09 December 2022: Original Paper
Modification of Venous Outflow to Avoid Thrombotic Graft Failure in Pancreas Transplantation
Je Ho Ryu1ABE, Jae Ryong Shim1ABC, Tae Beom Lee1BCD, Kwangho Yang1ADE, Taeun Kim2ABF, Seo Rin KimDOI: 10.12659/AOT.937514
Ann Transplant 2022; 27:e937514
Abstract
BACKGROUND: Even with recent data, 5-10% of pancreas transplants experience early technical failure. Graft thrombosis is the primary cause of early technical failure, even when only optimal grafts are used, as is the case in Korea. The purpose of this study was to determine whether we can avoid thrombotic graft failure by modifying venous outflow.
MATERIAL AND METHODS: Between March 2017 and December 2021, a total of 59 pancreas transplantations were performed. Until May 2019, 31 cases of fence-angioplasty with cadaveric vena cava were performed to graft portal veins (the vena cava group). Since then, a total of 28 aortic interposition grafts have been performed to graft portal veins (the aortic group).
RESULTS: Between the 2 groups, there was no significant difference in baseline characteristics. Each group had 1 instance of technical failure. Early graft failure rates were 3.2% and 3.4%, respectively (P=1.000), while 1-year graft survival rates were 96.8% and 94.4%, respectively (P=0.991). Although a graft-threatening thrombosis occurred in the vena cava group, neither group experienced thrombotic graft failure, despite the decreased (vena cava group) or absence of heparin use (aorta group).
CONCLUSIONS: When the optimal graft is used, both techniques of modifying venous outflow can significantly reduce thrombotic graft failure.
Keywords: Pancreas Transplantation, Postoperative Complications, Thrombosis, Humans
Background
According to the OPTN/SRTR 2020 annual data report, the rate of pancreatic graft failure within the first 90 days post-transplant was 4.7~8.8% between 2018 and 2020 [1].It is widely accepted that the primary cause of early technical failure is thrombotic graft failure [2]. Indeed, over the last 4 decades, the incidence of thrombotic graft failure has remained unchanged [3]. Numerous risk factors for early graft thrombosis have been identified. Age >50 years, high body mass index (BMI) (>30 kg/m2), and a cardiovascular cause of death are risk factors for donors. Age >50 years, a high BMI (>30 kg/m2), and a prolonged cold ischemic time (>24 h) are factors associated with recipients [4].
By contrast, the surgical technique of venous outflow to avoid graft congestion has been extensively studied in liver transplantation, particularly in living donor liver transplantation [5–8]. The method of right and middle hepatic vein reconstruction is essential for the best outcome in living donor liver transplantation [9]. However, only a few studies of surgical modification to prevent graft thrombosis in pancreas transplantation have been conducted [10–14]. The reason for this relatively low level of attention to surgical technique could be the widely held belief that graft thrombosis occurs as a result of poor graft quality or the recipient’s medical condition, and thus surgical modification cannot be considered a solution for thrombosis. Only 7 transplant centers in Korea performed pancreas transplants in 2019, whereas 80 centers performed kidney transplants. Thus, centers capable of performing pancreas transplants can select the optimal graft from a relatively large pool of cadaveric donors [15]. Additionally, due to the developed transportation system and the country’s small size, cold ischemic time should be short. In this case, the surgical technique is more critical than the quality of the graft. The best outcome is possible when the optimal graft is used and no technical failures occur.
The purpose of this study was to determine whether modifying venous outflow can reduce thrombotic graft failure.
Material and Methods
STUDY DESIGN AND POPULATION:
We retrospectively reviewed 59 pancreas transplantations that were performed between March 2017 and December 2021 at Pusan National University Yangsan Hospital. There were 6 cases of simultaneous pancreas and kidney transplantation (SPK), 9 cases of simultaneous deceased pancreas and living kidney transplantation (SPLK), 15 cases of pancreas transplantation after kidney transplantation (PAK), and 29 cases of pancreas transplantation alone (PTA).
Technical failure was defined as graft loss within 90 days of transplantation as a result of non-immunologic factors such as thrombosis, infection, leakage, pancreatitis, bleeding, and patient death with a functional graft. Technical failure due to graft thrombosis was defined as thrombotic graft failure. Graft failure was defined by the absence of any evidence for clinically significant C-peptide production [16].
The Pusan National University Yangsan Hospital’s Institutional Review Board (IRB) approved this study (IRB number: 05-2022-081). The Ethics Committee waived the requirement for informed consent due to the study’s retrospective design and the absence of diagnostic or therapeutic interventions.
PROCUREMENT:
The procedure for recovering the pancreas from a deceased donor is conventional. During the bench procedure, we obtained the remnant inferior vena cava (IVC) to perform the fence-angioplasty and the supra-celiac aorta to perform the aortic interposition graft. In Korea, histidine-tryptophan-ketoglutarate (HTK) solution is the only solution available for preserving abdominal organs. No additional washout of preservation fluid occurred during the bench procedure.
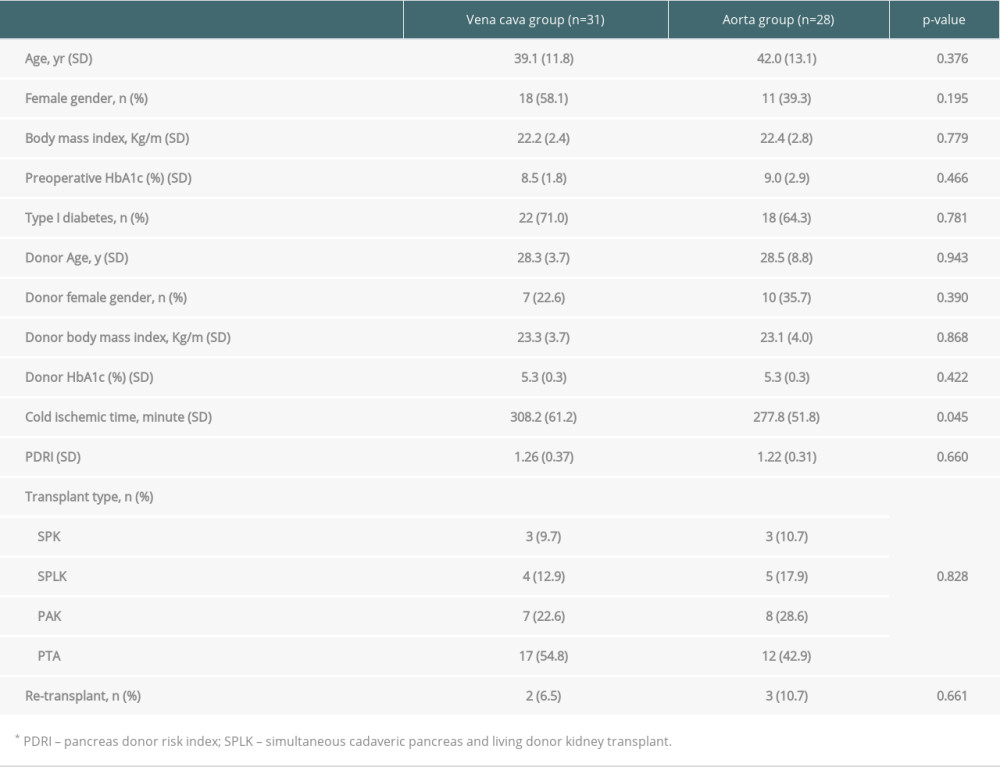
THE FENCE-ANGIOPLASTY OR AORTIC INTERPOSITION GRAFT TO GRAFT PORTAL VEIN IN BENCH PROCEDURE: In the first 31 cases of pancreas transplantation, we invented fence-angioplasty with cadaveric vena cava from the same donor (vena cava group, Figure 1A). This procedure resembled the quilt venoplasty technique used in living donor liver transplantation [11,17]. Additionally, in the last 28 cases of pancreas transplantation, the aortic interposition graft was used to graft the portal vein (aorta group, Figure 1B). This was a simple end-to-end anastomosis between the graft portal vein and the aortic graft.
THE RECIPIENT PROCEDURE: We have always used the recipient’s IVC for venous anastomosis via a modified procedure that includes both groups of fence-angioplasty using the vena cava and aortic interposition graft (Figure 2). Intraoperative Doppler ultrasound was always performed to assess the graft pancreas’s circulation prior to wound closure (Figure 3). We performed duodeno-duodenostomy in most cases for exocrine drainage; small bowel drainage was performed in only 2 cases in the vena cava group.
Our surgical procedure was described in full in a previous paper [10].
ANTICOAGULATION:
During their hospital stay, the 25 recipients in the vena cava group received low-molecular-weight heparin (LMWH, 1 mg/kg/day for the first 7 recipients and 0.5 mg/kg/day or less for the remaining 18 recipients). No heparin was prescribed postoperatively in the last 6 cases in the vena cava group and in all cases in the aorta group.
STATISTICAL ANALYSIS:
Absolute numbers and relative frequencies were used to express categorical variables. Mean and standard deviation were used to express quantitative variables. The
Results
DEMOGRAPHICS AND BASELINE CHARACTERISTICS:
Table 1 summarizes the baseline characteristics of transplant recipients. There were no significant differences in the profiles of donors or recipients. Although the cold ischemic time was longer in the vena cava group, neither group had a cold ischemic time greater than 7 h. In both groups, the pancreas donor risk index (PDRI) suggested by Axelrod et al was determined [18]. The short cold ischemic time and donor and recipient lean body mass contributed to our excellent results.
SURGICAL OUTCOME:
In the vena cava group, we encountered 1 case of immediate postoperative death due to hypoxic brain damage caused by an anesthetic accident. And, in the aorta group, immediate graftectomy was performed following reperfusion due to unexplained subcapsular petechiae, subcapsular hematoma, and absence of venous flow in the pancreatic graft. Apart from these 2 technical failures, neither group experienced any other failures. In the vena cava group, an emergent thrombectomy was conducted (the last case of the vena cava group), which resulted in the venous outflow modification technique being changed to the aortic interposition graft from the fence-angioplasty, although the graft was saved following thrombectomy. All surgical complications, including these, are listed in Table 2. Heparin was used throughout the hospital stay only in the vena cava group.
On the first day of transplantation, all recipients underwent CT angiography. CT scans of both groups revealed seemingly harmless thrombi of the graft vein in some cases, except for 1 case in the vena cava group in which the thrombus threatened graft survival.
METABOLIC FINDINGS AFTER TRANSPLANT:
In both groups, postoperative C-peptide levels were increased, indicating a well-functioning graft. Following transplantation, fasting glucose and HbA1c levels were rapidly normalized. The postoperative 1-month C-peptide level was significantly higher in the aorta group, owing to the inclusion of PTA recipients with chronic kidney disease, including dialysis, who had impaired endogenous insulin metabolism (Figure 4). Except for the 2 technical failures, all recipients met Igls criteria for optimal β-cell graft function [15].
GRAFT SURVIVAL:
Graft survival at 1 year was 96.8% in the vena cava group and 94.4% in the aorta group. At 3 years, graft survival rates were 87.1% and 87.7%, respectively. Between the 2 groups, there was no significant difference (P=0.991, Figure 5). Within 1 year, SARS-CoV-2 virus infection resulted in 1 extra graft loss, in addition to a case of technical failure in the aorta group.
No graft failure was observed in the 8 recipients who received grafts from donors with a higher PDRI (>1.57) in both groups (5 in the vena cava group, 3 in the aorta group).
Discussion
Early pancreas transplant graft failure occurred at a rate of 7.2% in SPK, 4.7% in PAK, and 6.5% in PTA in 2020, according to the most recent report. Additionally, the failure rate of PAK grafts within 1 year was reported to be greater than 15% in 2018, and PTA grafts had a failure rate over 10% in 2019 in the United States [1]. If the pancreatic graft survived 1 year after transplant, the half-life of the graft was significantly increased. This means that minimizing graft loss during the first year was critical for long-term graft survival [19]. The primary cause of graft loss in the first year is technical failure, and the most common cause of technical failure is graft thrombosis [20,21]. As a result, if graft thrombosis can be reduced by modifying venous outflow, we can also improve long-term graft survival.
The proposed causes of graft thrombosis, which included donor age, donor cerebrovascular history, cold ischemic time, and prolonged cold ischemic time, were associated with graft quality [3,21]. Can we then anticipate the best outcome when the optimal graft is used for transplantation? Our data indicate that the graft was optimal in Korea in terms of donor age, body mass index, and cold ischemic time. This situation was similar in other transplant centers throughout Korea. However, the 1-year survival rate for grafts at other centers was 88.1% in 2020 [22]. Additionally, the postoperative thrombosis rate (not thrombotic graft failure) was 34% in Korea between 2015 and 2020 [22]. Without technological innovation, achieving the optimal result may be difficult.
Axelrod et al proposed PDRI as an indicator of allograft quality that predicts the risk of allograft failure at 1 year [18]. The mean PDRI among the 9401 pancreatic transplant recipients was 1.00. According to the authors’ proposed equation, Asian ethnicity is a risk factor. In addition, some donors had acute kidney injury, which was also regarded as a risk factor for graft failure. Consequently, they were responsible for the elevated PDRI in our study. However, there was no graft failure in the group with a higher PDRI (>1.57). This result may suggest that our modifications to venous outflow are superior.
In a previous article, we discussed graft pancreas thrombosis and prevention [10]. In situations involving pancreatic transplantation, there is no blood flow from the spleen, small intestine, or colon. The abrupt reduction in venous blood flow could be the risk factor for thrombosis. In addition, we have previously reported that if the portal vein is directly anastomosed with the recipient vein, postoperative CT scans always reveal a narrowing of the anastomotic site [11]. The constriction is not the result of a technical error, but rather decreased blood flow. Our experience indicates that a wide and sustainable venous anastomosis prevents graft thrombotic failure.
Due to our experience with the last recipient in the vena cava group, the venous outflow technique was changed from fence-angioplasty to aortic interposition graft. Re-exploration and thrombectomy were performed on the patient, resulting in a transient decrease in insulin secretion that resolved 1 week after re-exploration. However, graft function deteriorated over time, and one and a half years later, the patient required re-transplantation of the pancreas. Following this experience, we used an aortic interposition graft for venous outflow. The aortic interposition graft has several advantages over fence-angioplasty, including a faster procedure, less suture material remaining in the vascular lumen, and maintaining the orifice size of venous anastomosis. Figure 6 shows the schematic picture of our final version of the operative procedure. The vena cava used for venous anastomosis demonstrated outstanding short-term graft survival without graft thrombosis [23].
There was no need to prescribe any type of heparin to prevent thrombosis in the postoperative period for 6 recipients in the vena cava group and in all recipients in the aorta group (total 34/59). Our experiences led to a reduction in heparin use, and intraoperative Doppler findings indicated sufficient venous outflow to support discontinuation of heparin. Appropriate modification of venous outflow may result in a reduction in the need for anticoagulation.
Hakeem et al proposed a thrombosis grading system based on CT scans. In grade 1 (peripheral) and grade 2 (intermediate non-occlusive) thrombosis, anticoagulation therapy was unnecessary [24]. These criteria included all 3 of the thrombi detected by the CT scan in the aorta group.
Th present study has several limitations. This was a retrospective, single-center study involving a small number of pancreas transplants. The follow-up period was different between the 2 groups, as the fence-angioplasty was performed prior to the aortic interposition graft. We would appreciate the reader’s understanding of this as an evolution of operative procedure.
Despite these limitations, we are convinced that the modification of venous outflow, specifically the aortic interposition graft to the portal vein of the graft pancreas, as a simpler procedure, can significantly reduce thrombotic graft failure when the optimal graft is used. This technique could be validated in cases where a suboptimal graft was used in the United States or Europe.
Conclusions
In pancreas transplantation, we modified venous outflow with 2 surgical techniques. The first was a fence-angioplasty, and the second was an aortic interposition graft. Through these technical modifications, we were able to avoid early graft failure.
Figures
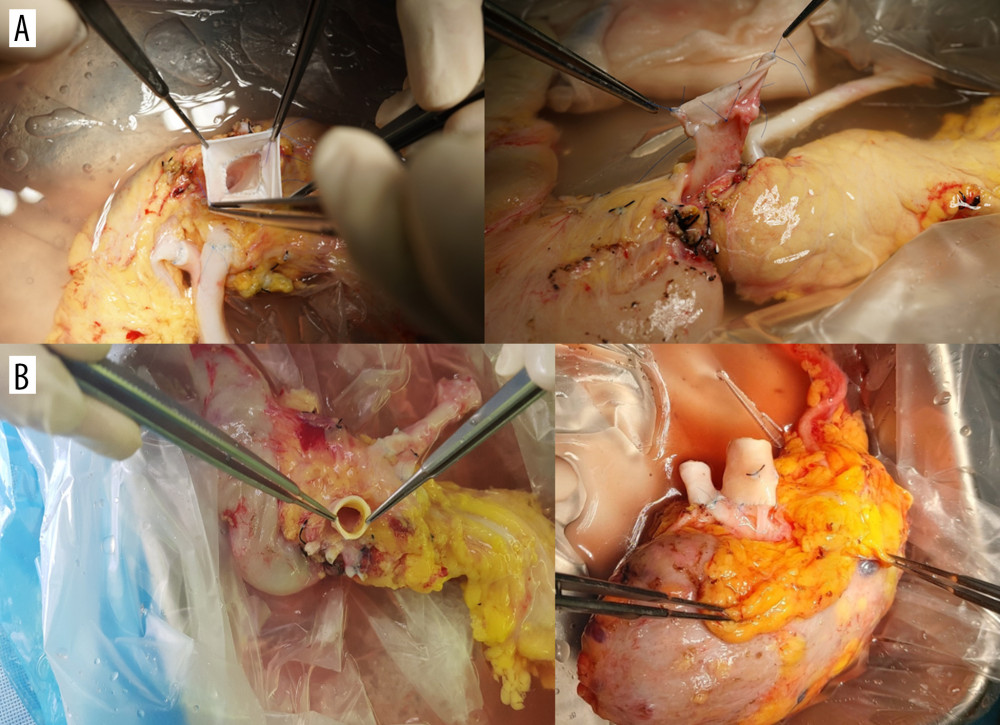 Figure 1. The modification of venous outflow. (A) The fence-angioplasty. To enlarge the anastomotic opening, the cadaveric vena cava was divided longitudinally and anastomosed to the graft portal vein horizontally. (B) The aortic interposition graft. The cadaveric aorta was anastomosed end-to-end to the portal vein of pancreatic graft.
Figure 1. The modification of venous outflow. (A) The fence-angioplasty. To enlarge the anastomotic opening, the cadaveric vena cava was divided longitudinally and anastomosed to the graft portal vein horizontally. (B) The aortic interposition graft. The cadaveric aorta was anastomosed end-to-end to the portal vein of pancreatic graft. 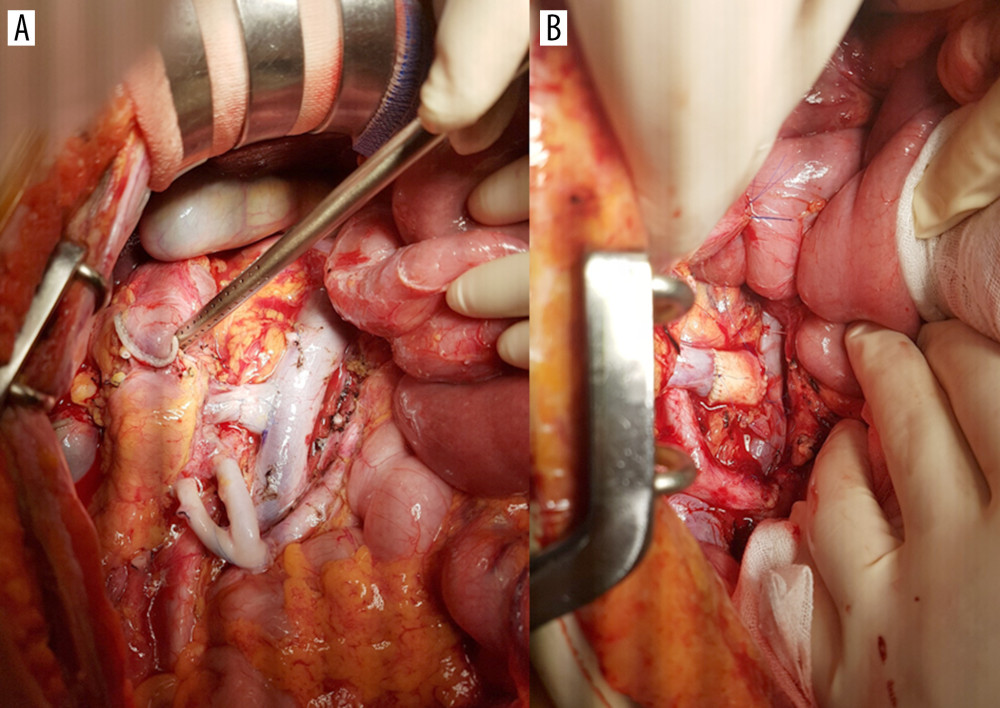 Figure 2. End-to-side anastomosis of the fence-angioplasty (A) and the aortic interposition graft (B) to the recipient’s vena cava.
Figure 2. End-to-side anastomosis of the fence-angioplasty (A) and the aortic interposition graft (B) to the recipient’s vena cava. 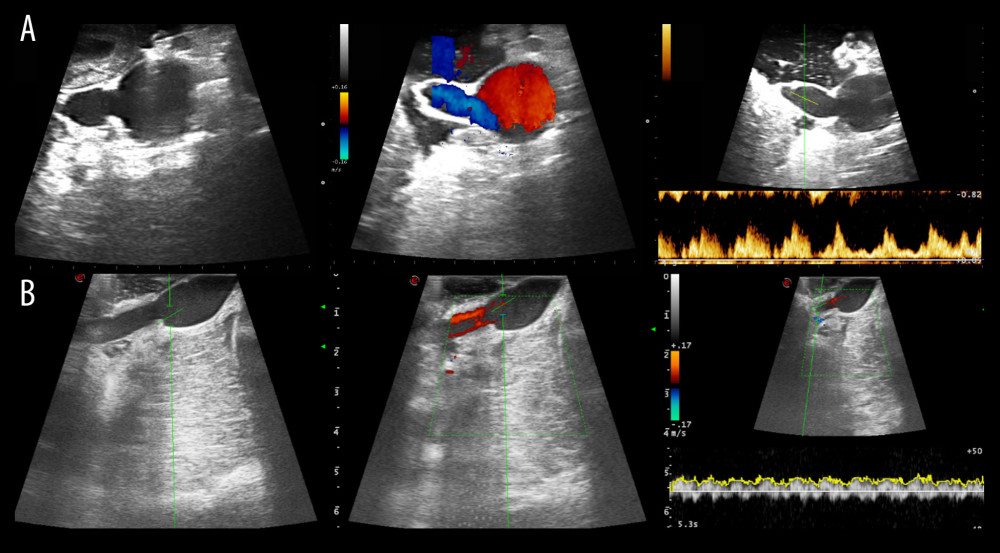 Figure 3. Intraoperative ultrasound findings. Anastomosis site structure, color Doppler and spectral wave of flow were evaluated in both groups (A, the vena cava group; B, the aorta group).
Figure 3. Intraoperative ultrasound findings. Anastomosis site structure, color Doppler and spectral wave of flow were evaluated in both groups (A, the vena cava group; B, the aorta group). 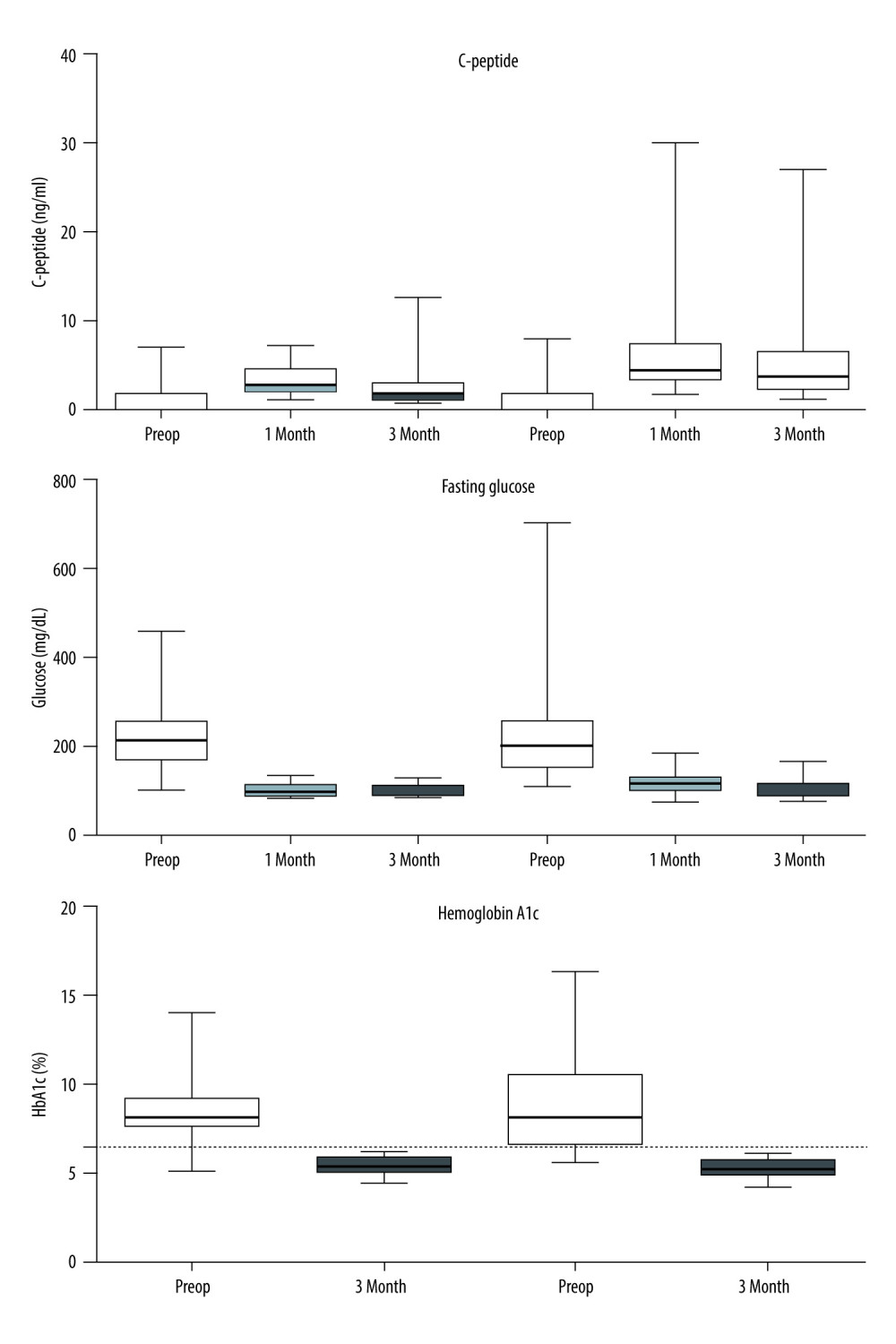 Figure 4. Metabolic findings in both groups. Empty boxes represent the IVC group; hatched boxes represent the aorta group. After transplantation, C-peptide levels were increased and fasting glucose levels were rapidly normalized in each group. The postoperative 1-month C-peptide level was significantly higher in the aorta group owing to the inclusion of PTA recipients with chronic kidney disease, including dialysis, who had impaired endogenous insulin metabolism. Hemoglobin A1c levels were all normalized (<6.5%) after transplant in both groups of recipients.
Figure 4. Metabolic findings in both groups. Empty boxes represent the IVC group; hatched boxes represent the aorta group. After transplantation, C-peptide levels were increased and fasting glucose levels were rapidly normalized in each group. The postoperative 1-month C-peptide level was significantly higher in the aorta group owing to the inclusion of PTA recipients with chronic kidney disease, including dialysis, who had impaired endogenous insulin metabolism. Hemoglobin A1c levels were all normalized (<6.5%) after transplant in both groups of recipients. 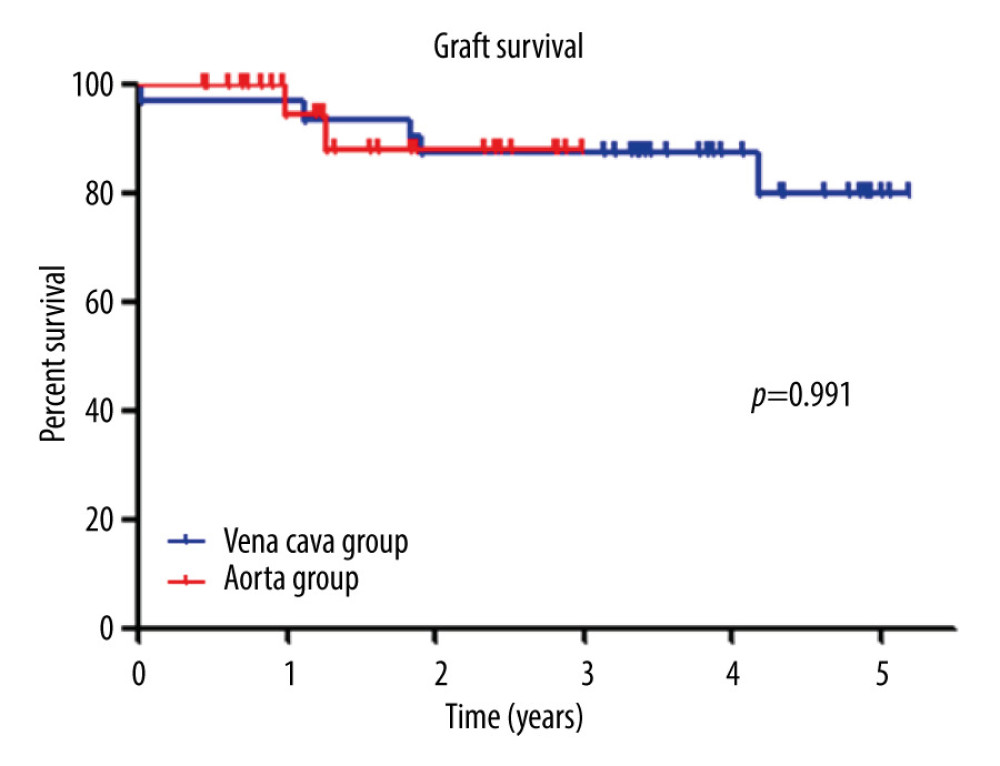 Figure 5. Graft survival. There was no difference in graft survival between the 2 groups (P=0.815). Graft survival at 1 year was 96.8% in the vena cava group and 92.6% in the aorta group.
Figure 5. Graft survival. There was no difference in graft survival between the 2 groups (P=0.815). Graft survival at 1 year was 96.8% in the vena cava group and 92.6% in the aorta group. 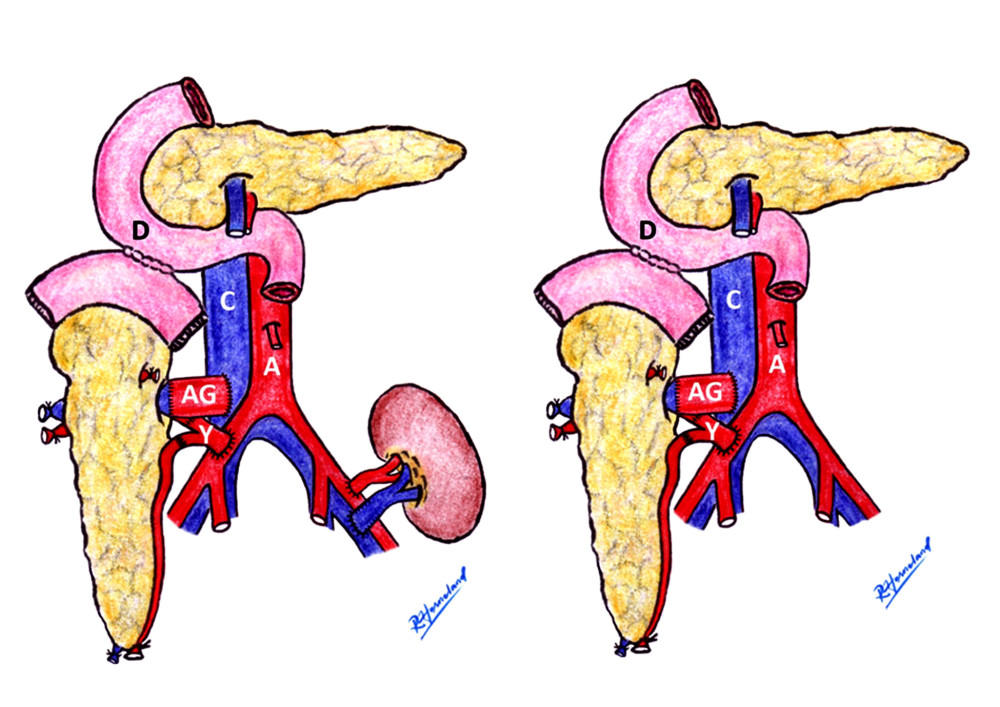 Figure 6. The aortic interposition graft schematic. Aortic interposition graft and duodenal exocrine drainage have been used to create a modified venous outlet. In the case of SPK or PAK, the transplanted kidney was implanted on the patient’s left side (left picture). The PTA case is shown on the right. Rune Horneland, a Norwegian transplant surgeon, created this illustration for this article. A – aorta; C – vena cava; D – duodenum, AG – aortic graft; Y – Y-graft.
Figure 6. The aortic interposition graft schematic. Aortic interposition graft and duodenal exocrine drainage have been used to create a modified venous outlet. In the case of SPK or PAK, the transplanted kidney was implanted on the patient’s left side (left picture). The PTA case is shown on the right. Rune Horneland, a Norwegian transplant surgeon, created this illustration for this article. A – aorta; C – vena cava; D – duodenum, AG – aortic graft; Y – Y-graft. References
1. Kandaswamy PGS, Miller J, White J, OPTN SRTR 2020 Annual Data Report: Pancreas: Am J Transplant, 2022; 22(Suppl 2); 137-203
2. Troppmann C, Complications after pancreas transplantation: Curr Opin Organ Transplant, 2010; 15(1); 112-18
3. Farney AC, Rogers J, Stratta RJ, Pancreas graft thrombosis: Causes, prevention, diagnosis, and intervention: Curr Opin Organ Transplant, 2012; 17(1); 87-92
4. Ramessur Chandran S, Kanellis J, Early pancreas allograft thrombosis: Clin Transplant, 2013; 27(3); 410-16
5. Balci D, Kirimker EO, Hepatic vein in living donor liver transplantation: Hepatobiliary Pancreat Dis Int, 2020; 19(4); 318-23
6. Hwang S, Jung DH, Ha TY, Usability of ringed polytetrafluoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation: Liver Transpl, 2012; 18(8); 955-65
7. Hwang S, Ha TY, Ahn CS, Hemodynamics-compliant reconstruction of the right hepatic vein for adult living donor liver transplantation with a right liver graft: Liver Transpl, 2012; 18(7); 858-66
8. Hwang S, Lee SG, Ahn CS, Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts: Liver Transpl, 2005; 11(6); 644-49
9. Hwang S, Ahn CS, Kim KH, Standardization of modified right lobe grafts to minimize vascular outflow complications for adult living donor liver transplantation: Transplant Proc, 2012; 44(2); 457-59
10. Ryu JH, Ko HJ, Shim JR, Technical factors that minimize the occurrence of early graft failure in pancreas transplantation: Clin Transplant, 2021; 35(11); e14455
11. Ryu JH, Lee TB, Yang KH, Fence angioplasty prevents narrowing of venous anastomosis in solitary pancreas transplant: Ann Transplant, 2018; 23; 681-90
12. Ryu JH, Lee TB, Park YM, Pancreas transplant with duodeno-duodenostomy and caval drainage using a diamond patch graft: A single-center experience: Ann Transplant, 2017; 22; 24-34
13. Aboalsamh G, Anderson P, Al-Abbassi A, Heparin infusion in simultaneous pancreas and kidney transplantation reduces graft thrombosis and improves graft survival: Clin Transplant, 2016; 30(9); 1002-9
14. Szmidt J, Lao M, Grochowiecki T, Pancreas transplantation: Four vascular anastomoses: Transplant Proc, 1996; 28(6); 3511-13
15.
16. Rickels MR, Stock PG, de Koning EJP, Defining outcomes for beta-cell replacement therapy in the treatment of diabetes: A consensus report on the Igls criteria from the IPITA/EPITA Opinion Leaders Workshop: Transplantation, 2018; 102(9); 1479-86
17. Hwang S, Lee SG, Ahn CS, Outflow vein reconstruction of extended right lobe graft using quilt venoplasty technique: Liver Transpl, 2006; 12(1); 156-58
18. Axelrod DA, Sung RS, Meyer KH, Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization: Am J Transplant, 2010; 10(4); 837-45
19. Redfield RR, Rickels MR, Naji A, Odorico JS, Pancreas transplantation in the modern era: Gastroenterol Clin North Am, 2016; 45(1); 145-66
20. Wallace DF, Bunnett J, Fryer E, Early allograft pancreatectomy – technical failure or acute pancreatic rejection?: Clin Transplant, 2019; 33(10); e13702
21. Finger EB, Radosevich DM, Dunn TB, A composite risk model for predicting technical failure in pancreas transplantation: Am J Transplant, 2013; 13(7); 1840-49
22.
23. Laftavi MR, Pankewycz O, Kohli R, Short and long-term outcomes of systemic drainage to IVC: A new technique for pancreas transplantation: Transplant Proc, 2014; 46(6); 1900-4
24. Hakeem A, Chen J, Iype S, Pancreatic allograft thrombosis: Suggestion for a CT grading system and management algorithm: Am J Transplant, 2018; 18(1); 163-179
Errate
Figures
 Figure 1. The modification of venous outflow. (A) The fence-angioplasty. To enlarge the anastomotic opening, the cadaveric vena cava was divided longitudinally and anastomosed to the graft portal vein horizontally. (B) The aortic interposition graft. The cadaveric aorta was anastomosed end-to-end to the portal vein of pancreatic graft.
Figure 1. The modification of venous outflow. (A) The fence-angioplasty. To enlarge the anastomotic opening, the cadaveric vena cava was divided longitudinally and anastomosed to the graft portal vein horizontally. (B) The aortic interposition graft. The cadaveric aorta was anastomosed end-to-end to the portal vein of pancreatic graft. Figure 2. End-to-side anastomosis of the fence-angioplasty (A) and the aortic interposition graft (B) to the recipient’s vena cava.
Figure 2. End-to-side anastomosis of the fence-angioplasty (A) and the aortic interposition graft (B) to the recipient’s vena cava. Figure 3. Intraoperative ultrasound findings. Anastomosis site structure, color Doppler and spectral wave of flow were evaluated in both groups (A, the vena cava group; B, the aorta group).
Figure 3. Intraoperative ultrasound findings. Anastomosis site structure, color Doppler and spectral wave of flow were evaluated in both groups (A, the vena cava group; B, the aorta group). Figure 4. Metabolic findings in both groups. Empty boxes represent the IVC group; hatched boxes represent the aorta group. After transplantation, C-peptide levels were increased and fasting glucose levels were rapidly normalized in each group. The postoperative 1-month C-peptide level was significantly higher in the aorta group owing to the inclusion of PTA recipients with chronic kidney disease, including dialysis, who had impaired endogenous insulin metabolism. Hemoglobin A1c levels were all normalized (<6.5%) after transplant in both groups of recipients.
Figure 4. Metabolic findings in both groups. Empty boxes represent the IVC group; hatched boxes represent the aorta group. After transplantation, C-peptide levels were increased and fasting glucose levels were rapidly normalized in each group. The postoperative 1-month C-peptide level was significantly higher in the aorta group owing to the inclusion of PTA recipients with chronic kidney disease, including dialysis, who had impaired endogenous insulin metabolism. Hemoglobin A1c levels were all normalized (<6.5%) after transplant in both groups of recipients. Figure 5. Graft survival. There was no difference in graft survival between the 2 groups (P=0.815). Graft survival at 1 year was 96.8% in the vena cava group and 92.6% in the aorta group.
Figure 5. Graft survival. There was no difference in graft survival between the 2 groups (P=0.815). Graft survival at 1 year was 96.8% in the vena cava group and 92.6% in the aorta group. Figure 6. The aortic interposition graft schematic. Aortic interposition graft and duodenal exocrine drainage have been used to create a modified venous outlet. In the case of SPK or PAK, the transplanted kidney was implanted on the patient’s left side (left picture). The PTA case is shown on the right. Rune Horneland, a Norwegian transplant surgeon, created this illustration for this article. A – aorta; C – vena cava; D – duodenum, AG – aortic graft; Y – Y-graft.
Figure 6. The aortic interposition graft schematic. Aortic interposition graft and duodenal exocrine drainage have been used to create a modified venous outlet. In the case of SPK or PAK, the transplanted kidney was implanted on the patient’s left side (left picture). The PTA case is shown on the right. Rune Horneland, a Norwegian transplant surgeon, created this illustration for this article. A – aorta; C – vena cava; D – duodenum, AG – aortic graft; Y – Y-graft. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860