular carcinoma after liver transplantation: Preliminary experience: Hepatobiliary Pancreat Dis Int, 2020; 19(4); 365-70
128. Xu SL, Zhang YC, Wang GY, Survival analysis of sirolimus-based immunosuppression in liver transplantation in patients with hepatocellular carcinoma: Clin Res Hepatol Gastroenterol, 2016; 40(6); 674-81
129. De Simone P, Crocetti L, Pezzati D, Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation: Transplant Proc, 2014; 46(1); 241-44
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860